Introduction
Hypopharyngeal carcinoma has one of the lowest
5-year-survival rates of head and neck tumors (1). Early diagnosis of hypopharyngeal
cancer is difficult, therefore the majority of these tumors are at
an advanced stage when they are diagnosed. The 5-year-survival
rates for patients treated with radiotherapy is 10–20%, with
surgery is 30–40%, and with a combination of surgery and
radiotherapy is 40–60% (2).
Despite improved therapeutic strategies in previous years, there
remains no effective treatment for this type of tumor. Thus, it is
important to identify markers associated with the pathogenesis and
development of hypopharyngeal carcinoma to enable earlier diagnosis
and treatment.
Angiogenesis, the process whereby new capillaries
form from pre-existing blood vessels, is key in physiological and
pathological conditions. Angiogenesis is important in normal
development and wound healing, and is critical for tumor growth,
invasion and metastasis (3–5).
Vascular endothelial growth factor (VEGF) stimulates tumor
angiogenesis (6). Three types of
VEGF receptor (VEGFR) tyrosine kinases have been identified,
including VEGFR-l, VEGFR-2 (also termed kinase insert domain
receptor) and VEGFR-3 (7,8). The VEGF gene encodes five polypeptide
growth factors, VEGF-A, -B, -C, -D and -E. VEGF-C, also termed
VEGF-related protein, has been characterized as a lymphangiogenic
and angiogenic growth factor (9).
The binding of VEGF-C to VEGFR results in phosphorylation of the
receptor. Activation of tyrosine kinases stimulates endothelial
cell mitosis, promotes tumor vascular endothelial cell
proliferation and induces tumor angiogenesis. VEGF-C and the VEGFR
are expressed in lymphatic endothelial cells and a variety of tumor
cells (10). Activation of the
VEGF-C/VEGFR signaling pathway stimulates the formation of blood
vessels and enhances cancer cell migratory and invasive abilities.
Thus, activation of this signaling pathway facilitates cancer cell
metastasis (8).
Down syndrome critical region 1 (DSCR1), induced
during cell adaptation to oxidative stress, belongs to a family of
evolutionarily conserved small proteins. The DSCR1 gene, located on
human chromosome 21, encodes a protein, which binds directly to the
calcineurin catalytic A subunit (11). DSCR1 also has a regulatory role in
calcineurin-mediated signaling (11,12).
It is expressed at high levels in the brain, heart and skeletal
muscle (11,13). DSCR1 has been implicated in several
diseases, including Alzheimer's disease, Down syndrome, cardiac
hypertrophy, gastrointestinal abnormalities and immune system
deficiencies (11,14–16).
In addition to its involvement in endothelial cell migration and
angiogenesis (17,18), DSCR1 has also been associated with
carcinogenesis and tumor growth. Epidemiological studies have
demonstrated that individuals with Down's syndrome are at higher
risk of leukemia and testicular cancer, compared with the general
population (19–21). However, certain studies have
suggested that DSCR1 exerts a tumor suppressive effect in cancer
via inhibiting tumor growth (22,23).
Therefore, the role of Down's syndrome and DSCR1 in cancer
development remains controversial.
Our previous study demonstrated that the protein
expression levels of DSCR1 were significantly higher in cancerous
tissues, compared with paracancerous tissues in patients with
hypopharyngeal cancer (24). DSCR1
was expressed in 94.9% of the cancerous tissues and in 35.9% of the
paracancerous tissues examined. The elevated protein levels of
DSCR1 were positively correlated with poorly differentiated tumors
and advanced tumor-lymph node-metastasis (TNM) stage in these
patients. Whether DSCR1 is also upregulated at the transcriptional
level in hypopharyngeal cancer remains to be elucidated. Studies
have demonstrated that the DSCR1 gene is a downstream target of
VEGF signaling, whereby activation upregulates the expression of
DSCR1 (18,25,26).
However, whether the mRNA levels of DSCR1 positively correlate with
the mRNA levels of VEGF-C in hypopharyngeal cancer remains to be
elucidated. The present study hypothesized that the mRNA levels of
DSCR1 in hypopharyngeal cancerous tissues are increased, and that
this elevation in the expression of DSCR1 is associated with
increased expression levels of VEGF-C. Therefore, the aims of the
present study were to determine the expression levels of DSCR1 and
VEGF-C in hypopharyngeal cancer, and to investigate the association
between DSCR1 and angiogenesis in this disease.
Patients and methods
Patients
The present study was approved by the Institutional
Ethics Committee of Qilu Hospital, Shandong University, (Jinan,
China), and complied with the 1964 Helsinki declaration (27) and its later amendments or
comparable ethical standards. Written informed consent was obtained
by the recruited patients or their family members. Between February
2009 and October 2010, 94 cases of pathologically confirmed
hypopharyngeal squamous cell carcinoma were examined in the
Department of Otolaryngology and Head and Neck Surgery, Qilu
Hospital, Shandong University (Jinan, China). The inclusion
criteria were as follows: Age >18 years; tumor type, primary
hypopharynx carcinoma; patients underwent surgical treatment;
pathological diagnosis of squamous cell carcinoma; patients
underwent radiotherapy and chemotherapy. The patients were divided
into two groups, according to age (<60 or ≥60 years old).
