Introduction
Tuberculosis (TB), caused by Mycobacterium
tuberculosis (M. tb), is one of the most important
infectious diseases of humans, with an estimated 9 million new
cases and 1.5 million fatalities worldwide in 2013 (1). The treatment of TB requires a
six-month program; the treatment for drug-resistant TB is longer
(at least 18 months) and requires the use of more toxic drugs
(2). Consequently, early and
accurate diagnosis of TB may potentially increase treatment
efficacy and reduce patient suffering. However, conventional
diagnostic methods, including culture and microscopic examination
of sputa, the tuberculin skin test (TST), chest X-ray, bronchial
endoscopy and polymerase chain reaction (PCR) typically produce
high proportions of false negative and positive results (3,4).
Blood-based laboratory tests, including the interferon-γ (IFN-γ)
in vitro release assay and antibody detection, have greater
sensitivity and specificity compared with the conventional
diagnostic methods; however, they do not differentiate between
latent and active infections (5).
Therefore, novel diagnostic biomarkers with high sensitivity and
specificity are urgently required to accurately diagnose TB, and to
differentiate between the different forms of TB (6).
Recently, tissue inhibitors of metalloproteinases
(TIMPs) have been suggested as potential biomarkers for TB
(7,8). TIMPs (TIMP-1, −2 and −3) facilitate
the remodeling and repair of tissue following destruction by matrix
metalloproteinases (MMPs). For example, the concentrations of
MMP-1, −3 and −8 have been demonstrated to decrease rapidly during
TB treatment, in contrast to transient increases in the
concentrations of TIMP-1 and −2 at week 2 of treatment (7). In addition, TIMP-1 has been revealed
to be responsible for residual pleural thickening in pleural
tuberculosis (9).
Our previous investigations to identify potential TB
biomarkers in the sera of patients using protein microarrays
demonstrated that TIMP-1 is a primary component associated with the
occurrence of disease (unpublished data). However, the role of
TIMP-1 in TB diagnosis has rarely been studied. Therefore, the
present study aimed to evaluate the association of TIMP-1 with TB,
and to investigate the potential of TIMP-1 as a biomarker to aid in
the diagnosis of TB.
Materials and methods
Subjects
A total of 122 patients who were confirmed to have
active TB based upon presenting clinical symptoms and/or culture
and/or chest X-ray at the Tuberculosis Department, Wuhan Medical
Treatment Center (Wuhan, China), were recruited. A further 37
pneumonia patients were enrolled at Zhongnan Hospital (Wuhan,
China). In addition, 128 healthy volunteers from Huazhong
Agricultural University (Wuhan, China), who had a negative TST, a
negative chest X-ray and had no known exposure to TB, were included
in the present study as a control group. All subjects tested
negative for human immunodeficiency virus. Informed consent was
obtained from all participants. The present study was approved by
the Research Ethics Committee of Huazhong Agricultural
University.
Blood collection
Heparinized venous blood (5 ml) was collected from
the antecubital vein of all subjects. For each sample, plasma was
isolated and stored at −20°C for the subsequent detection of
TIMP-1. Peripheral blood mononuclear cells (PBMCs) were isolated
from blood samples and stimulated with CFP-10/ESAT-6 (stocked in
our own lab), or mock-stimulated with PBS, overnight (16 h) as
described previously (10).
Measurement of TIMP-1
concentrations
Commercial ELISA kits for TIMP-1 (RayBiotech, Inc.,
Norcross, GA, USA, cat. no. ELH-TIMP1) were used according to the
manufacturer's protocol, to analyze the levels of TIMP-1 in plasma.
Briefly, 100 µl of each sample was added to each well of an ELISA
plate. The plates were incubated for 2.5 h at room temperature and
washed. A biotin-conjugated antibody (100 µl) was added to each
well and the plates incubated for a further 1 h at room
temperature. Following washing, 100 µl horseradish
peroxidase-streptavidin solution was added to each well and the
plate was incubated at room temperature for 45 min. The color was
developed with the substrate
3,3′,5,5′-tetramethylbenzidine/H2O2 for 30
min at room temperature, and the reaction was terminated by adding
the stop solution. The optical density was measured at a wavelength
of 450 nm using a microplate reader and the concentrations were
calculated based on the standard curve.
