Introduction
With an increase in the elderly population, the
occurrence of bone-associated diseases including osteoporosis,
osteoarthritic bone and joint disease will increase, and these
diseases affect the quality of life of the patients affected
(1). In particular, osteoporosis
is a metabolic bone disorder characterized by a reduction in bone
density and an increase in the fracture risk, which may influence
up to 50% women and 20% men over 50 years of age (2,3).
Bone remodeling occurs continuously throughout our lifetime and
unbalance of bone remodeling is the cause of osteoporosis (4). Treatment for osteoporosis is focused
on drug therapy using bone resorption inhibitors. Although this
drug therapy aids in the prevention of osteoporosis, side effects
are present, and there are limitations in the regeneration of bone
tissue (5). Regenerative medicine
with autologous transplantation such as embryonic stem cell,
induced pluripotent stem cell and adult stem cell is an alternative
method to repair bone loss of osteoporosis (6).
Mesenchymal stem cells (MSCs) are characterized by
their ability to give rise to differentiated stromal cells, which
belong to the osteogenic, chondrogenic, adipogenic, myogenic and
fibroblastic lineages, and their capacity for self-renewal
potential (7–9). MSCs maintain a balance between stem
cell and differentiated cell populations by asymmetrical cell
division (10). Their multipotency
and self-renewal potential makes them attractive targets for cell
and gene therapy, and the differentiation of MSCs into the
osteogenic lineage is important for bone regeneration, as observed
in osteogenesis imperfecta, which is caused by abnormal collagen
production or hypophosphatasia, a disorder of osteoblasts and
chondrocytes (11,12). However, the application of gene and
cell therapy methodologies to MSCs is limited by the reduced
differentiation and proliferation potential of these cells in
culture.
Herbal Epimedium (HE) is used as a tonic,
aphrodisiac and antirheumatic in Korea, and consists of flavonoids,
alkaloids, lignans and terpenic compounds. HE has been demonstrated
to decrease the total cholesterol and triglyceride levels, however
to increase estradiol (13), and
flavonoid components of HE have been reported to have antioxidant
properties (14). HE flavonoids
stimulate osteoblast formation and suppress the process of
osteoclastogenesis in ovariectomized mice, and thus prevent bone
loss in this model (15). However,
little is known about the effects of HE extract formula on the
balance between proliferation and differentiation potential of
mouse bone marrow MSCs (mBMMSCs). The present study aimed to
identify the effects of HE on proliferation and differentiation
potential of mBMMSCs using a series of experiments including
viability assays, cell cycle analysis, immunohistochemistry and
differentiation assays.
Materials and methods
mBMMSC culture
Mouse stromal cells were isolated according to a
modification of the protocol of Nadri and Soleimani (16). A total of three C57BL/6 mice (male,
8–12 weeks of age; weight, 20–22 g; SLC Inc., Hamamatsu, Japan)
were used. The animals were housed in a specific pathogen-free
environment with 12/12 h light/dark cycles at the Center for
Laboratory Animal Care and Use at Kyung Hee University (Seoul,
Korea). Animals had free access to standard rodent pellets and
water (Purine, Seoul, Korea). The mice were anesthetized by
intraperitoneal injection of urethane (100 mg/kg; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany). Bone marrow cells were
obtained by flushing the femurs and tibias of the C57BL/6 mice with
Dulbecco's Modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 15% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin. Single-cell suspensions were
prepared from clumps of bone marrow by resuspending the cells using
a syringe mounted with a 26-gauge needle, and passing them through
a 70-mm cell strainer (Falcon; BD Biosciences, Heidelberg,
Germany). The cells were cultured in DMEM with 15% FBS, 100 U/ml
penicillin and 100 mg/ml of streptomycin on 0.1% gelatin-coated
10-cm dishes at 37°C in a humidified atmosphere of 5%
CO2.
Preparation of HE extract
HE was purchased from Wonkwang Herbal Drug Co., Ltd.
(Seoul, Korea). Samples of 300 g of dried medicinal herbs were
boiled in 6 l of water for 2 h at 100°C, and the suspensions were
then filtered and evaporated under reduced pressure. The filtrates
were lyophilized and yielded 37.1 g of powder. Dried extracts were
dissolved in distilled deionized water (EMD Millipore, Billerica,
MA, USA) and vortexed for 2 min at room temperature.
