Introduction
Sensorineural deafness develops with age and has a
severe impact on human health and quality of life (1). The precise underlying mechanisms and
etiology of the disease remain to be elucidated, and its prevention
and treatment are current areas of research interest in otology
(2). Apoptosis or injury of spiral
ganglion neurons (SGNs) in the inner ear are considered the primary
causes of sensorineural deafness (3). Thus, it is important to investigate
potential anti-apoptotic effects on SGNs to identify potential
therapeutic targets for the prevention and treatment of
sensorineural deafness.
A previous study has suggested that erythropoietin
(EPO) exhibits neuroprotective properties via the erythropoietin
receptor (EPOR) (4). EPO and its
receptor EPOR are the primary factors promoting erythroid
progenitor cell proliferation and differentiation in mammals. EPO
was previously considered to be a single functional hematopoietic
factor. A previous study suggested that EPO is widely distributed
throughout the body, in the brain, liver, uterine tissue and
kidneys, and demonstrates protective effects on the brain, spinal
cord and heart (5).
Furthermore, previous studies have suggested that
EPO is expressed in the inner ear of rodents and may play a
protective role. Cayé-Thomasen et al (6) first reported the expression of EPO
and EPOR in the inner ear of guinea pigs, suggesting that EPO and
its receptor may be important in the regulation of SGNs. Berkingali
et al (7) revealed that
although EPO treatment of cultured SGNs did not improve the
survival rate, the neurite length increased significantly, and the
role of EPO in SGNs may therefore be primarily associated with
neurite extension. Monge Naldi et al (8) demonstrated that EPO had a protective
effect on ischemia- and gentamicin-induced hair cell and neuron
injury. These studies suggested that the inner ear may be a target
for EPO, and that EPO may have a neuroprotective effect on
SGNs.
During the progression of sensorineural deafness,
the number of apoptotic SGNs increases, despite the presence of
EPO. The present study investigated the expression of EPO and EPOR
in rat SGNs during development, to determine whether their
expression alters throughout the aging process and affects their
neuroprotective roles. In addition, it was examined how and at what
point they exhibit anti-apoptotic effects.
Materials and methods
Animals
A total of 15 healthy wild-type Sprague Dawley (SD)
rats of either sex at different ages were provided by the Third
Military Medical University (Chongqing, China) for use in the
present study. The experimental animals were divided into three
groups: i) An infant group at post-natal day (PND) 12–14 around the
onset of hearing, ii) an adult group at PND 60 and iii) an aged
group at ~3-years postnatal. The present study was approved by the
Laboratory Animal Welfare and Ethics Committee of the Third
Military Medical University. Guidelines from this Ethics Committee
to ensure the welfare of laboratory animals were followed in this
study, and the welfare of the rodents was optimized to improve
their living environments. Rats were maintained under standard
laboratory conditions, at 18–27°C and 40–70% humidity under a 12-h
light/dark cycle, with free access to clean water and food. All
rats were sacrificed by rapid decapitation.
Tissue preparation and
immunohistochemistry
All rats were sacrificed by rapid decapitation.
Bilateral temporal bones of each rat were dissected to remove the
cochleae, and the capsules were fractured to reveal the membranous
cochlear duct and the bony modiolus. The modiolus with the encased
acoustic ganglion neurons and the attached cochlear duct was
dissected and opened in 37°C Dulbecco's phosphate-buffered saline.
The middle ear was then opened, and the cochlea was carefully
perfused with 1.5% paraformaldehyde. The fixative was slowly
injected into the tympanic scale of the basal turn via the
round-window plasma membrane. A small opening was made at the
cochlear apex to allow cochlear perfusion. The specimens were
submersed in the same fixative at 4°C to ensure complete fixation
of the osseous tissue components. The cochlea was split into two
parts by a longitudinal mid-modiolar transaction and was
subsequently embedded in paraffin. Sections of 5–10 µm thickness
were cut from the embedded tissue and dried at 60°C to melt the
paraffin.
De-paraffinization of the tissues was performed
using xylene, and rehydration was achieved using decreasing
concentrations of ethanol. A 10-min Tris-buffered saline rinse was
performed, and the endogenous peroxidase activity was blocked by
incubation in 3% H2O2 for 5 min followed by a
30-min incubation with the following primary antibodies: Goat
anti-EPO (1:50-1:500; N-19; catalog no. sc-1310; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and rabbit anti-EPOR
(1:10-1:500; catalog no. bs-1424R; Bioss Inc., Woburn, MA, USA).
