Introduction
Temporal lobe epilepsy (TLE) is the most prevalent
type of partial complex seizures in the clinical setting; seizures
in 20–30% of TLE patients are poorly controlled by anti-epileptic
drugs (1). An alternative method
to examine the potential mechanisms underlying epileptogenesis is
through the use of animal models. The pilocarpine model of TLE in
mice was first described in 1983 (2), and is an animal model that mimics the
pathology of human TLE. Initially, the mice undergo an acute period
that begins with pilocarpine-induced status epilepticus (SE), which
is followed by a latent period lasting no more than 14 days in
which mice demonstrate normal behavior and electroencephalograph
activity, although the pathophysiological processes associated with
epileptogenesis may occur. Subsequently, the mice exhibit
spontaneous recurrent seizures (SRSs), described as the key
characteristic of the chronic period (3). Following SE, there are a series of
pathophysiological processes in the hippocampal Cornu Ammonis (CA)
areas that reorganize the epileptogentic neurological networks.
Neurodegeneration (4,5), neurogenesis (6), mossy fiber sprouting (MFS) (3,7) and
dendrite spine plasticity (8,9) are
the most extensively described pathological changes. These
neurobiological phenomena require excessive oxygen and glucose. It
has been observed that the hippocampal cerebral blood volume of
mice remained elevated for two weeks following pilocarpine-induced
SE using magnetic resonance imaging, and that the hippocampal
vessel morphology of the rats altered using immunochemistry
(10); however, the cerebral blood
flow was not altered (10). The
molecular mechanisms underlying the angiogenesis of epilepsy remain
to be fully elucidated.
The erythropoietin-producing hepatocellular family
of receptor tyrosine kinases (Eph receptors) is one of the largest
families of receptor tyrosine kinases. They comprise a glycosylated
extracellular domain including an immunoglobulin-like
ligand-binding site, a cysteine-rich region and two fibronectin
(FN) type III repeats. This is connected via a transmembrane domain
to the intracellular domain, consisting of a juxtamembrane region,
a protein tyrosine kinase region, a sterile alpha motif, and a
postsynaptic density 95-discs large-zonula occludentes-1 binding
motif (11). The ephrins, as the
ligands of Eph receptors, are divided into two subfamilies:
Ephrin-As associate with the cell membrane via a
glycosylphosphatidylinositol anchor, and ephrin-Bs span the cell
membrane and contain a cytoplasmic domain. EphA receptors
preferentially bind all ephrin-As and EphB receptors bind all
ephrin-Bs; however, EphA4 binds ephrin-As and -Bs. To date, three
interaction sites have been identified between EphA4 and ephrin-As:
The high affinity interface of G -HL loop in ephrin-As
and the hydrophobic pocket in EphA4, the low affinity interface of
solvent-excluded regions at the ephrin-docking site along the upper
convex surface of the receptor, and the clustering interface
between the ligand binding domain and the second FN located in the
adjacent cysteine-rich region of the receptor (12,13).
EphA4 has a series of crucial functions in the brain during
embryonic development and postnatal plasticity, and is expressed in
the cortex, hippocampus, corpus striatum and spinal cord (14,15).
As a mediator of axon repulsion, EphA4 contributes to the
localization of the position of growth cone and nerve projection
(16,17). During neurogenesis, EphA4 is
considered to regulate neural stem/progenitor cell proliferation,
differentiation and migration (18). Furthermore, previous studies have
suggested that EphA4 may regulate angiogenesis in the neural system
(19,20). Following deletion of ephrin-A5 in
mice, EphA4 was blocked and hippocampal vessels were narrowed
(19). In addition, EphA4 was
revealed to regulate vascular smooth muscle contractility (20). As vessel remodeling is induced by
EphA4, this receptor may be a key mediator in angiogenesis during
epileptogenesis. Our previous study demonstrated that in the
hippocampal dentate gyrus the microvessel density decreased
following EphA4 reduction (21).