Liquid chromatography-tandem mass
spectrometry (LC-MS/MS)
Stock solution of reference standard, icarrin was
prepared in DMSO at a concentration of 1 mg/ml. The working
solutions for MS analysis were prepared in 80% acetonitrile at
concentration of 1000 ng/ml. For each standard curve, six
calibration standards (at 50, 100, 250, 500 and 1000 ng/ml) were
analyzed. For HE analysis, 10 mg/ml HE was dissolved in 80%
acetonitrile and was separated by centrifugation at 1,152 ×
g for 10 min at 25°C. The clear supernatant extract was
filtered through 0.45-µm membrane filter prior to LC-MS/MS
determination.
Chromatography was performed on a liquid
chromatography system (Agilent Technologies, Inc., Santa Clara, CA,
USA). A 500 µl aliquot of diluted HE was injected onto a 100×2.1
mm, Atlantis DC18 column (Waters Corporation, Milford, MA, USA).
The column was maintained at 20°C. Mobile phases A and B consisted
of 0.1% aqueous formic acid and acetonitrile, respectively, and
were eluted with a linear gradient of 5–95% in 20 min at a flow
rate of 0.2 ml/min.
The QTRAP 3200 mass spectrometer system (Ab Sciex,
Framingham, MA, USA) operated in positive ionization modes was used
in the current study and processed with multiple reactions
monitoring (MRM) scanning. The mobile phase contained acetonitrile
in water with 0.1% (v/v) formic acid. Under the optimized condition
for positive mode, nitrogen was used as drying gas, 10 l min-1 and
nebulizer gas, 50 psi, while the voltage of ion spray in source was
set at 5500 V and the gas temperature was 400°C.
Cell viability, proliferation,
proliferating cell nuclear antigen (PCNA) detection and apoptosis
assays
Cell proliferation was determined by MTT assay.
mBMMSCs were starved for 24 h and then treated with the indicated
concentrations of HE (1, 10, 50, 75, 100 or 200 mg/ml). After 12,
24 and 48 h, the medium was removed and the cells were incubated
with MTT to measure metabolic activity. For detection of PCNA,
mBMMSCs in starvation medium were incubated with HE (100 ug/ml)
basic fibroblast growth factor (bFGF; Gibco; Thermo Fisher
Scientific, Inc.) and various inhibitors (SB202190 and PD98059;
Cell Signaling Technology, Danvers, MA, USA) for 24 h, before they
were fixed and permeabilized in cold methanol, washed three times
and blocked for 1 h, at room temperature in DMEM-based buffer
containing 5% FBS. Samples were washed and incubated overnight with
an anti-PCNA antibody (dilution, 1:200; cat. no. M0879; Dako;
Agilent Technologies, Inc.) at 4°C. The cells were then washed
three times and incubated for 4 h with the goat anti-mouse Alexa
488 antibody (excitation 488 nm, emission 519 nm; dilution, 1:200;
cat. no. A21202; Invitrogen; Thermo Fisher Scientific, Inc.). For
detection of nuclei, cells were incubated for 5 min with propidium
iodide (PI; 3.75 mg/ml; excitation 540 nm, emission 630 nm).
Apoptosis was evaluated using a commercial Annexin V/Fluorescein
Isothiocyanate (FITC) Apoptosis detection kit (BD Biosciences, San
Jose, CA, USA) on cells stained with annexin V according to the
manufacturer's instructions. Cells positive for annexin V were
counted by fluorescence-activated cell sorting and analyzed using
CellQuest Pro software (version 5.1; BD Biosciences) to determine
the percentage of cells undergoing early apoptosis and late
apoptosis.
Bone marrow MSC differentiation
assays
The potential of the isolated cells to differentiate
into osteogenic and adipogenic lineages was examined. For
osteogenesis, the cultured cells were incubated in osteogenic
conditioned medium, as described previously by Eslaminejad and
Nadri (17). Briefly, DMEM was
supplemented with 10 mM β-glycerol phosphate (Sigma-Aldrich; Merck
Millipore), 50 mg/ml ascorbate-2-phosphate (Sigma-Aldrich; Merck
Millipore), and 10−7 M dexamethasone (Sigma-Aldrich;
Merck Millipore). The cells were fixed with methanol for 10 min at
room temperature and stained with Alizarin red (pH 4.0) for 5 min
at room temperature and with Von Kossa staining for bone nodule
formation. For adipogenesis, the cultured cells were incubated in
adipogenic medium consisting of DMEM supplemented with 50 mg/ml
indomethacin (Sigma-Aldrich; Merck Millipore), 10−7 M
dexamethasone, and 50 mg/ml ascorbate-2-phosphate. The cells were
then fixed in methanol for 45 min and stained with Oil red O
(Sigma-Aldrich; Merck Millipore).