Sections were subsequently incubated with anti-goat (1:150; catalog
no. SP9000) or anti-rabbit (1:150; catalog no. SP9001) secondary
antibodies from immunohistochemistry staining kits purchased from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China). Color was developed with reagents from these kits,
according to the manufacturer's protocol. The tissue was rinsed in
running tap water for 5 min. Staining was observed under a light
microscope.
Apoptosis detection by terminal
deoxynucleotidyl transferase-mediated X-dUTP nick end labeling
(TUNEL) assay
TUNEL assays were performed on paraffin sections to
detect apoptosis, according to the manufacturer's protocol (In
Situ Apoptosis Detection kit; Roche Molecular Diagnostics,
Pleasanton, CA, USA). The transforming agent peroxidase (POD) was
applied so that the staining could be visualized under a light
microscope. POD (In Situ Cell Death Detection kit; OriGene
Technologies, Inc., Beijing, China) stained positive nuclei brown.
The proportion of apoptotic SGNs was calculated by counting 5
randomly selected fields under a microscope. The average proportion
represented the ratio of apoptotic neurons to surviving
neurons.
Auditory brainstem response (ABR)
recording
All animals were used for recording of the ABR,
detected in total from 30 ears of 15 rats (n=5 at PND 12–14, n=5 at
PND 60 and n=5 at 3-year postnatal). The rats were sedated with 400
mg/kg chloral hydrate (BBI Life Sciences Corporation, Shanghai,
China) injected intraperitoneally, in a soundproof chamber. A total
of four subcutaneous stainless-steel needle electrodes were
positioned at the vertex (positive), left mastoid (negative), right
mastoid (negative) and upper/lower lip (ground) of the animal. The
resistance between each electrode and the ground electrode was less
than 10 kΩ. The ABRs to the probe tone were recorded using a
Nicolet Spirit (Natus Medical, Inc., Pleasanton, CA, USA) and
stored on a computer for off-line analysis. The recording sampling
frequency was 25 kHz, and the signal was notch-filtered at 50 Hz,
high pass filtered at 0.1 kHz and low pass filtered at 3 kHz. The
ABR consisted of five vertex-positive peaks, labeled I–V. The
latency of peak I exponentially decreased with increasing stimulus
intensity. ABR latency was reduced by 2 ms as the correction for
the acoustic travel time from the loudspeakers to the ear. The
hearing thresholds, including the amplitude and latency of ABRs,
were detected and recorded for analysis.
Statistical analysis
The hearing thresholds and latency of peak I of ABRs
for statistical analysis were expressed as the mean ± standard
deviation. Data were analyzed using one-way analysis of variance
followed by the least significant difference post hoc test. For
immunohistochemistry, five sections from each rat were analyzed by
Image-Pro® Plus software version 5.0 (Media Cybernetics,
Inc., Rockville, MD, USA), and the average optical densities of
positive cells were measured. The percentages of apoptotic SGNs in
all groups were analyzed. Data analysis was performed using SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of EPO and EPOR during
development
EPO expression was observed in the neurons of the
inner ear as brown coarse granulated staining on the plasma
membrane and in the cytoplasm surrounding the nuclei. In addition,
EPO was present in the organ of Corti, including the hair cells,
and in the stria vascularis (Fig.
1A). The expression of EPO in SGNs was detected during
different developmental stages without significant alterations
(Fig. 1B), and a similar
expression pattern was observed in Corti's organ and the stria
vascularis. However, the EPO expression level in the SGNs was
significantly reduced compared with the other two areas (P=0.0423
in infant group; P=0.00724 in adult and aged groups). EPOR
expression was observed as clear granulated staining in the plasma
membrane and cytoplasm of SGNs in the inner ear, in the phalangeal
cells, in the organ of Corti, in the hair cells and in the stria
vascularis (Fig. 2A). The
expression of EPOR in the SGNs, Corti's organ and stria vascularis
increased significantly from the infant to the adult group and this
increased expression was maintained in the aged group. There were
no significant differences between the adult and aged groups in any
of the three areas (Fig. 2B).