Ephrin-A-Fc is a genetically-engineered immunoadhesin that targets
EphA receptors; it contains the natural receptor binding domain of
endogenous ephrin-A to interact with EphA receptors and it is
capable of eliciting dimerization and thus phosphorylation of its
receptor (22). There are two
types of ephrin-A-Fc: The unclustered form and the pre-clustered
form. The former is typically an antagonist that blocks EphA
expression and the latter is an agonist that activates EphA
expression (23–25). However, our previous study revealed
that the unclustered and pre-clustered ephrin-A5-Fc had the same
inhibition effect on EphA4 (21).
Therefore, the present study investigated the
expression of the angiogenesis-associated protein EphA4 and its
ligand ephrin-A5, and the plasticity of platelet endothelial cell
adhesion molecule-1 (PECAM-1)-labeled microvessels in the
hippocampal CA1 and CA3 areas in a mouse model of TLE induced by
pilocarpine. The results of the present study demonstrated the
effects of two opposite types of ephrin-A5-Fc on EphA4 and
microvessel remodeling.
Materials and methods
Animal model
A total of 91 male C57BL/6 mice (weight, 18–21 g;
age, 5–6 weeks; specific pathogen-free) were purchased from the
Experimental Animal Center of Central South University (Changsha,
China). Mice were housed at 5–6 per cage, at a temperature of
23±1°C under a 12-h light/dark cycle, and had ad libitum
access to food and water. The animals were maintained in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals, and the present study was approved by the
Animal Ethics Committee of Central South University. The animals
were randomly divided into experimental and control groups and
treated with pilocarpine as previously described (26). All animals received an
intraperitoneal injection of 1 mg/kg scopolamine hydrobromide
(Shanghai Harvest Pharmaceutical Co., Ltd., Shanghai, China) 30 min
prior to pilocarpine injection. Subsequently, a total of 67 mice
received an intraperitoneal injection of 320–340 mg/kg pilocarpine
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) diluted in
sterile saline, which induced SE followed by hippocampal damage and
the development of SRSs. In the present study, 36 mice recovered
from SE and 31 mice died. Mice in the control group (n=24) received
an injection of the same volume of sterile saline instead of
pilocarpine. All mice received an intraperitoneal injection of 7.5
mg/kg diazepam (Abbott Laboratories, Chicago, IL, USA) 2 h
following the onset of SE to stop or limit behavioral seizures.
Additional diazepam was administered if seizures were not
attenuated sufficiently or recurred within 1 h following the first
diazepam injection. The present study used the minimum number of
animals required.
The first SRS appeared in mice 3–10 days following
pilocarpine injection. A total of 24 experimental mice and the same
number of control mice were sacrificed at 7, 14, 28 and 56 days
post-SE (n=6 per group), which meant that none of them was removed
from analysis during the study. Mice were anesthetized with an
intraperitoneal injection of 10% chloral hydrate (0.05–0.1 ml/10 g;
Department of Pharmacy, The Third Xiangya Hospital, Changsha,
China), and perfused through the left ventricle with 0.9% sterile
saline at 4°C, followed by 4% paraformaldehyde. Following
perfusion, the animals were maintained at 4°C for 30 min,
decapitated and the brains removed from the skull and postfixed in
4% paraformaldehyde for 12–16 h at 4°C. Subsequent to thorough
rinsing in phosphate buffer, the brains were cryoprotected in a 30%
sucrose solution for 48–72 h and sectioned at 30 µm on a cryostat.
Sections were preserved in a cryoprotectant solution (a mixture of
glycerine, ethanediol and 0.1 M phosphate buffer, at a volume ratio
of 5:6:9) at −20°C until processing.