Histological differentiation of the cancer was defined as
well-differentiated, moderately differentiated or poorly
differentiated. TNM staging was performed, according to the 1997
criteria of the Union for International Cancer Control (28). The tumors were also classified by
their mode of growth, as either exogenous or infiltrative. In
addition, patient smoking histories were recorded.
Tissue specimen collection
All tissue specimens were collected from the
patients with primary hypopharyngeal tumors during surgery. The
cancerous tissue was collected from the center of the
hypopharyngeal tumor. The tissue adjacent to the tumor
(paracancerous tissue) was collected, from a site 10–20 mm from the
margin of the tumor. Following removal of the tissue, the specimens
were immediately rinsed in RNA enzyme-free saline (Shanghai Sangon
Biotechnology Co., Shanghai, China), treated with 0.1%
diethylpyrocarbonate, and the impurities, including blood clots and
eschar, were carefully removed. The specimens were immediately
transferred to RNA enzyme-free Eppendorf tubes and preserved in
liquid nitrogen. The remaining tumor tissue was prepared for
histological or immunohistochemical examination.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
A total of 65 frozen tissue samples were prepared. A
total of 100 mg of each tissue specimen was homogenized in a tissue
homogenizer containing 600 µl guanidium thiocyanate denaturing
solution. The organic and aqueous phases were separated by
centrifugation for 5 min at 13,350 × g. The RNA-containing aqueous
phase was carefully transferred into a fresh centrifuge tube, and
the RNA was precipitated by addition of an equal volume of cold
isopropanol. Subsequently, the samples were centrifuged ar 13,350 ×
g, 4°C for 20 min to pellet RNA. Following washing, RNA was
dissolved in RNase free water and stored at −20°C. Total RNA from
the cancerous and paracancerous tissues was extracted using a
TRIzol total RNA extraction kit (Shanghai Shenggong Biology
Engineering Technology Service, Ltd., Shanghai, China). First
strand complementary DNA (cDNA) was reverse transcribed using a
PrimeScript™ High Fidelity RT-PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China). The cDNA of the target genes was amplified
using PCR master mix purchased from Takara Bio, Inc. (Otsu, Japan).
The target gene primers (1 µl) were purchased from Shanghai Sangon
Biotechnology Co. Reverse transcription reactions were performed at
30°C for 15 min, 56°C for 35 min, 99°C for 5 min and 5°C for 5 min.
The conditions used for the PCR were as follows: 94°C for 2 min;
94°C for 45 sec, 55°C for 45 sec, 72°C for 90 sec for 30 cycles and
72°C for 5 min. The sequences of the primers were as follows: Sense
5′-TGCGACCCCAGTCATAAACTA-3′ and antisense 5′-CCATTTCCTCTTCTTCCT-3′
for DSCR1; sense 5′-CTCAAAAGTTATTTTAATAACAGG-3′ and antisense
5′-GTTAGGACTTATTCCTGTCATTA-3′ for VEGF-C; and sense
5′-ATCATGTTTGAGACCTTCAACA-3′ and antisense
5′-CATCTCTTGCTCGAAGTCCA-3′ for β-actin. The mRNA expression of
β-actin served as a housekeeping control. Following amplification,
the PCR products were resolved by running them on a 2% agarose gel
(Shanghai Sangon Biotechnology Co.), and the relative mRNA
expression was calculated using an electrophoresis gel quantitative
image analyzer (Kodak, Rochester, NY, USA).