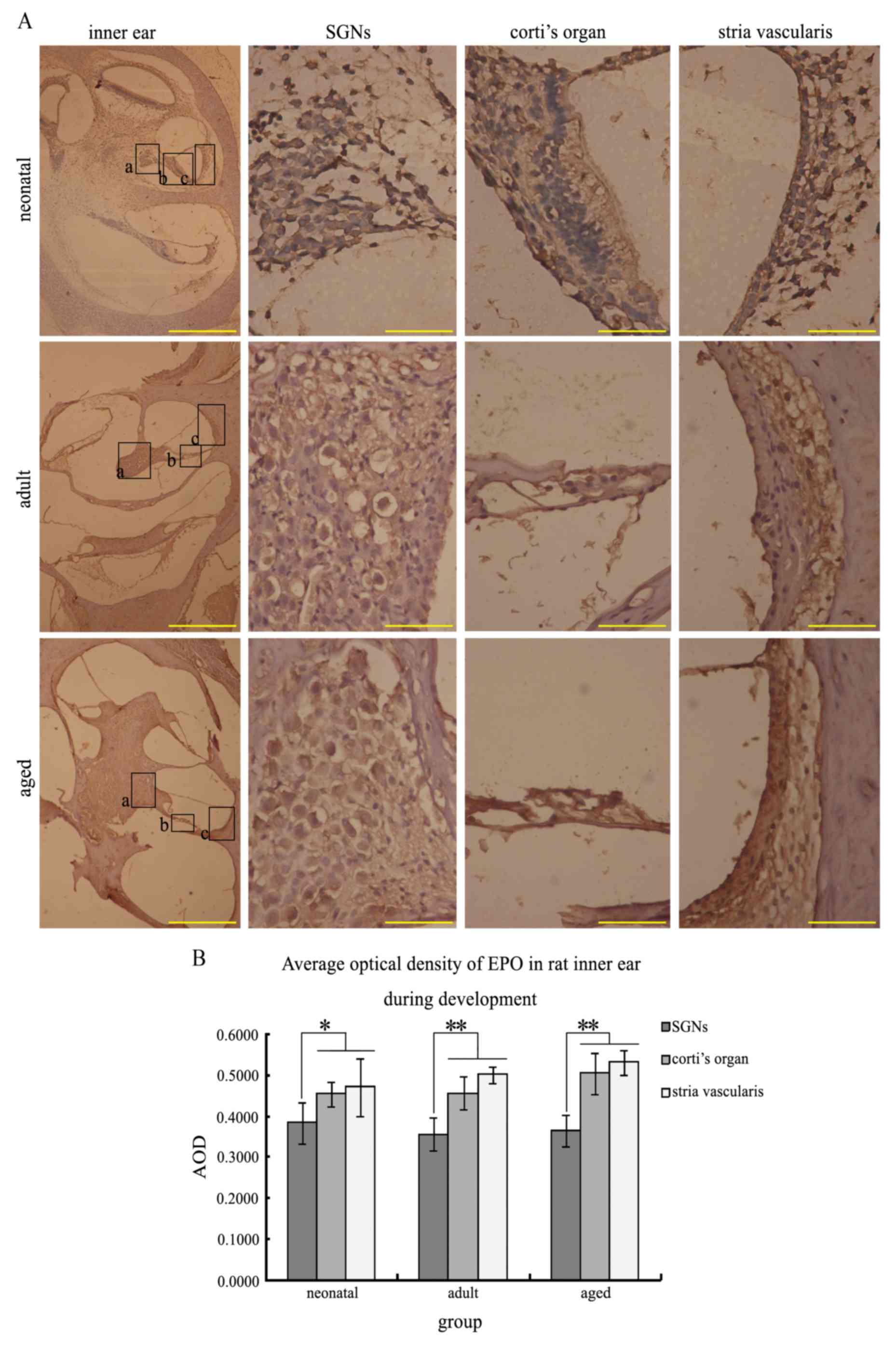 | Figure 1.EPO immunohistochemistry in the rat
inner ear during development. EPO-positive cells were stained brown
primarily in the membrane and cytoplasm. (A) Longitudinal sections
of EPO immunohistochemistry in the inner ear, SGNs, Corti's organ
and stria vascularis in infant, adult and aged rats. Higher
magnification images of the boxed areas a, b and c, in the inner
ear images are presented as SGNs, Corti's organ and stria
vascularis, respectively. (B) Average optical density of EPO in the
rat inner ear during development. The EPO expression levels in the
SGNs were significantly reduced compared with the other two areas
at all stages. *P<0.05 and **P<0.01. Inner ear images, scale
bar=500 µm; all other images, scale bar=50 µm. EPO, erythropoietin;
SGNs, spiral ganglion neurons; AOD, average optical density. |
 | Figure 2.EPOR immunohistochemistry in the rat
inner ear during development. EPOR-positive cells were stained
brown primarily in the membrane and cytoplasm. (A) Longitudinal
sections indicating the EPOR immunohistochemistry reaction in the
inner ear, Corti's organ and stria vascularis in infant, adult and