Bacterial culture
Mycobacterium bovis [M. bovis;
American Type Culture Collection (ATCC) 19210] and Bacillus
Calmette-Guérin (BCG) Tokyo strain (ATCC 35737) were donated by Dr
Chuan-You Li (Beijing Tuberculosis & Thoracic Tumor Research
Institute, Beijing, China). The two strains were cultured in 250 ml
Middlebrook 7H9 broth (BD Biosciences, Franklin Lakes, NJ, USA)
supplemented with 10% oleic acid-albumin-dextrose-catalase (BD
Biosciences) and 0.05% Tween 80 (Amresco, LLC, Solon, OH, USA),
with shaking in the Biosafey Level 3 facility at Huazhong
Agricultural University. Following incubation at 37°C for 7 days
until growth reached the log phase, the bacilli were harvested from
the media and centrifuged at 5,000 × g for 10 min at room
temperature. The pellets were transferred into a mortar grinder and
homogenized, and subsequently resuspended in 40 ml Middlebrook 7H9
broth. Following a 5-min incubation, the supernatant was collected,
mixed, aliquoted into 1 ml tubes and stored at −80°C until further
use. The middle tube was taken and the number of colony forming
units was determined via a dilution-plating assay, as previously
described (11).
Analysis of TIMP-1 mRNA expression
levels
The THP-1 human monocytic cell line was provided by
Dr Chuan-You Li (Beijing Tuberculosis & Thoracic Tumor Research
Institute, Beijing, China) and was prepared and the infection
procedures were conducted as previously described (12). Briefly, the bacteria (M.
bovis and BCG) were added to 24-well cell culture plates and
cultured for 24 h at a multiplicity of infection rate of 10. Total
RNA was extracted as described previously (12), and subjected to reverse
transcription-quantitative PCR (RT-qPCR). RNA was
reverse-transcribed using a Reverse Transcription kit (Toyobo Co.,
Ltd., Osaka, Japan). During the RNA isolation and reverse
transcription, RNase-free reagents and consumables were used.
Real-time quantitative PCR (qPCR) was performed using THUNDERBIRD
SYBR qPCR mix (Toyobo Co., Ltd., Osaka, Japan). The volume of each
reaction was 25 µl, including 100 ng cDNA, 200 nmol of each primer
and 12.5 µl 2xSYBR-Green dye. Reactions were programmed in Roche
LightCycler® 480 (Roche Diagnostics, Basel, Switzerland)
as follows: 95°C for 10 min, followed by 30 cycles of 95°Cfor 30
sec, 58°C for 30 sec and 72°C for 45 sec. The fluorescence signal
was detected at the end of each elongation step. Primers (presented
in Table I) were designed and
commercially synthesized by the Beijing Genomics Institute
(Beijing, China) for RT-qPCR to determine the mRNA expression
levels of TIMP-1 in BCG- and M. bovis-infected THP-1 cells;
µ-actin served as the internal reference, the relative expression
levels were quantified using the 2−∆ΔCq method (13).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence (5′-3′) | Product size
(bp) | GenBank accession
no. |
|---|
| β-actin | F |
AGCGAGCATCCCCCAAAGTT | 285 | BC002409 |
|
| R |
GGGCACGAAGGCTCATCATT |
|
|
| TIMP-1 | F |
CTGCGGATACTTCCACAGGTC | 168 | NM_003254 |
|
| R |
TTCTGGATGTGACAACCGACAACCGACACT |
|
|
Statistical analysis
Data were analyzed using a Student's t-test or
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference. Cut-off values and
corresponding test sensitivity and specificity were calculated
through receiver operating characteristic (ROC) curve analysis and
assessing the area under the curve using Microsoft Excel software,
version 2013 (Microsoft Corporation, Redmond, WA, USA), as
previously described (14).