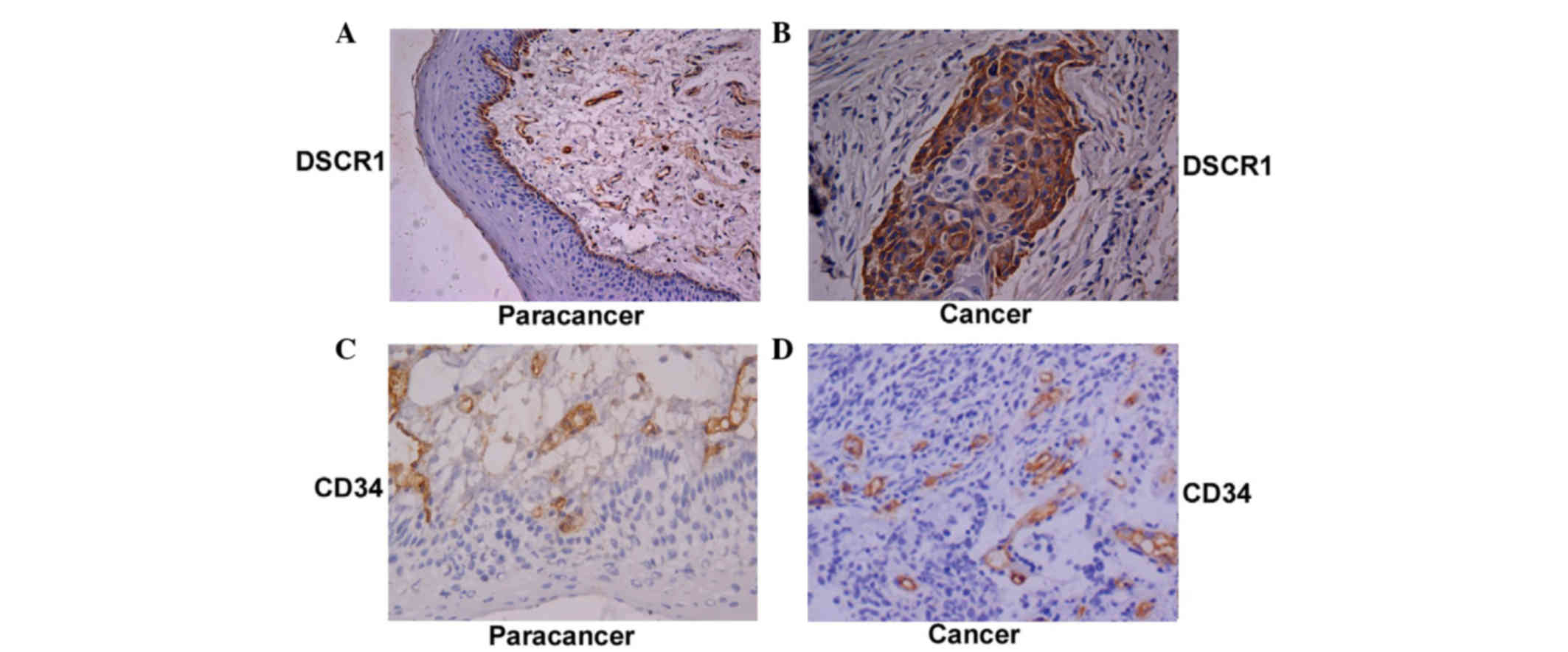
Immunohistochemistry
Immunohistochemistry was performed using a PV9000
staining kit (Beijing Zhongpin Jinqiao, Biotechnology Co., Ltd.,
Beijing, China), according to the manufacturer's protocols.
Paraffin sections were placed in xylene solution for 10–15 min
twice at room temperature. Subsequently, sections were dehydrated
in a gradient series of 70–100% ethanol. Following
deparaffinization and hydration, tissue sections were incubated for
10 min in 3% hydrogen peroxide in methanol and were washed three
times with phosphate-buffered saline (PBS). Subsequent to antigen
retrieval, the slides were again washed three times for 5 min with
PBS. The slides were then blocked with blocking solution A (normal
goat serum; Beijing Zhongshan Jinqiao Biological Technology Co.,
Ltd., Beijing, China) for 15 min at room temperature, and incubated
with specific primary antibodies overnight at 4°C. The antibodies
used were as follows: Rat monoclonal anti-human cluster of
differentiation (CD)34 (QBEnd/10; 1:250; cat. no. ZM-0046; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.) and polyclonal rabbit
anti-human DSCR1 (DCT3; 1:250; provided by Prof Philip Brandt,
Columbia University, New York, NY, USA). The slides were then
incubated with solution B for 20 min at room temperature. Following
washing with PBS three times for 5 min, the slides were incubated
with solution C for 20 min at room temperature. The slides were
stained with Dolichos biflorus agglutinin (Shanghai Chemical
Reagent Co., Ltd., Shanghai, China) and hematoxylin and eosin and
dehydrated prior to observation under a light microscope (Olympus
CX31; Olympus Corporation, Tokyo, Japan). PBS served as the blank
control, normal goat serum as the negative control and human brain
tissue as the positive control. Brain tissue was obtained from the
pathological tissue library of Shandong University (Jinan,
China).
Determination of microvessel density
(MVD)
In the CD34 antibody-stained sections, the presence
of brown granules in the cytoplasm indicated positively stained
vascular endothelial cells. Positive cells with a clear background
and a distinct boundary or basement membrane, including
single-stained endothelial cells, were classed as one vessel unit,
regardless of the formation of the vascular lumen or whether red
blood cells were present within the lumen. For vessels containing
lumens over eight red blood cells in size, the muscular layer was
not counted. The tumor region and the interstitial region in all
tissue sections were examined, with the exception of the
surrounding areas, disrupted tissue or areas of tumor necrosis.
LEICA Qwin 3.31 automatic image software (Leica Microsystems GmbH,
Wetzlar, Germany) was used to count the MVD. The MVD was counted in
five randomly-selected separate fields in each section using a
light microscope (Olympus CX31; Olympus Corporation) at
magnification, ×400. The mean value of the five counts was
determined to be the MVD.