Immunohistochemistry
Free-floating sections were processed for
immunohistochemistry. Following washing in phosphate-buffered
saline (PBS; 0.1 M, pH 7.5) twice, sections were immersed in 1%
H2O2 for 30 min, and antigen-retrieval was
performed in sodium citrate solution (pH 6.0) using heat in a
microwave for 20 min. Sections were incubated in 10% normal goat or
rabbit serum in PBS containing 200 µl/ml avidin (Avidin/Biotin
Blocking kit; catalog no. SP-2001; Vector Laboratories, Inc.,
Burlingame, CA, USA) for 2 h. The sections were rinsed gently in
PBS twice between steps. The primary antibodies goat anti-ephrin-A5
(1:50; catalog no. AF3743; R&D Systems, Inc., Minneapolis, MN,
USA), rabbit anti-EphA4 (1:100; catalog no. ab5396; Abcam,
Cambridge, MA, USA) or rat anti-PECAM-1 (1:100; catalog no. 550274;
BD Biosciences, Franklin Lakes, NJ, USA) were diluted in PBS with
10% normal serum and 200 µl/ml biotin (Avidin/Biotin Blocking kit)
and the sections were subsequently incubated in the mixed solution
for 48 h at room temperature. Following rinsing in PBS, sections
were incubated in PBS containing the corresponding
peroxidase-labeled anti-goat, anti-rabbit, and anti-rat secondary
antibody (all 1:200; catalog nos. 14-13-06, 074–1506 and 14-16-06;
KPL, Inc., Gaithersburg, MD, USA) at room temperature for 1 h.
VECTASTAIN® ABC kit (catalog no. PK-4000; Vector
Laboratories, Inc.) and 3,3′-diaminobenzidine solution (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
were used for visualization of the immunolabeled products. Sections
were counterstained with hematoxylin following PECAM-1 staining.
Subsequent to dehydration, clearing and coverslipping, the sections
were imaged with a DM5000 B light microscope (Leica Microsystems
GmbH, Wetzlar, Germany). Imaging-Pro Plus software version 6.0 (IPP
6.0; Media Cybernetics, Inc., Rockville, MD, USA) was used to
calculate the optical density (OD) values of immunolabeling
products. Three unilateral hippocampal CA1 and CA3 regions were
analyzed for each mouse and the mean OD value was obtained.
The microvessel densities (MVDs) and diameters of
microvessels in the CA1 and CA3 hippocampal areas were calculated
on a 2D plane of the images using IPP 6.0. The MVD was defined as
the numbers of microvessels/total area. The mean diameter (µm) was
defined as the sum of diameters/number of diameter-calculated
microvessels. A total of three hippocampal sections from each mouse
were examined. All longitudinal vessels were measured. Each branch
of the vessel was considered as an individual vessel. Vessels
crossing the section were not calculated to avoid any false
positive due to edge effects around potential holes in the tissue.
All measurements were conducted by researchers blinded to the
animal treatment groups.
Intrahippocampal infusion
A total of 3 days following pilocarpine treatment,
12 of the treated mice were divided into 4 groups (n=3 per group),
which subsequently received continuous intrahippocampal infusion in
the left side using ALZET® 1007D micro-osmotic pumps
(DURECT Corporation, Cupertino, CA, USA) with standard ALZET brain
infusion kits. The contents of the pumps were as follows:
Artificial cerebrospinal fluid (ACSF) negative control group, in
which the pump contained only ACSF (pH 7.42) containing 124 mM
NaCl, 3 mM KCl, 2.4 mM CaCl2, 2.4 mM MgSO4,
1.25 mM KH2PO4, 26 mM NaHCO3 and
10 mM D-glucose; IgG2A control group, using a
biologically non-active IgG in ACSF, in which the pump contained 50
µg/ml mouse IgG2A isotype control (catalog no. MAB003;
R&D Systems, Inc.) in ACSF; non-clustered (NC)-ephrin-A5-Fc
group, in which the pump contained 50 µg/ml unclustered
ephrin-A5-Fc (catalog no. 7396-EA-050; R&D Systems, Inc.) in
ACSF; and clustered (C)-ephrin-A5-Fc group, in which the pump
contained pre-clustered ephrin-A5-Fc (50 µg/ml ephrin-A5-Fc and 500
µg/ml goat anti-mouse IgG-Fc, catalog no. G-202-C; R&D Systems,
Inc.) in ACSF.