Results
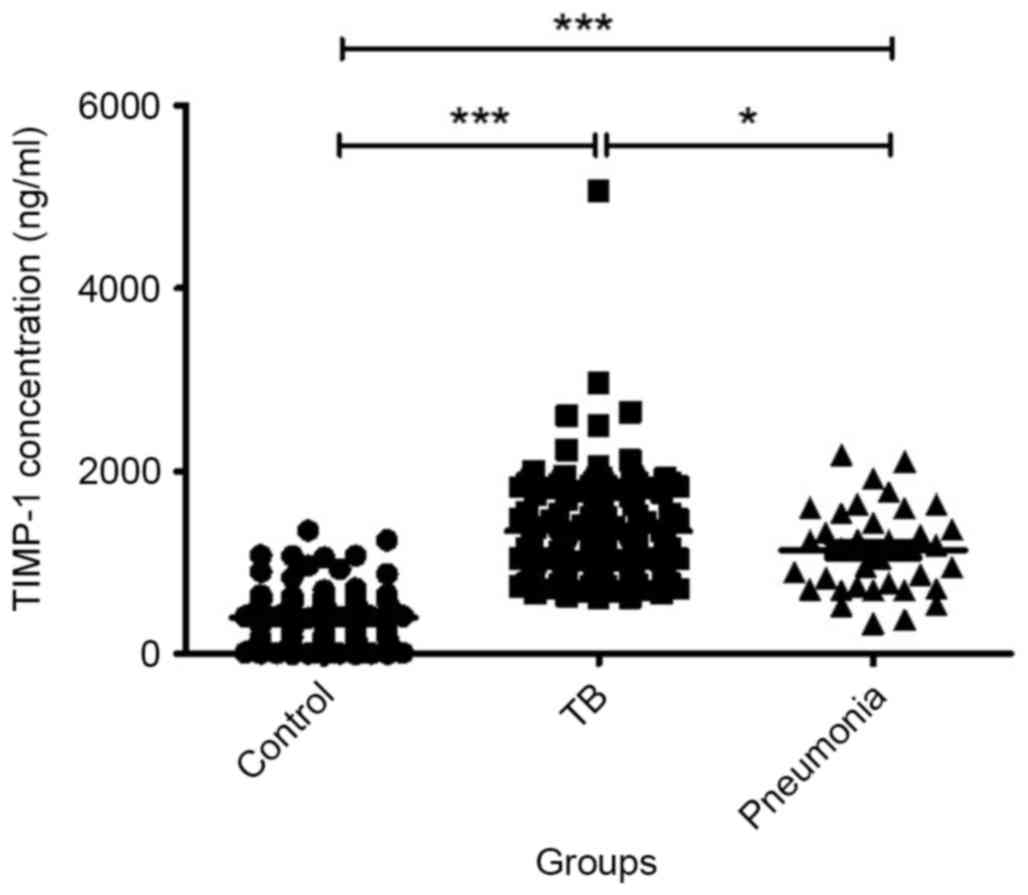
TB patients have increased serum
levels of TIMP-1 compared with healthy controls and pneumonia
patients
The baseline serum TIMP-1 levels of patients with
tuberculosis were significantly greater compared with the pneumonia
(P=0.02) and healthy control groups (P=8×10−4), with
median values of 1201, 1140 and 415.4 ng/ml, respectively (Fig. 1). In addition TIMP-1 levels in
pneumonia patients were significantly greater compared with healthy
controls (P=2×10−11).
According to the ROC, a cut-off value of 727 ng/ml
was set to maximize discrimination between positive and negative
results for TIMP-1 in the TB patients group. TIMP-1 levels were
significantly greater in 112 TB patients [mean ± standard deviation
(SD), 1348±607.3 ng/ml] compared with healthy controls (mean ± SD,
400.4±292.2 ng/ml; P<0.0001). At this cut-off point, 91.80%
[112/122; 95% confidence interval (CI): 85.44, 96.00] of TB
patients were classified as test-positive compared with only 8.59%
(11/128; 95% CI: 4.37, 14.86) of healthy controls. Using clinical
diagnosis as the gold standard, the TIMP-1 ELISA had a sensitivity
of 91.80% (95% CI: 85.44, 96.00) and a specificity of 91.41% (95%
CI: 85.14, 95.63; Fig. 2).
Furthermore, 1037 ng/ml was set as the cut-off point
to distinguish TB and pneumonia patients according to ROC, with a
sensitivity of 62.3% (95% CI: 53.07, 70.91), and a specificity of
45.95% (95% CI: 29.49, 63.08; Fig.
3).