Calculations of DSCR1-positive
cells
In the DSCR1 antibody-stained sections, the presence
of brown granules in the cytoplasm of the cells was considered to
be positive for DSCR1. Tumor cells in each field (500) were
examined from a total of five randomly-selected separate fields in
each section at magnification, ×400. The positive cells were
counted by two pathologists using a double-blind method, and the
mean values were calculated. Sections containing <10% positively
stained cells were defined as negative (−), those with 10–20%
positively stained cells were weakly positive (±), those with
20–40% positively stained cells were positive (+), those with
40–60% positively stained cells were medium positive (++) and those
with >60% positively stained cells were considered strongly
positive (+++).
Statistical analysis
Data were analyzed using SAS, version 8.2
statistical software (SAS Institute, Inc., Cary, NC, USA). All
continuous data are expressed as the mean ± standard deviation.
Student's t-test was used for the comparison between two groups.
Spearman's correlation analysis was utilized to analyze the
association between DSCR1 and angiogenesis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 94 cases of hypopharyngeal squamous cell
carcinoma were examined in the present study. The mean age of the
patients was 47 years, 52.13% were <60 years, 47.87% were ≥60
years; 94.68% were males and 5.34% were females (Table I). Based on histological
differentiation, there were 30 well-differentiated cases (31.91%),
43 moderately differentiated cases (45.74%) and 21 poorly
differentiated cases (22.34%). A total of eight cases (8.51%) were
stages I–II and 86 (91.49%) were stages III–IV. Lymph node
metastasis was observed in 54 cases (57.45%). Exogenous tumor
growth was observed in 49 cases, whereas infiltrative growth was
observed in 45 cases. The majority of the tumors were located at
the piriform fossa (79.79%), and the remaining were localized to
the posterior pharyngeal wall (15.96%) or the postcricoid region
(4.26%). Of the patients included in the present study 69 (73.4%)
had a history of smoking (Table
I).
 | Table I.Characteristics of patients with
hypopharyngeal cancer. |
Table I.
Characteristics of patients with
hypopharyngeal cancer.
| Variable | Number | % |
|---|
| Total | 94 | 100 |
| Age (years) |
|
|
|
<60 | 49 | 52.13 |
|
≥60 | 45 | 47.87 |
| Gender |
|
|
|
Male | 89 | 94.68 |
|
Female | 5 | 5.34 |
| Histological
differentiation |
|
|
|
Well-differentiated | 30 | 31.91 |
|
Moderately differentiated | 43 | 45.74 |
| Poorly
differentiated | 21 | 22.34 |
| TNM stage |
|
|
|
I–II | 8 | 8.51 |
|
III–IV | 86 | 91.49 |
| Lymph node
metastasis |
|
|
|
Yes | 54 | 57.45 |
| No | 40 | 42.55 |
| Growth mode |
|
|
|
Exogenous | 49 | 52.13 |
|
Infiltrative | 45 | 47.87 |
| Location |
|
|
|
Piriform fossa | 75 | 79.79 |
|
Posterior pharyngeal wall | 15 | 15.96 |
|
Postcricoid region | 4 | 4.26 |
| Smoking |
|
|
|
Yes | 69 | 73.40 |
| No | 25 | 26.60 |
mRNA expression levels of DSCR1 and
VEGF-C, and MVD are increased in cancerous tissues, compared with
paracancerous tissue in hypopharyngeal cancer
Cancerous tissue was collected from 43 patients and
paracancerous tissue was collected from 21 of these patients. The
tissue adjacent to the cancer was pathologically confirmed as
normal pharyngeal mucosa. In patients with hypopharyngeal squamous
cell carcinoma, the overall mean relative mRNA expression levels of
DSCR1 in the cancerous tissue were significantly higher than in the
paracancerous tissue (0.91±0.10 vs. 0.61±0.08, respectively;
P=0.011; Fig. 1). In addition, the
overall mean relative mRNA expression levels of VEGF-C in the
cancerous tissue was significantly higher than in the paracancerous
tissue (1.21±0.41 vs. 0.56±0.19, respectively; P=0.011; Fig. 1). The MVD in the 78 cancerous and
paracancerous tissue samples from the patients with hypopharyngeal
squamous cell carcinoma patients were also examined. The MVD was
significantly higher in the cancerous tissues, compared with the
paracancerous tissue (17.61±10.59 vs. 12.59±7.77, respectively;
P=0.011; Fig. 2).