The pumps were filled, connected to a cannula and
immersed in PBS at 37°C overnight. Mice were anesthetized with an
intraperitoneal injection of 10% chloral hydrate 4 days following
pilocarpine treatment. Using a stereotaxic apparatus a cannula was
placed into the left hippocampus of the mice at the following
position: Anteriorposterior = −1.5 mm, medial lateral = −1.8 mm
from bregma and dorsal ventral = 1.8 mm from flat skull surface.
The cannula was cemented onto the skull with dental acrylic, the
pump was placed subcutaneously in the back of mouse, and the
incision was sutured. The osmotic pumps were taken out under the
anesthesia 7 days later. For the following 2 weeks the SRSs were
observed and recorded using video monitoring. All mice survived,
and no apparent behavioral discomfort was observed. At day 28
following pilocarpine injection, the mice were sacrificed for
immunohistochemistry.
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed in GraphPad Prism software version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA). Dynamic temporal expression
patterns in EphA4 and ephrin-A5 proteins and vascular parameters
were performed with two-way analyses of variance (ANOVA) followed
by the Bonferroni correction. Statistical analyses of other data
were performed with one-way ANOVA followed by Tukey's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
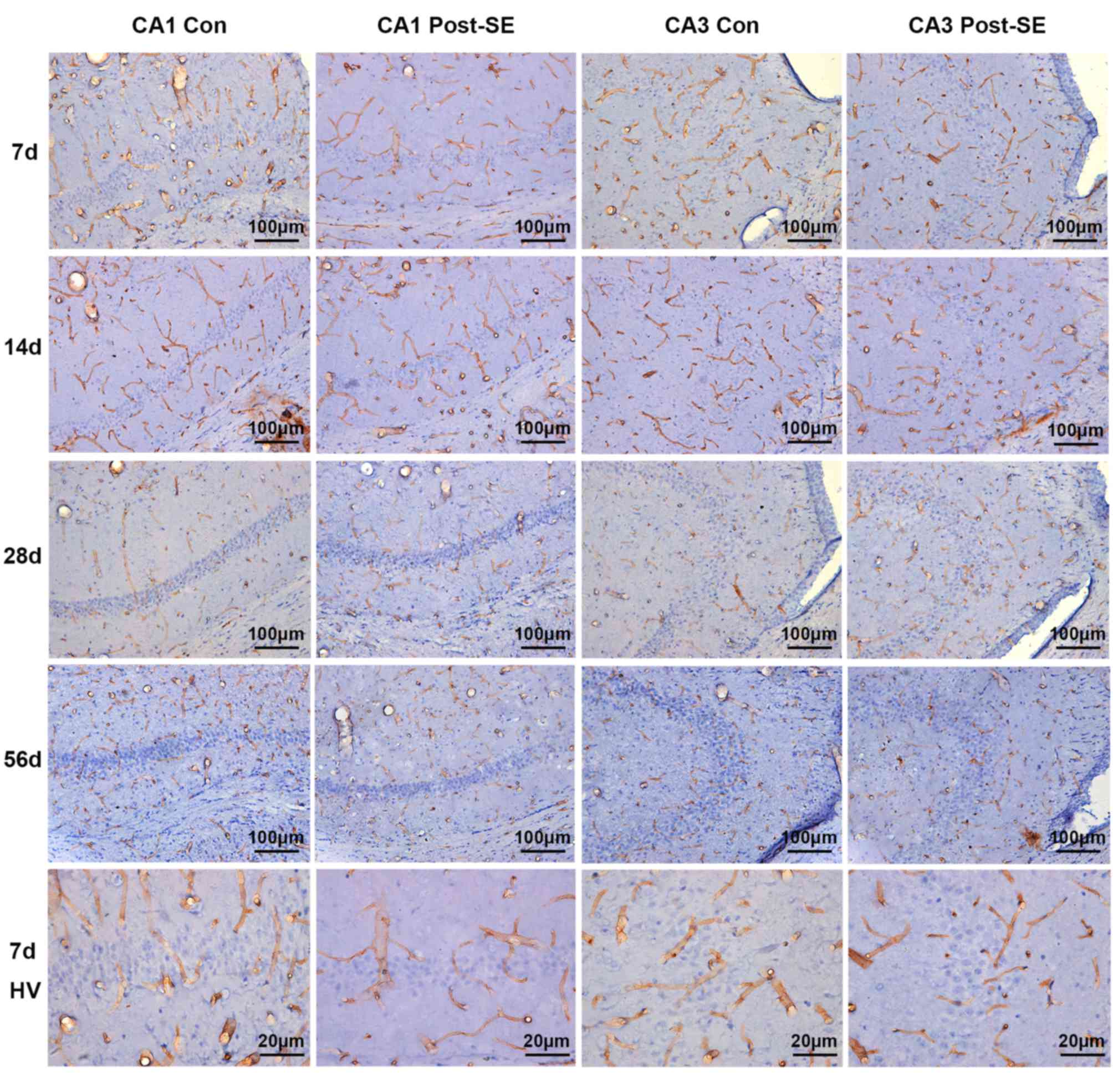
Density and diameter of microvessels
post-SE
In the control group, PECAM-1-labeled microvessels
appeared to primarily orient perpendicular to the pyramidal cell
layer (PCL) in the CA1 and CA3 areas. They mostly originated in the
fissure around the cell layer. In addition, there were certain
microvessels aligned in parallel to the PCL in the two areas. No
differences were observed in the distribution of PECAM-1-labeled