Statistical analysis
Statistical analysis was performed using GraphPrism
software, version 4.0.3 (GraphPad Software, Inc., La Jolla, CA,
USA). Data from the MTT assay are presented as the mean and
standard deviation, and were analyzed using SPSS, version 12.0
(SPSS, Inc., Chicago, IL, USA).
Results
Detection and quantification of HE
using LC-MS/MS
To identify and confirm the compound of HE extract
was analyzed using an LC-MS/MS method. Icariin was used as an
indicative compound investigated in the positive ionization mode of
LC-MS/MS. Quantification was performed using MRM mode at m/z 677 →
396.2 for icariin and detected at a retention time of 10.54 min. 10
mg/ml HE extract containing 29.9 µg/ml icariin (Fig. 1).
HE inhibits mBMMSC proliferation
The effects of HE on the proliferation of mBMMSCs
were determined from cell growth kinetics with the MTT assay
measuring the metabolic activity of viable cells. Growth-arrested
mBMMSCs, cultured in starvation medium for 24 h prior to the
experiment, were incubated for 12, 24 or 48 h with HE. The results
indicated that HE-treated cells exhibited reduced cell viability
compared with untreated controls. As presented in Fig. 2, treatment with HE at
concentrations up to 100 mg/ml for up to 48 h inhibited cell
proliferation in a dose- and time-dependent manner to a maximum of
45.43±3.33% (P<0.001; Fig.
2A).
 | Figure 2.(A) Growth inhibitory effect of HE on
mBMMSC proliferation. mBMMSCs were starved for 24 h and treated
with HE as indicated for 12, 24 or 48 h. Normal (0 µg/ml),
PBS-treated cells. Each column represents the mean ± standard
deviation (n=3). *P<0.05, **P<0.01 vs. normal value. (B)
Effects of HE on PCNA immunoreactivity in mBMMSCs. mBMMSCs grown in
complete medium were incubated in the presence or absence of either
SB202190 (30 mM, 2 h) or PD98059 (30 mM, 2 h) prior to incubation
with HE (100 mg/ml, 24 h). Cells were then stained with PI for
detection of nuclei (red) or with an anti-PCNA antibody (green).
Overlay images of both stains are also presented. bFGF was used as
a positive control. All images were obtained using magnification of
×20 with an Olympus BX-61 fluorescence microscope. (C) HE induced
mBMMSCs apoptosis. Apoptosis was measured by annexin V-FITC/PI
staining and analyzed by flow cytometry. Horizontal and vertical
axes represent labeling with annexin V-FITC and PI, respectively.
Lower left, live cells; lower right, early apoptotic cells; upper
left, necrotic cells; upper right, late apoptotic cells. HE, herbal
Epimedium; mBMMSCs, mouse bone marrow mesenchymal stem
cells; PCNA, proliferating cell nuclear antigen; PI, propidium
iodide; bFGF, basic fibroblast growth factor; FITC, fluorescein
isothiocyante. |
Measurement of PCNA immunoreactivity
in proliferating mBMMSCs treated with HE
The antiproliferative effects of HE were further
confirmed using PCNA, a protein that participates in DNA
replication. In these experiments, cells grown in complete medium
were treated with HE (100 mg/ml, 24 h) in the presence or absence
of SB202190 or PD98059, inhibitors of p38 and extracellular
signal-related kinase 1/2, respectively, and then incubated with
the fluorescent DNA binding dye, PI, for nuclear staining (red) and
with an antibody directed against PCNA (green). As expected,
treatment with bFGF, an inducer of cell growth and proliferation,
resulted in an increase in PCNA staining (Fig. 2B). In contrast, HE reduced the
level of PCNA expression, and pretreatment with SB202190 and
PD98059 further reduced the level of PCNA compared with cells
treated with HE alone (Fig.
2B).
Effects of HE on apoptosis
MSCs exhibited a reduction in the number of live
cells and increases in both apoptotic and dead cells (early or late
apoptosis) at both 24 and 48 h after treatment with HE. The
percentages of MSCs undergoing late apoptosis at 24 and 48 h, as
determined by annexin V-FITC/PI staining, were increased in
HE-treated cells (71.93 and 67.03% vs. 14.93%, respectively), while
the percentages of HE-treated MSCs undergoing early apoptosis were
similar to that of untreated cells (11.78 and 14.3% vs. 11.13%,
respectively) (Fig. 2C).