aged rats. Higher magnification images of the boxed areas a, b and
c, in the inner ear images are presented as SGNs, Corti's organ and
stria vascularis, respectively. (B) Average optical density of EPOR
in the rat inner ear during development. The expression of EPOR
increased significantly from the infant to the adult group and this
increased expression was maintained in the aged group. There were
no significant differences between the adult and aged groups or in
EPOR expression levels in the SGNs, Corti's organ or the stria
vascularis. *P<0.05 vs. infant SGNs; **P<0.01 vs. infant
SGNs; ΔΔP< 0.01 vs. infant Corti's organ;
▲P<0.05 vs. infant stria vascularis;
##P<0.01 vs. infant total. Inner ear images, scale
bar=500 µm; all other images, scale bar=50 µm. EPOR, erythropoietin
receptor; SGNs, spiral ganglion neurons; AOD, average optical
density. |
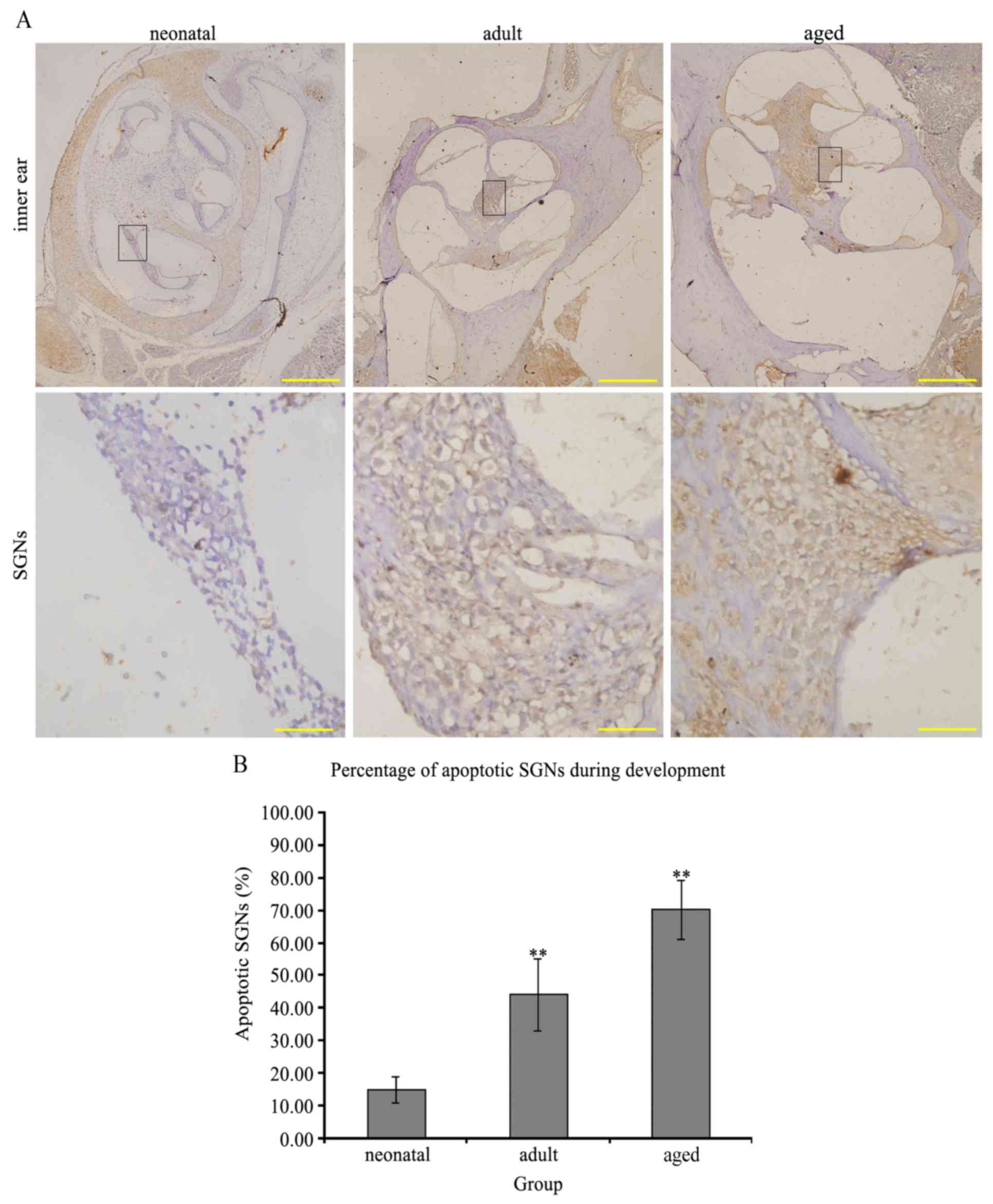
Apoptosis of neurons
The nuclei of apoptotic neurons were stained brown
(Fig. 3A). The percentage of
apoptotic SGNs in the infant rat group was 15.11±0.04%, which was
significantly decreased compared with 44.25±0.11% in the adult
group and 70.23±0.09% in the aged group (P<0.01 infant vs.