Expression levels of DSCR1 correlate
with the progression of hypopharyngeal cancer
To define the role of DSCR1 in the progression of
cancer of the laryngopharynx, the expression profiles of DSCR1
(Fig. 2) were analyzed, according
to tumor differentiation and TNM stage. The results indicated that
higher mRNA expression levels of DSCR1 were associated with poor
tumor differentiation (P=0.033) and lymph node metastasis (P=0.042;
Table II). No statistically
significant associations were found between the protein levels of
DSCR1 and the different modes of tumor growth or patient smoking
history (Table II).
 | Table II.mRNA and protein levels of DSCR1 in
patients with hypopharyngeal cancer. |
Table II.
mRNA and protein levels of DSCR1 in
patients with hypopharyngeal cancer.
|
| DSCR1 mRNA |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Cancer tissue
(n=43) | P-value | n | % |
|---|
| Total | 0.91±0.10 |
| 35 | 81.40 |
| Histological
differentiation |
| 0.033a |
|
|
|
Well-differentiated | 0.73±0.11 |
| 8 | 18.60 |
|
Moderately-differentiated | 0.79±0.09 |
| 19 | 44.19 |
|
Poorly-differentiated | 0.93±0.15 |
| 7 | 16.28 |
| TNM stage |
| 0.121 |
|
|
|
I–II | 0.89±0.13 |
| 4 | 9.30 |
|
III–IV | 0.92±0.08 |
| 31 | 72.09 |
| Lymph node
metastasis |
| 0.042a |
|
|
|
Yes | 0.93±0.11 |
| 26 | 60.47 |
| No | 0.79±0.07 |
| 9 | 20.93 |
| Growth mode |
| 0.138 | – | – |
|
Exogenous | 0.90±0.07 |
| – | – |
|
Infiltrative | 0.89±0.17 |
| – | – |
MVD is correlated with the progression
of hypopharyngeal cancer
To determine the association between tumor
angiogenesis or lymphangiogenesis with the clinical characteristics
of patients with hypopharyngeal squamous cell carcinoma, the MVDs
in tumor tissues from 94 patients were examined (Fig. 2). Compared with patients with a
lower intratumoral MVD, the tumor tissues in patients with a higher
intratumoral MVD were poorly differentiated with a higher TNM stage
and increased lymph node metastasis (all P<0.05; Table III). The MVDs were similar in
patients with different tumor growth modes and in smokers, compared
with non-smokers.
 | Table III.MVD in patients with hypopharyngeal
cancer. |
Table III.
MVD in patients with hypopharyngeal
cancer.
| Variable | Number | MVD | P-value |
|---|
| Total | 94 | 17.57±10.61 |
|
| Age (years) |
|
| 0.639 |
|
<60 | 49 | 17.99±10.49 |
|
|
≥60 | 45 | 17.23±11.34 |
|
| Gender | 94 |
| 0.588 |
|
Male | 89 | 17.64±10.53 |
|
|
Female | 5 | 18.88±9.89 |
|
| Histological
differentiation |
|
| 0.037 |
|
Well-differentiated | 30 | 20.48±13.61 |
|
|
Moderately differentiated | 43 | 18.58±10.35 |
|
| Poorly
differentiated | 21 | 16.29±9.98 |
|
| TNM stage |
|
| 0.045 |
|
I–II | 8 | 16.13±9.61 |
|
|
III–IV | 76 | 18.43±11.61 |
|
| Lymph node
metastasis |
|
| 0.031 |
|
Yes | 54 | 18.96±11.63 |
|
| No | 40 | 16.17±9.61 |
|
| Location |
|
| 0.386 |
|
Piriform fossa | 75 | 17.20±10.23 |
|
|
Posterior pharyngeal wall | 15 | 18.10±11.13 |
|
|
Postcricoid region | 4 | 18.75±11.88 |
|
| Smoking |
|
| 0.874 |
|
Yes | 69 | 17.79±10.21 |
|
| No | 25 | 17.11±10.79 |
|
DSCR1 is positively correlated with
tumor angiogenesis in hypopharyngeal cancer
The mRNA expression levels of DSCR1 were positively
correlated with the mRNA levels of VEGF-C in the cancerous tissue
of patients with hypopharyngeal squamous cell carcinoma, (r=0.578;
P<0.001). As shown in Fig. 3,
the MVDs in the cancerous tissue were 10.7±4.44 (DSCR1, -),
13.7±7.45 (DSCR1, ±), 17.9±10.96 (DSCR1, +), 26.5±10.55 (DSCR1, ++)
and 29.1±11.65 (DSCR1, +++). The MVDs increased significantly with
increasing protein levels (P<0.05). A significant correlation
was found between the protein levels of DSCR1 and MVD in the
patients with cancer (r=0.689; P<0.001). These results indicated
that higher protein expression levels of DSCR1 were significantly
associated with angiogenesis in laryngopharynx cancer.
 | Figure 3.MVD, determined by the number of
CD34-positive cells, in cancer tissue is positively correlated with
protein levels of DSCR1. Data are expressed as the mean ± standard
deviation (*P<0.05). -, negative; ±, weakly positive; +,
positive; ++, medium positive; +++, strongly positive; MVD,
microvessel density; CD34, cluster of differentiation 34; DSCR1,
Down syndrome critical region 1. |
Discussion
In the present study, the expression levels of DSCR1
in patients with hypopharyngeal squamous cell carcinoma were
examined. The results demonstrated that the relative mRNA
expression levels of DSCR1 in the cancerous tissues were
significantly higher than those in the paracancerous tissues. This
finding is consistent with a previous study, which demonstrated
that the protein levels of DSCR1 were significantly increased in
hypopharyngeal cancer (24). This
previous study also indicated that elevated protein expression
levels of DSCR1 was correlated with poor tumor differentiation and
advanced TNM stage (28). In the
present study, elevated mRNA expression levels of DSCR1 were also
associated with poor tumor differentiation and advanced disease
stage, which suggested that the upregulation of DSCR1 is important
in the pathogenesis and progression of hypopharyngeal cancer. DSCR1
may serve as a useful marker in the early diagnosis and monitoring
of this type of cancer.