TIMP-1 production by PBMC following
stimulation with CFP-10/ESAT-6
PBMCs isolated from the blood of 38 TB patients and
38 healthy controls were stimulated with CFP-10/ESAT-6 or
mock-stimulated with PBS. CFP-10/ESAT-6 did not induce PBMCs to
produce TIMP-1 in TB patients (P=0.3051). Similarly, there was no
difference in TIMP-1 levels between healthy control PBMCs incubated
with CFP-10/ESAT-6 or PBS (P=0.1158). The TB samples treated with
CFP-10/ESAT-6 or PBS had significantly greater TIMP-1 levels
compared with healthy controls (P<0.0001; Fig. 4).
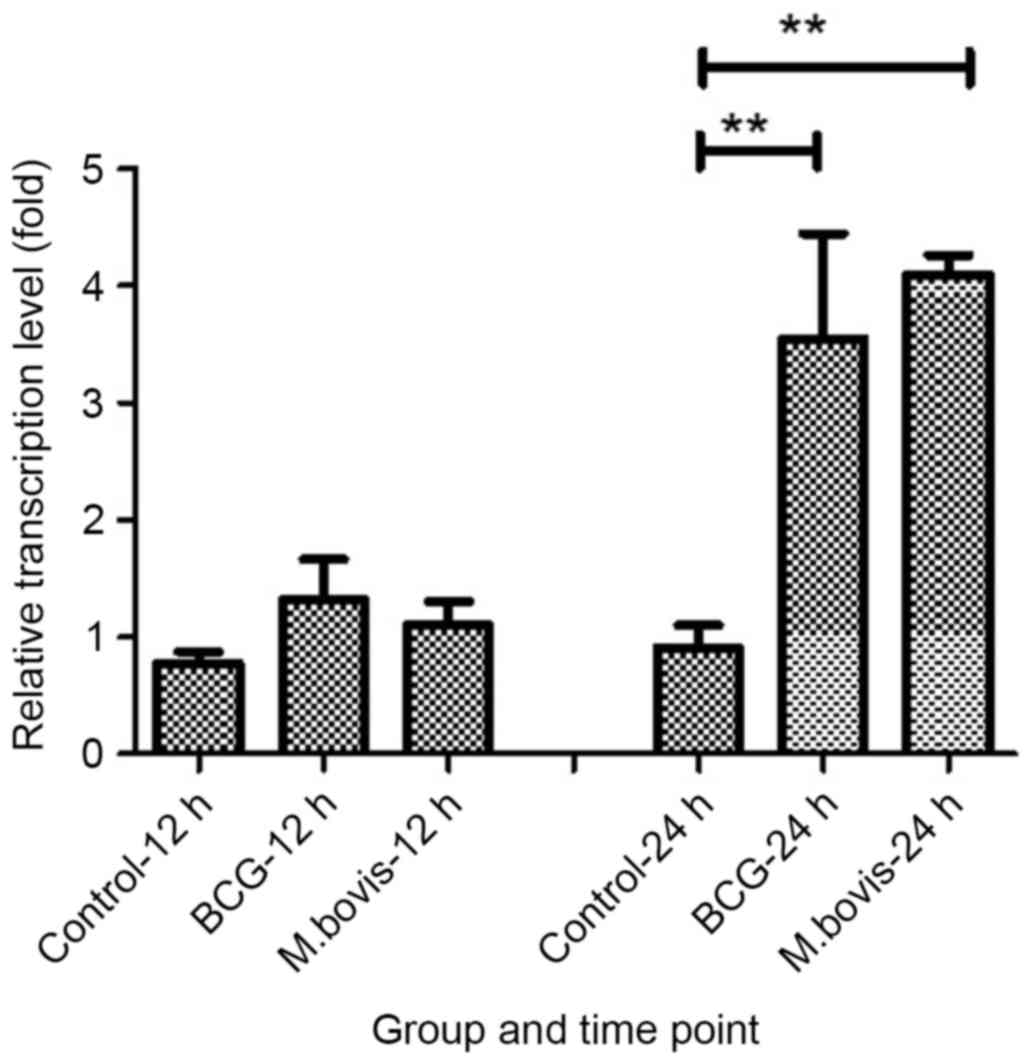
mRNA expression levels of TIMP-1
following infection with BCG or M
bovis. TIMP-1 mRNA expression levels were
detected by RT-qPCR at 12 and 24 h post infection and normalized to
µ-actin mRNA expression levels. TIMP-1 mRNA expression levels were
significantly upregulated in a time-dependent manner following BCG
and M. bovis infection. BCG and M. bovis infection
significantly increased TIMP-1 mRNA expression levels at 24 h post
infection (P=0.006 for BCG 24 h PI; P=3.2×10−7 for
M.bovis 24 PI; Fig. 5).