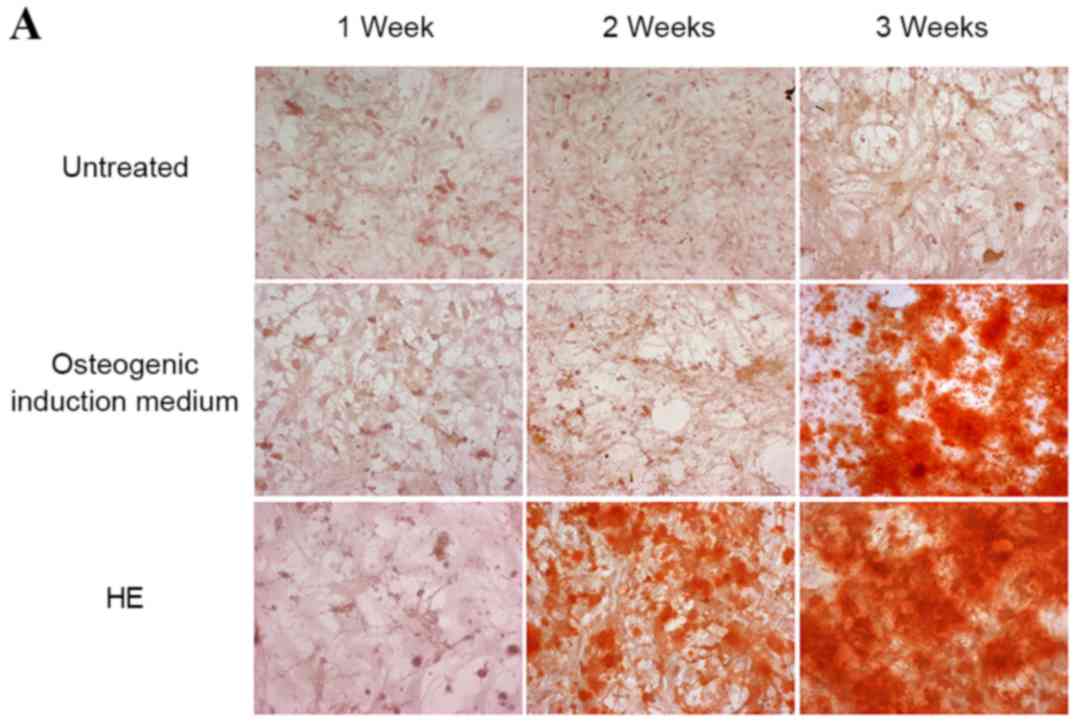
An important feature of mBMMSCs is their ability to
differentiate into osteoblasts, chondroblasts and adipocytes. To
determine whether HE affects the osteogenic and adipogenic
differentiation capacity of the cells, mBMMSCs were treated with HE
at a concentration of 100 mg/ml for three weeks. Both Alizarin red
and Von Kossa staining assays clearly indicated strong induction of
differentiation of mBMMSCs to the osteogenic lineage by HE
treatment (Fig. 3A and B). Adipose
droplets, a representative marker of adipogenic induction of
mBMMSCs, was not detected up to 3 weeks subsequent to HE treatment
(Fig. 3C).
Discussion
HE has been widely used in the treatment of
bone-associated diseases, including osteoporosis (14). However, the molecular mechanisms
underlying the effects of HE remain to be fully elucidated. In the
present study, the effects of HE on MSC differentiation into
osteogenic lineage cells were investigated.
The results of cell viability assays indicated that
HE inhibited cell proliferation of mBMMSCs in a dose-dependent
manner. And this was confirmed through the examination of PCNA, a
protein that participates in DNA replication. The results indicated
that HE reduced the level of PCNA expression compared with that of
untreated cells. With appropriate stimulation, adult stem cells
proceed though the cell cycle to replace the stem cell population,
and then give rise to a variety of differentiated cell types for
tissue regeneration and homeostasis (18). By contrast, cell cycle or growth
arrest can lead to tumor suppression and normal cell
differentiation (19).
Apoptosis, which is programmed cell death, is a
normal physiological process involved in embryonic development in
addition to adult tissue homeostasis. Undifferentiated human MSCs
(hMSCs) are resistant to apoptosis. By contrast, hMSCs during
differentiation exhibit increased apoptosis regulated by members of
the BCL-2 family (20). The
present study demonstrated that HE could additionally induce
apoptosis in MSCs; the percentage of MSCs undergoing apoptosis was
markedly increased in HE-treated cells. As cell cycle arrest or
apoptosis can trigger cell differentiation and gene expression
involved in differentiation (21),
it is suggested that HE may induce differentiation instead of
promoting the cell cycle and proliferation of mBMMSC.
HE induced mBMMSCs to undergo differentiation into
osteogenic lineage cells, however not into adipogenic lineage
cells, suggesting that HE is a potential candidate for promoting
MSC into osteogenic differentiation to improve metabolic bone
diseases including osteoporosis.