A previous study examining cancer incidence in
individuals with Down's syndrome indicated that the overall risk of
cancer was similar between the these individuals and the general
population. However, the individuals with Down's syndrome had a
significantly higher risk of leukemia, with a standardized
incidence ratio (SIR) of 10.5, 95% confidence interval (CI) of
6.6–15.8, and testicular cancer (SIR, 4.8; 95% CI, 1.8–10.4)
(21). Another study demonstrated
that the targeted deletion of DSCR1 in mice inhibited the formation
of new tumor vasculature and suppressed tumorigenesis. Treatment
with the calcineurin-specific inhibitor, cyclosporin A attenuated
this endothelial defect and promoted tumor growth (29). However, Baek et al (22) reported that the incidence of cancer
was significantly reduced in individuals with Down's syndrome, and
that the upregulation of DSCR1 inhibited tumor growth. Sussan et
al (23) also suggested that
trisomy reduced the numbers of intestinal tumors in a mouse model
of Down's syndrome. These reports indicate that the role of DSCR1
in cancer is controversial, and further investigations are required
to elucidate the function of DSCR1 in carcinogenesis.
It is well-known that the expression of VEGF-C is
increased in various types of human malignancy (30,31).
The levels of VEGF-C in tumor tissues are significantly correlated
with regional angiogenesis (30,31).
Bunone et al (30)
demonstrated that the mRNA and protein expression levels of VEGF-C
were upregulated in thyroid carcinoma, and Yonemura et al
(31) reported that the mRNA and
protein expression levels of VEGF-C were also increased in gastric
carcinoma. Similarly, the present study demonstrated that, in
patients with hypopharyngeal cancer, the relative mRNA expression
levels of VEGF-C were significantly higher than in the normal
tissues suggesting that the levels of VEGF-C are elevated in
hypopharyngeal cancer, concordant with previous reports in other
types of cancer (30,31).
In the present study, a statistically significant
positive correlation was observed between the mRNA expression
levels of DSCR1 and VEGF-C in the cancerous tissue from patients
with hypopharyngeal cancer. The mRNA expression levels of DSCR1
were significantly higher in tissues expressing upregulated levels
of VEGF-C. In addition, the MVD in the laryngopharynx cancerous
tissue was significantly increased, compared with that in the
paracancerous tissue (P<0.05), and the protein expression of
DSCR1 in the cancerous tissue were significantly correlated with
MVD (r=0.689; P<0.001). Yao et al (18) demonstrated that DSCR1 is
upregulated in endothelial cells upon stimulation with VEGF, TNF-α
and A23187 treatment. The upregulation of DSCR1 is suppressed by
inhibitors of the calcineurin-nuclear factor of activated T cell
(NFAT) signaling pathway, inhibition of protein kinase C (PKC) and
Ca2+ chelation (32).
Furthermore, Hesser et al (13) conducted a genome-wide analysis of
genes, which are regulated by VEGF in endothelial cells. This
analysis identified DSCR1 as one of the most frequently induced
targets of VEGF. The results of our previous and present studies
are consistent with the report by Yao et al (18). These findings indicated that DSCR1
is involved in tumor angiogenesis, and suggested that DSCR1 is
important in angiogenesis, specifically in hypopharyngeal
carcinoma, and may be a promising therapeutic target.
In response to various stimuli, tumor cells secrete
a large quantity of VEGF via either paracrine or autocrine
mechanisms. VEGF binds to VEGFR-1 and VEGFR-2, leading to the
activation of downstream signaling, including phosphatidylinositol
3-kinase/protein kinase B, phospholipase C-γ, PKC, mitogen
activated protein kinases and calcineurin (33–36).
Abe and Sato (26) reported that
the DSCR1 gene is an important downstream target of VEGF in human
endothelial cells. VEGF enhances calcineurin activity (18). Calcineurin is involved in the
regulation of a variety of cellular activities, including cell
maturation, survival, proliferation and functional activities
(18). Calcineurin binds to and
activates NFAT, which subsequently translocates to the nucleus.
NFAT promotes the transcription of various target genes by binding
to the promoter and enhancer regions of these genes (11,15,37).