Discussion
Tuberculosis has been recognized in humans for
centuries and is a potentially life-threatening or debilitating
disease. Advances in the diagnosis of the disease may result in
control measures being implemented more rapidly (15), reducing the impact of the disease
on the community. In the present study the plasma TIMP-1 ELISA was
revealed to have a sensitivity of 91.80% (95% CI: 85.44, 96.00) and
a specificity of 91.41% (95% CI: 85.14, 95.63) according to
ROC.
MMPs are involved in TB in the migration of
leukocytes to infection sites and tissue destruction (16). Cytokines, including tumor necrosis
factor-α and IFN-γ, which may be induced by M. tb infection,
upregulate MMP production in recruited monocytes and macrophages
(8). TIMP-1 is an inhibitor of
MMPs, and controls MMP activity by forming 1:1 complexes with MMPs,
regulating the proteolysis of connective tissues and controlling
tissue damage (17). Previous
studies have demonstrated that concentrations of MMP-1, −2, −3, −8
and −9, as well as TIMP-1/2, are significantly greater in TB
patients compared with healthy controls (7,18–20).
In the present study, serum TIMP-1 levels were significantly
increased in TB patients compared with healthy controls, similar to
previous studies (9,18,19).
As TIMP production is associated with tissue destruction, tests for
TIMP levels have the potential to differentiate active TB from
latent infection. This represents a potential advantage over the
commonly used IFN-γ in vitro release assay, which is based
on cellular immunity memory to M. tb infection and which
does not differentiate active TB from latent infection (21).
To support this hypothesis indirectly, the present
study used the M. tb-specific antigen CFP-10/ESAT-6 to
stimulate PBMCs. However, CFP-10/ESAT-6 stimulation did not
significantly alter the plasma TIMP-1 concentrations in TB
patients. These results indicated that TIMP-1 was not produced by
PBMCs following M. tb antigen stimulation, and was affected
by the tissue damage induced by virulent bacteria or BCG. However,
this finding requires confirmation in a larger cohort.
In conclusion, the present study demonstrated that
TIMP-1 is present at high levels in TB patient sera, and that
expression of TIMP-1 mRNA is induced by mycobacteria. TIMP-1 may
therefore be a potential biomarker of TB in humans.
Acknowledgements
The present study was supported by the Key Special
Science and Technology Program for Important Infectious Diseases
Such as AIDS and Viral Hepatitis (grant nos. 2012ZX10003 and
2012ZX10004214), the China National Basic Research (973) Program
(grant no. 2012CB518801), the National Natural Science Foundation
of China (grant no. 31421064) and the Fok Ying Tung Education
Foundation (grant no. 132026).
References
|
1
|
World Health Organisation (WHO), . Global
tuberculosis report. 2014.
|
|
2
|
Qadeer E, Fatima R, Fielding K, Qazi F,
Moore D and Khan MS: Good quality locally procured drugs can be as
effective as internationally quality assured drugs in treating
multi-drug resistant tuberculosis. PLoS One. 10:e01260992015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tiwari D, Tiwari RP, Chandra R, Bisen PS
and Haque S: Efficient ELISA for Diagnosis of Active Tuberculosis
Employing a Cocktail of Secretory Proteins of Mycobacterium
tuberculosis. Folia Biol (Praha). 60:10–20. 2014.PubMed/NCBI
|
|
4
|
Hajiabdolbaghi M, Rasoulinejad M, Davoudi
AR, Alikhani A and Najafi N: Application of peripheral blood
Mycobacterium tuberculosis PCR for diagnosis of tuberculosis
patients. Eur Rev Med Pharmacol Sci. 18:185–189. 2014.PubMed/NCBI
|
|
5
|
Chen Y, Deng Q, Zhan Z, Guo A, Xiang J,
Chen J, Zhou J, Zeng Q, Wei W, Tong Q, et al: Establishment of
human IFN-gamma in vitro release assay and its application in
tuberculosis diagnosis. Sheng Wu Gong Cheng Xue Bao. 24:1653–1657.