adult/aged groups; Fig. 3B),
demonstrating an age-associated increase in neuronal apoptosis.
Audiometric testing of the ABR
The hearing thresholds of rats were recorded in
audiograms (Fig. 4A). The average
hearing thresholds of the ABR of each ear in the infant, adult and
aged groups were 5.625±4.955, 14.500±8.960 and 23.500±13.134
decibels above normal hearing level, respectively. In the adult
rats, the ABR was close to three times that of the infant rats, and
in the aged rats, it was close to five times that of the infant
rats. Hearing thresholds for adult (P=0.0375 vs. infant rats) and
aged rats (P=0.00832 vs. infant rats) increased significantly
(Fig. 4B). Latencies were
expressed as the mean ± standard deviation (infant, 1.796±0.375 ms;
adult, 2.036±0.093 ms; aged, 1.948±0.452 ms; Fig. 4C) and amplitudes of peak I in these
three groups (infant, 2.036±0.905 µV; adult, 3.089±1.308 µV; aged,
2.181±1.162 µV; Fig. 4D) were not
significant (P=0.079).
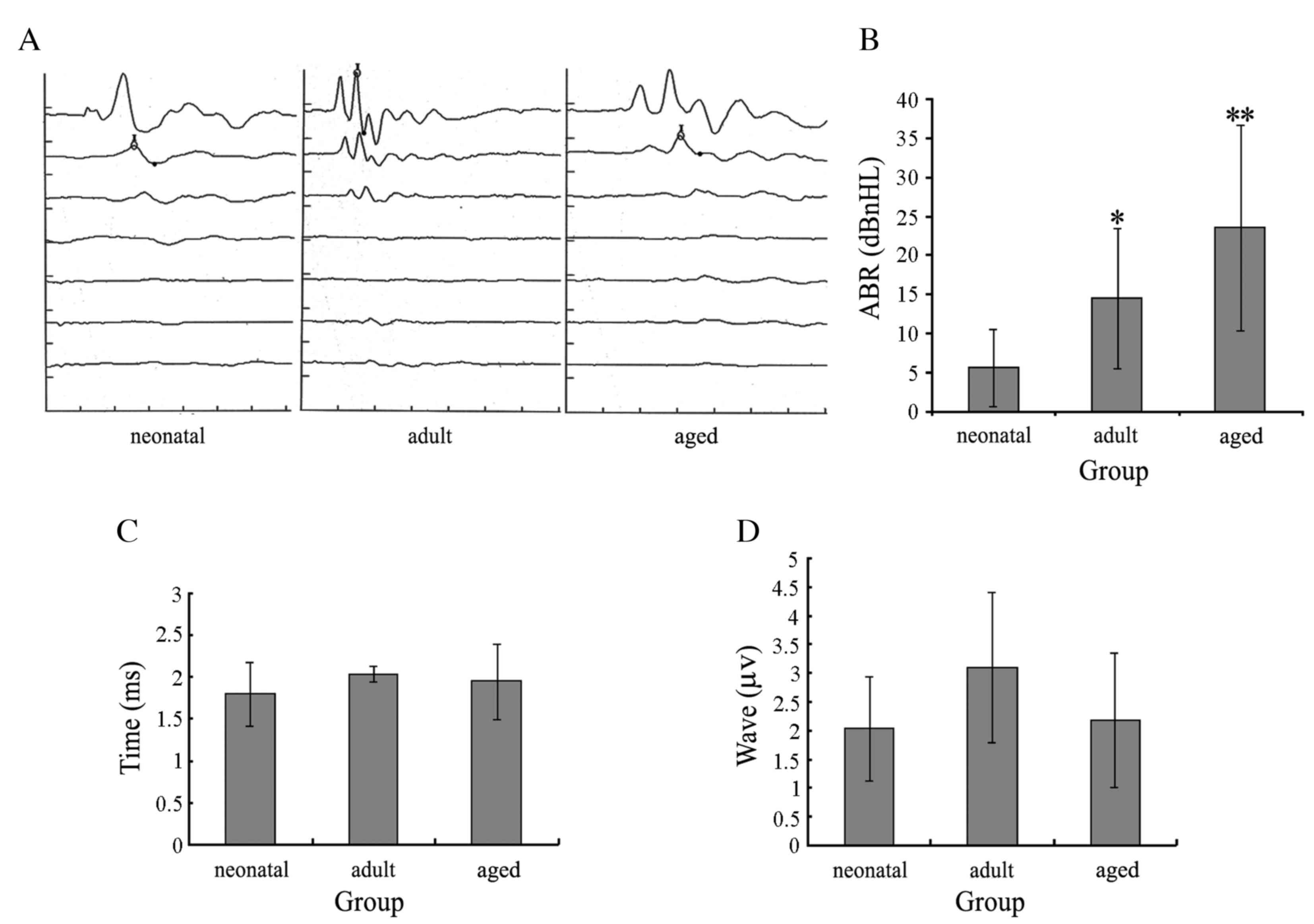 | Figure 4.ABRs of rats. (A) Audiograms of the
infant, adult and aged groups revealed typical peaks of ABR. (B)
Average hearing thresholds of infant, adult and old groups were
5.625±4.955, 15.000±8.498 and 23.500±13.134 decibels above normal
hearing level, respectively. Hearing thresholds of adult and aged
rats increased significantly. *P<0.05; **P<0.01. (C) Average
latencies of peak I in the infant, adult and aged groups were
1.796±0.375, 2.036±0.093 and 1.948±0.452 ms, respectively. (D)
Average amplitudes of peak I in the infant, adult and aged group
were 2.036±0.905, 3.089±1.308 and 2.181±1.162 µV, respectively. The
differences in average time and amplitude of peak I latencies in
the three groups were not significant. ABR, audio brainstem
response. |
Discussion
Sensorineural deafness, the underlying mechanism of
which remains to be fully elucidated, is typically characterized by
bilateral progressive hearing loss associated with the aging
process (9). Injury and apoptosis
to the SGNs in the inner ear are considered to be the primary
causes of sensorineural deafness (3). SGNs are the primary neurons of the
auditory pathway, which transmit signals from auditory hair cells
to the auditory center. SGNs are therefore key to successful
auditory conduction. The infant rats were assessed at PND 12–14,