Previous studies have demonstrated that the binding of calcineurin
to DSCR1 enhances the expression of DSCR1, and the upregulation of
DSCR1 serves as a negative regulator of calcineurin activity,
resulting in a negative feedback loop between DSCR1 and calcineurin
A signaling (11,15,37).
This may represent a potential molecular mechanism underlying the
association between DSCR1 and angiogenesis.
It has been suggested that exposure of the active
site during the binding of calcineurin to
Ca2+/calmodulin promotes calcineurin to bind with DSCR1.
In addition to directly inhibiting calcineurin activity, mutational
studies have demonstrated that DSCR1 competes with NFAT in binding
to the same anchor points on the calcineurin protein. This is one
of the molecular mechanisms by which DSCR1 inhibits the activity of
the calcineurin/NFAT signaling pathway (38). DSCR1 also inhibits the phosphatase
activity of calcineurin (36). As
a downstream target of the calcineurin/NFAT signaling pathway,
DSCR1 is activated by a series of factors associated with NFAT
signaling, including VEGF, angiotensin-II, TNF-α, thrombin and
calcium ion carriers (39).
Although the present study demonstrated important
findings, including the correlation between the mRNA and protein
levels of DSCR1, angiogenesis and the progression of hypopharyngeal
squamous cell carcinoma progression, the study had certain
limitations. For example, the sample size was relatively small and
patient follow-up information was absent; therefore, evaluation of
correlations between patient survival times and the expression
levels of DSCR1 or VEGF-C were not performed. In addition, the
present study did not investigate in detailed the molecular
mechanism(s) by which DSCR1 regulates angiogenesis in
hypopharyngeal cancer. Further investigations are ongoing to
determine the molecular signaling pathways involved in
DSCR1-induced tumor angiogenesis and lymphangiogenesis.
In conclusion, the present study revealed that the
mRNA expression levels of DSCR1 and VEGF-C were upregulated in
cancerous tissue samples from patients with hypopharynx cancer.
These elevated expression levels were correlated with poorly
differentiated tumors and advanced TNM stage, and the expression
levels of DSCR1 were positively correlated with angiogenesis in the
tumor tissues. DSCR1 is likely to be important during the
progression of hypopharyngeal cancer by regulating tumor
angiogenesis and lymphangiogenesis. Thus, DSCR1 may be a promising
marker for early diagnosis and early management of hypopharyngeal
cancer.
Acknowledgements
The authors would like to thank Medjaden Bioscience
Limited for assisting in the preparation of this manuscript.
References
|
1
|
Newman JR, Connolly TM, Illing EA, Kilgore
ML, Locher JL and Carroll WR: Survival trends in Hypopharyngeal
cancer: A population-based review. Laryngoscope. 125:624–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berrino F and Gatta G: Variation in
survival of patients with head and neck cancer in Europe by the
site of origin of the tumours. EUROCARE working group. Eur J
Cancer. 34:2154–2161. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Liu X, Wang J, Wang Z, Jiang W, Reed
E, Zhang Y, Liu Y and Li QQ: Thalidomide down-regulates the
expression of VEGF and bFGF in cisplatin-resistant human lung
carcinoma cells. Anticancer Res. 23:2481–2487. 2003.PubMed/NCBI
|
|
4
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su JL, Yen CJ, Chen PS, Chuang SE, Hong
CC, Kuo IH, Chen HY, Hung MC and Kuo ML: The role of the
VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 96:541–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plate K: From angiogenesis to
lymphangiogenesis. Nat Med. 7:151–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuentes JJ, Genescà L, Kingsbury TJ,
Cunningham KW, Pérez-Riba M, Estivill X and de la Luna S: DSCR1,
overexpressed in Down syndrome, is an inhibitor of
calcineurin-mediated signaling pathways. Hum Mol Genet.
9:1681–1690. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harris CD, Ermak G and Davies KJ: Multiple
roles of the DSCR1 (Adapt78 or RCAN1) gene and its protein product
calcipressin 1 (or RCAN1) in disease. Cell Mol Life Sci.
62:2477–2486. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hesser BA, Liang XH, Camenisch G, Yang S,
Lewin DA, Scheller R, Ferrara N and Gerber HP: Down syndrome
critical region protein 1 (DSCR1), a novel VEGF target gene that
regulates expression of inflammatory markers on activated
endothelial cells. Blood. 104:149–158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aynaci FM, Orhan F, Celep F and Karagüzel
A: Frequency of cardiovascular and gastrointestinal malformations,
leukemia and hypothyroidism in children with Down syndrome in
Trabzon, Turkey. Turk J Pediatr. 40:103–109. 1998.PubMed/NCBI
|
|
15
|
Rothermel B, Vega RB, Yang J, Wu H,
Bassel-Duby R and Williams RS: A protein encoded within the Down
syndrome critical region is enriched in striated muscles and
inhibits calcineurin signaling. J Biol Chem. 275:8719–8725. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vega RB, Rothermel BA, Weinheimer CJ,
Kovacs A, Naseem RH, Bassel-Duby R, Williams RS and Olson EN: Dual
roles of modulatory calcineurin-interacting protein 1 in cardiac
hypertrophy. Proc Natl Acad Sci USA. 100:669–674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iizuka M, Abe M, Shiiba K, Sasaki I and
Sato Y: Down syndrome candidate region 1, a downstream target of
VEGF, participates in endothelial cell migration and angiogenesis.