2008.(In Chinese). PubMed/NCBI
|
|
6
|
Bwanga F, Hoffner S, Haile M and Joloba
ML: Direct susceptibility testing for multi drug resistant
tuberculosis: A meta-analysis. BMC Infect Dis. 9:672009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ugarte-Gil CA, Elkington P, Gilman RH,
Coronel J, Tezera LB, Bernabe-Ortiz A, Gotuzzo E, Friedland JS and
Moore DA: Induced sputum MMP-1, −3 & −8 concentrations during
treatment of tuberculosis. PLoS One. 8:e613332013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sundararajan S, Babu S and Das SD:
Comparison of localized versus systemic levels of Matrix
metalloproteinases (MMPs), its tissue inhibitors (TIMPs) and
cytokines in tuberculous and non-tuberculous pleuritis patients.
Hum Immunol. 73:985–991. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang KE, Shon YJ, Cha BK, Park MJ, Chu
MS, Kim YJ, Jeong ET and Kim HR: Tissue inhibitor of
metalloproteinase-1 is responsible for residual pleural thickening
in pleural tuberculosis. Tohoku J Exp Med. 235:327–333. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Chao Y, Deng Q, Liu T, Xiang J,
Chen J, Zhou J, Zhan Z, Kuang Y, Cai H, et al: Potential challenges
to the Stop TB Plan for humans in China; cattle maintain M. bovis
and M. tuberculosis. Tuberculosis (Edinb). 89:95–100. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Li S, Luo Y, Chen Y, Cheng S,
Zhang G, Hu C, Chen H and Guo A: Mycobacterium bovis and BCG induce
different patterns of cytokine and chemokine production in
dendritic cells and differentiation patterns in CD4+ T cells.
Microbiology. 159:366–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fontán P, Aris V, Ghanny S, Soteropoulos P
and Smith I: Global transcriptional profile of Mycobacterium
tuberculosis during THP-1 human macrophage infection. Infect Immun.
76:717–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weglarz L, Molin I, Orchel A, Parfiniewicz
B and Dzierzewicz Z: Quantitative analysis of the level of p53 and
p21(WAF1) mRNA in human colon cancer HT-29 cells treated with
inositol hexaphosphate. Acta Biochim Pol. 53:349–356.
2006.PubMed/NCBI
|
|
14
|
Gall D and Nielsen K: Comparison of some
methods for determing cutoff values for serological assays: A
retrospective study using the fluorescence polarization assay. J
Immunoassay Immunochem. 22:85–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Small PM and Pai M: Tuberculosis
diagnosis-time for a game change. N Engl J Med. 363:1070–1071.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rand L, Green JA, Saraiva L, Friedland JS
and Elkington PT: Matrix metalloproteinase-1 is regulated in
tuberculosis by a p38 MAPK-dependent, p-aminosalicylic
acid-sensitive signaling cascade. J Immunol. 182:5865–5872. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anand SP and Selvaraj P: Effect of 1, 25
dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and
the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol.
133:126–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elkington P, Shiomi T, Breen R, Nuttall
RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F,
Lipman M, et al: MMP-1 drives immunopathology in human tuberculosis
and transgenic mice. J Clin Invest. 121:1827–1833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walker NF, Clark SO, Oni T, Andreu N,
Tezera L, Singh S, Saraiva L, Pedersen B, Kelly DL, Tree JA, et al:
Doxycycline and HIV infection suppress tuberculosis-induced matrix
metalloproteinases. Am J Respir Crit Care Med. 185:989–997. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoheisel G, Sack U, Hui DS, Huse K, Chan
KS, Chan KK, Hartwig K, Schuster E, Scholz GH and Schauer J:
Occurrence of matrix metalloproteinases and tissue inhibitors of
metalloproteinases in tuberculous pleuritis. Tuberculosis (Edinb).
81:203–209. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tincati C, Iii AJ Cappione and
Snyder-Cappione JE: Distinguishing Latent from Active Mycobacterium
tuberculosis Infection Using Elispot Assays: Looking Beyond
Interferon-gamma. Cells. 1:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|