around the onset of hearing in the present study and compared with
mature and aged rats. SGNs in the aged group began to demonstrate a
high degree of apoptosis, which was significantly increased
compared with the adult group. The hearing of the rats was recorded
by ABR in all groups, in a series of five to seven consistent waves
that were electrically recorded from the early neural responses to
acoustic signals (10,11). The hearing threshold increased
significantly with age and was almost 3 and 5 times greater in the
adult and aged groups, respectively, compared with the infant group
(1,2). Alleviating hearing loss by protecting
SGNs has become a primary focus of current research.
The latencies of ABR waves are important
otologically and neurophysiologically, and the SGNs (primarily type
I), which innervate the inner hair cells, may predominantly
contribute to peak I of the ABR (12). Differences in the timing and
altitude of the latencies of peak I in the present study were not
significantly associated with aging. A previous study has
demonstrated that peak latencies are associated with numerous
events, from the distance of the recording electrodes to the
current source (for example, on the scalp), to local transmembrane
postsynaptic currents and the activity of hair cells (13). These alternative explanations may
help to explain the findings of the present study.
EPO has demonstrated multiple neuroprotective
effects, and has therefore been investigated for its ability to
protect SGNs. EPO blocks the translation of apoptosis genes via
inhibition of the nuclear translocation of transcription factor 3a,
and activates anti-apoptosis genes by promoting the nuclear
transfer of nuclear factor (NF)-κB (14). Sims et al (15) revealed that the cysteine-glutamate
transformation ability of neurons differentiating from neural stem
cells may be increased by EPO, and thus the neural antioxidation
ability may be enhanced. A Previous study demonstrated that the
neuroprotective effects of EPO may include at least two mechanisms;
a decrease in the hair cell death rate and the induction of
angiogenic genes, including the vascular endothelial growth factor
and C-X-C chemokine receptor type 4 (16). This data indicates that
brain-derived neurotrophic factor and EPO may promote neuronal
survival in the inner ear in vitro (17).