J Vasc Res. 41:334–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao YG and Duh EJ: VEGF selectively
induces Down syndrome critical region 1 gene expression in
endothelial cells: A mechanism for feedback regulation of
angiogenesis? Biochem Biophys Res Commun. 321:648–656. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki K, Nishimi D, Yagishita T, Takanami
M and Hiruta N: Testicular tumor in Down syndrome. Int J Urol.
12:925–927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Satgé D, Sommelet D, Geneix A, Nishi M,
Malet P and Vekemans M: A tumor profile in Down syndrome. Am J Med
Genet. 78:207–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patja K, Pukkala E, Sund R, Iivanainen M
and Kaski M: Cancer incidence of persons with Down syndrome in
Finland: A population-based study. Int J Cancer. 118:1769–1772.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baek KH, Zaslavsky A, Lynch RC, Britt C,
Okada Y, Siarey RJ, Lensch MW, Park IH, Yoon SS, Minami T, et al:
Down's syndrome suppression of tumour growth and the role of the
calcineurin inhibitor DSCR1. Nature. 459:1126–1130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sussan TE, Yang A, Li F, Ostrowski MC and
Reeves RH: Trisomy represses Apc (Min)-mediated tumours in mouse
models of Down's syndrome. Nature. 451:73–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu C, Pan X, Gao J and Li Z: Study of
expression and significance of DSCR1 gene in laryngopharynx cancer
and peri-cancerous tissues. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke
Za Zhi. 25:848–850. 2011.(In Chinese). PubMed/NCBI
|
|
25
|
Liu D, Jia H, Holmes DI, Stannard A and
Zachary I: Vascular endothelial growth factor-regulated gene
expression in endothelial cells: KDR-mediated induction of Egr3 and
the related nuclear receptors Nur77, Nurr1 and Nor1. Arterioscler
Thromb Vasc Biol. 23:2002–2007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abe M and Sato Y: CDNA microarray analysis
of the gene expression profile of VEGF-activated human umbilical
vein endothelial cells. Angiogenesis. 4:289–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
World Medical Association: World Medical
Association Declaration of Helsinki, . Ethical principles for
medical research involving human subjects. JAMA. 310:2191–2194.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sobin L and Wittekind C: UICC TNM
classification of malignant tumours. 5th. Wiley Liss; New York, NY:
pp. 1381997
|
|
29
|
Ryeom S, Baek KH, Rioth MJ, Lynch RC,
Zaslavsky A, Birsner A, Yoon SS and McKeon F: Targeted deletion of
the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer
Cell. 13:420–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bunone G, Vigneri P, Mariani L, Butó S,
Collini P, Pilotti S, Pierotti MA and Bongarzone I: Expression of
angiogenesis stimulators and inhibitors in human thyroid tumors and
correlation with clinical pathological features. Am J Pathol.
155:1967–1976. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yonemura Y, Fushida S, Bando E, Kinoshita
K, Miwa K, Endo Y, Sugiyama K, Partanen T, Yamamoto H and Sasaki T:
Lymphangiogenesis and the vascular endothelial growth factor
receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 37:918–923.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan MG, Xiong Y and Chen F: NFAT gene
family in inflammation and cancer. Curr Mol Med. 13:543–554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kroll J and Waltenberger J: The vascular
endothelial growth factor receptor KDR activates multiple signal
transduction pathways in porcine aortic endothelial cells. J Biol
Chem. 272:32521–32527. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rousseau S, Houle F, Landry J and Huot J:
p38 MAP kinase activation by vascular endothelial growth factor
mediates actin reorganization and cell migration in human
endothelial cells. Oncogene. 15:2169–2177. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi T, Ueno H and Shibuya M: VEGF
activates protein kinase C-dependent, but Ras-independent
Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial
cells. Oncogene. 18:2221–2230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kingsbury TJ and Cunningham KW: A
conserved family of calcineurin regulators. Genes Dev.
14:1595–1604. 2000.PubMed/NCBI
|
|
38
|
Chan B, Greenan G, McKeon F and
Ellenberger T: Identification of a peptide fragment of DSCR1 that
competitively inhibits calcineurin activity in vitro and in vivo.
Proc Natl Acad Sci USA. 102:13075–13080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pinchai N, Perfect BZ, Juvvadi PR,
Fortwendel JR, Cramer RA Jr, Asfaw YG, Heitman J, Perfect JR and
Steinbach WJ: Aspergillus fumigatus calcipressin CbpA is involved
in hyphal growth and calcium homeostasis. Eukaryot Cell. 8:511–519.
2009. View Article : Google Scholar : PubMed/NCBI
|