Although further studies are required to determine
the signaling mechanism by which HE regulates mBMMSC growth and
differentiation, the current study provides information on how
limitations of clinical use of stem cell therapy such as
transplantation, in vitro induction and expansion of
differentiated cells, and endogenous induction of stem cell
differentiation into specific cell type lineages, might be
overcome.
References
|
1
|
Gennari L, Rotatori S, Bianciardi S,
Gonnelli S, Nuti R and Merlotti D: Appropriate models for novel
osteoporosis drug discovery and future perspectives. Expert Opin
Drug Discov. 10:1201–1216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sambrook P and Cooper C: Osteoporosis.
Lancet. 17:2010–2018. 2006.
|
|
3
|
Arceo-Mendoza RM and Camacho P: Prediction
of fracture risk in patients with osteoporosis: A brief review.
Womens Health (Lond Engl). 11:477–482. 2015. View Article : Google Scholar
|
|
4
|
Iñiguez-Ariza NM and Clarke BL: Bone
biology, signaling pathways, and therapeutic targets for
osteoporosis. Maturitas. 82:245–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mikami Y, Matsumoto T, Kano K, Toriumi T,
Somei M, Honda MJ and Komiyama K: Current status of drug therapies
for osteoporosis and the search for stem cells adapted for bone
regenerative medicine. Anat Sci Int. 89:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vitale AM, Wolvetang E and Mackay-Sim A:
Induced pluripotent stem cells: A new technology to study human
diseases. Int J Biochem Cell Biol. 43:843–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tögel F and Westenfelder C: Adult bone
marrow-derived stem cells for organ regeneration and repair. Dev
Dyn. 236:3321–3331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hentze H, Graichen R and Colman A: Cell
therapy and the safety of embryonic stem cell-derived grafts.
Trends Biotechnol. 25:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozawa K, Sato K, Oh I, Ozaki K, Uchibori
R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, et al: Cell and
gene therapy using mesenchymal stem cells (MSCs). J Autoimmun.
30:121–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jethva R, Otsuru S, Dominici M and Horwitz
EM: Cell therapy for disorders of bone. Cytotherapy. 11:3–17. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Damme A, Driessche T Vanden, Collen D
and Chuah MK: Bone marrow stromal cells as targets for gene
therapy. Curr Gene Ther. 2:195–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan FF, Liu Y, Liu YF and Zhao YX: Herba
epimedii water extract elevates estrogen level and improves lipid
metabolism in postmenopausal women. Phytother Res. 22:1224–1228.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sze SC, Tong Y, Ng TB, Cheng CL and Cheung
HP: Herba epimedii: Anti-oxidative properties and its medical
implications. Molecules. 15:7861–7870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen WF, Mok SK, Wang XL, Lai KH, Lai WP,
Luk HK, Leung PC, Yao XS and Wong MS: Total flavonoid action of the
Herba epimedii extract suppresses urinary calcium excretion and
improves bone properties in ovariectomised mice. Br J Nutr.
105:180–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nadri S and Soleimani M: Isolation murine
mesenchymal stem cells by positive selection. In Vitro Cell Dev
Biol Anim. 43:276–282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eslaminejad MB and Nadri S: Murine
mesenchymal stem cell isolated and expanded in low and high density
culture system: Surface antigen expression and osteogenic culture
mineralization. In Vitro Cell Dev Biol Anim. 45:451–459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonab MM, Alimoghaddam K, Talebian F,
Ghaffari SH, Ghavamzadeh A and Nikbin B: Aging of mesenchymal stem
cells in vitro. BMC Cell Biol. 7:142006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yee AS, Paulson EK, McDevitt MA,
Rieger-Christ K, Summerhayes I, Berasi SP, Kim J, Huang CY and
Zhang X: The HBP1 transcriptional repressor and the p38 MAP kinase:
Unlikely partners in G1 regulation and tumor suppression. Gene.
336:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oliver L, Hue E, Rossignol J, Bougras G,
Hulin P, Naveilhan P, Heymann D, Lescaudron L and Vallette FM:
Distinct roles of Bcl-2 and Bcl-Xl in the apoptosis of human bone
marrow mesenchymal stem cells during differentiation. PLoS One.
6:e198202011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Napolitano MA, Cipollaro M, Cascino A,
Melone MA, Giordano A and Galderisi U: Brg1 chromatin remodeling
factor is involved in cell growth arrest, apoptosis and senescence
of rat mesenchymal stem cells. J Cell Sci. 15:2904–2911. 2007.
|