Although EPO demonstrates the aforementioned
neuroprotective roles and may be detected in SGNs, it does not
demonstrate neuroprotective effects on the apoptosis of SGNs during
development (thereby ameliorating any sensorineural hearing loss).
To address this, the present study detected the expression of EPO
in the inner ear, particularly in SGNs during development. It was
observed that the expression of EPO did not alter with age in the
SGNs, Corti's organ or the stria vascularis. Furthermore, EPO
expression in the SGNs was reduced compared with the other two
areas, which may be associated with their greater blood flow, and
SGNs may be vulnerable and refractory as this area is not highly
vascularized.
Previous studies have suggested that circulating
EPO, produced primarily by the kidneys, may not cross the
blood-brain barrier (BBB), and thus only exerts its effects on
neurons in an autocrine or paracrine, but not endocrine, manner
(18,19). However, a separate study has
revealed that EPO may cross the BBB (20). The antibodies used in the present
study did not differentiate between circulating EPO and EPO
produced in the inner ear; however, it is possible that EPO
expression levels in Corti's organ and the stria vascularis were
greater compared with SGNs due to their containing two types of
EPO, that is, the circulating EPO and the EPO expressed by the
inner ear itself, whereas the SGNs contained reduced levels of
circulating EPO.
Frederiksen et al (21) reported contradictory effects of EPO
compared with those that had been previously reported. This study
observed that EPO augmented noise-induced hearing loss by altering
the dynamics of blood flow to the cochlear vascular bed. The
authors suggested an underlying mechanism of potentially induced
vasoconstriction, which included pathophysiologic alterations and
reduced cochlear blood flow, including localized periods of stasis,
vascular permeability alterations and local ischemia. These
alterations may result in temporary or even permanent deafness. The
discrepancies between the findings of this study and others may be
correlated with the results of the present study that the
expression of EPO and EPOR in the stria vascularis was greater
compared with the SGNs. Thus, further research is necessary to
investigate whether the expression patterns of EPO in the inner ear
impact its role in SGNs.
During development, the expression of EPO in SGNs
remained low and did not increase with age, whereas the number of
apoptotic SGNs increased with age. Thus, the neuroprotective
effects of naturally expressed EPO in SGNs may not be sufficient to
counter SGN apoptosis, particularly in aging, as the proposed
neuroprotective effects of EPO did not appear in the normal aging
process.
The neuroprotective effects of EPO may depend upon
the EPO-EPOR interaction. The EPO interaction with EPOR may
subsequently activate multiple downstream signaling pathways,
including Ras/mitogen activated protein kinase, phosphoinositide
3-kinase/protein kinase B, signal transducer and activator of
transcription 5 and NF-κB, resulting in a series of anti-apoptotic
priming mechanisms, which exert neuroprotective effects (22). Although in the present study the
expression of EPO in the inner ear, especially in the SGNs,
indicated no significant alterations during development, the
expression of EPOR was significantly increased from infant to adult
rats, and this increased expression was maintained in old age. The
significance of this alteration in expression is unknown. As
neurotrophic factors promote neural differentiation and survival
via the aforementioned signaling pathways, this increased
expression of EPOR may be associated with the expression of
neurotrophic factors; this requires further study. Although EPO
expression does not increase in the inner ear, particularly in
SGNs, during development, the increased EPOR expression level does
exhibit a further neuroprotective role by rendering a high
probability of abundant binding anchors for exogenous EPO.
In conclusion, the present study detected the
hearing threshold, apoptosis rate and expression of EPO and EPOR in
SGNs in infant, adult and aged rats using ABR, TUNEL assay and
immunohistochemistry, respectively. The present study demonstrated
that the number of apoptotic SGNs and the hearing threshold
increased significantly with age. However, the expression of EPO
did not alter significantly with age and was maintained at a
relatively low level in the SGNs. The present study hypothesized
that the neuroprotective effects of EPO expressed in SGNs may not
be sufficient to counter the apoptosis of SGNs, particularly in old
age. Thus, the neuroprotective effects of EPO were not revealed in
the aging process under natural conditions. The expression of EPOR
increased significantly from the infant to adult period, and this
increased expression was maintained at a high level from the adult
to the aged period, which may act as a potential novel therapeutic
strategy against age-associated sensorineural deafness via
administration of an exogenous supply of EPO.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81100720).
Glossary
Abbreviations
Abbreviations:
|
EPO
|
erythropoietin
|
|
EPOR
|
erythropoietin receptor
|
|
SGNs
|
spiral ganglion neurons
|
|
ABR
|
auditory brainstem response
|
References
|
1
|
Monini S, Filippi C, Baldini R and Barbara
M: Perceived disability from hearing and voice changes in the
elderly. Geriatr Gerontol Int. 15:147–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamasoba T, Lin FR, Someya S, Kashio A,
Sakamoto T and Kondo K: Current concepts in age-related hearing
loss: Epidemiology and mechanistic pathways. Hear Res. 303:30–38.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao J and Ohlemiller KK: Age-related loss
of spiral ganglion neurons. Hear Res. 264:93–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Byts N and Sirén AL: Erythropoietin: A
multimodal neuroprotective agent. Exp Transl Stroke Med. 1:42009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brines M and Cerami A: Discovering
erythropoietin's extra-hematopoietic functions: Biology and
clinical promise. Kidney Int. 70:246–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cayé-Thomasen P, Wagner N, Frederiksen B
Lidegaard, Asal K and Thomsen J: Erythropoietin and erythropoietin
receptor expression in the guinea pig inner ear. Hear Res.
203:21–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berkingali N, Warnecke A, Gomes P, Paasche
G, Tack J, Lenarz T and Stöver T: Neurite outgrowth on cultured
spiral ganglion neurons induced by erythropoietin. Hear Res.
243:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naldi A Monge, Gassmann M and Bodmer D:
Erythropoietin but not VEGF has a protective effect on auditory
hair cells in the inner ear. Cell Mol Life Sci. 66:3595–3599. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panza F, Solfrizzi V and Logroscino G:
Age-related hearing impairment-a risk factor and frailty marker for
dementia and AD. Nat Rev Neurol. 11:166–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jewett DL and Williston JS:
Auditory-evoked far fields averaged from the scalp of humans.
Brain. 94:681–696. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malherbe TK, Hanekom T and Hanekom JJ: Can
subject-specific single-fibreelectrically evoked auditory brainstem
response data be predicted from a model? Med Eng Phys. 35:926–936.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rattay F and Danner SM: Peak I of the
human auditory brainstem response results from the somatic regions
of type I spiral ganglion cells: Evidence from computer modeling.
Hear Res. 315:67–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown DJ and Patuzzi RB: Evidence that the
compound action potential (CAP) from the auditory nerve is a
stationary potential generated across dura mater. Hear Res.
267:12–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chong ZZ and Maiese K: Erythropoietin
involves the phosphatidylinositol 3-kinase pathway, 14-4-4 protein
and FOXO3a nuclear traffic-king to preserve endothelia cell
integrity. Br J Pharmacol. 150:839–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sims B, Clarke M, Njah W, Hopkins ES and
Sontheimer H: Erythropoietin-induced neuroprotection requires
cystine glutamate exchanger activity. Brain Res. 1321:88–95. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gross J, Moller R, Amarjargal N, Machulik
A, Fuchs J, Ungethüm U, Kuban RJ, Henke W, Haupt H and Mazurek B:
Expression of erythropoietin and angiogenic growth factors
following inner ear injury of newborn rats. Prague Med Rep.
110:310–331. 2009.PubMed/NCBI
|
|
17
|
Kaiser O, Paasche G, Stöver T, Ernst S,
Lenarz T, Kral A and Warnecke A: TGF-beta superfamily member
activin A acts with BDNF and erythropoietin to improve survival of
spiral ganglion neurons in vitro. Neuropharma. 75:416–425. 2013.
View Article : Google Scholar
|
|
18
|
Juul SE, Stallings SA and Christensen RD:
Erythropoieti n in the cerebrospinal fluid of neonates who
sustained CNS injury. Pediatr Res. 46:543–547. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saunders NR, Habgood MD and Dziegielewska
KM: Barrier mechanisms in the brain, II. Immature brain. Clin Exp
Pharmacol Physiol. 26:85–91. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banks WA, Jumbe NL, Farrell CL, Niehoff ML
and Heatherington AC: Passage of erythropoietic agents across the
blood-brain barrier: A comparison of human and murine
erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol.
505:93–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frederiksen BL, Cayé-Thomasen P, Lund SP,
Wagner N, Asal K, Olsen NV and Thomsen J: Does erythropoietin
augment noise induced hearing loss? Hear Res. 223:129–137. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maiese K, Chong ZZ, Li F and Shang YC:
Erythropoietin: Elucidating new cellular targets that broaden
therapeutic strategies. Prog Neurobiol. 85:194–213. 2008.
View Article : Google Scholar : PubMed/NCBI
|