microvessels in the control groups at different time points
(Fig. 1). The diameter of the
microvessels in CA1 was 9.272±0.3526 µm and in CA3 was 9.330±0.7638
µm. At day 7 post-SE, the mean diameter of microvessels remained
comparatively stable. Although certain vessels still emanated from
the fissure perpendicular to the PCL, certain others began to
traverse the cell layer and became entangled to form a disorganized
vascular plexus around the layer (Fig.
1). The microvessels enlarged and appeared more distorted
compared with the control at 14 days post-SE (Fig. 1). At 28 days post-SE, the enlarged
microvessels were more fibrous and fragmented. The individual
vessels perpendicular to the PCL almost disappeared and instead
intertwined vessels, which penetrated into the cell layer or
around, were observed (Fig. 1). At
56 days post-SE, the mean diameter and density of microvessels
recovered to a certain extent. Traversed and the vertical
microvessels were observed.
The MVD in the CA1 and CA3 areas gradually increased
from 7 days post-SE and peaked at 28 days post-SE (P<0.01). At
56 days post-SE the MVD was 70.46 and 64.98% in the CA1 and CA3
areas, respectively, of the levels at 28 days post-SE, but remained
greater compared with the control (Fig. 2A and B). In the CA1 area, the mean
diameters at 7, 14, 28 and 56 days post-SE were 8.9571±0.6714,
11.1698±0.3598, 10.7992±1.1180 and 9.9919±0.8019 µm, respectively;
this was 0.9947-, 1.2189-, 1.1030- and 1.0946-fold, respectively,
of the corresponding control groups (Fig. 2C). In the CA3 area, the mean
diameters at 7, 14, 28 and 56 days post-SE were 8.743±0.8527,
11.27±0.9481, 10.53±0.4096 and 9.996±1.231 µm; this was 1.0324-,
1.2515-, 1.0283- and 1.0408-fold, respectively, of the
corresponding control groups (Fig.
2D).
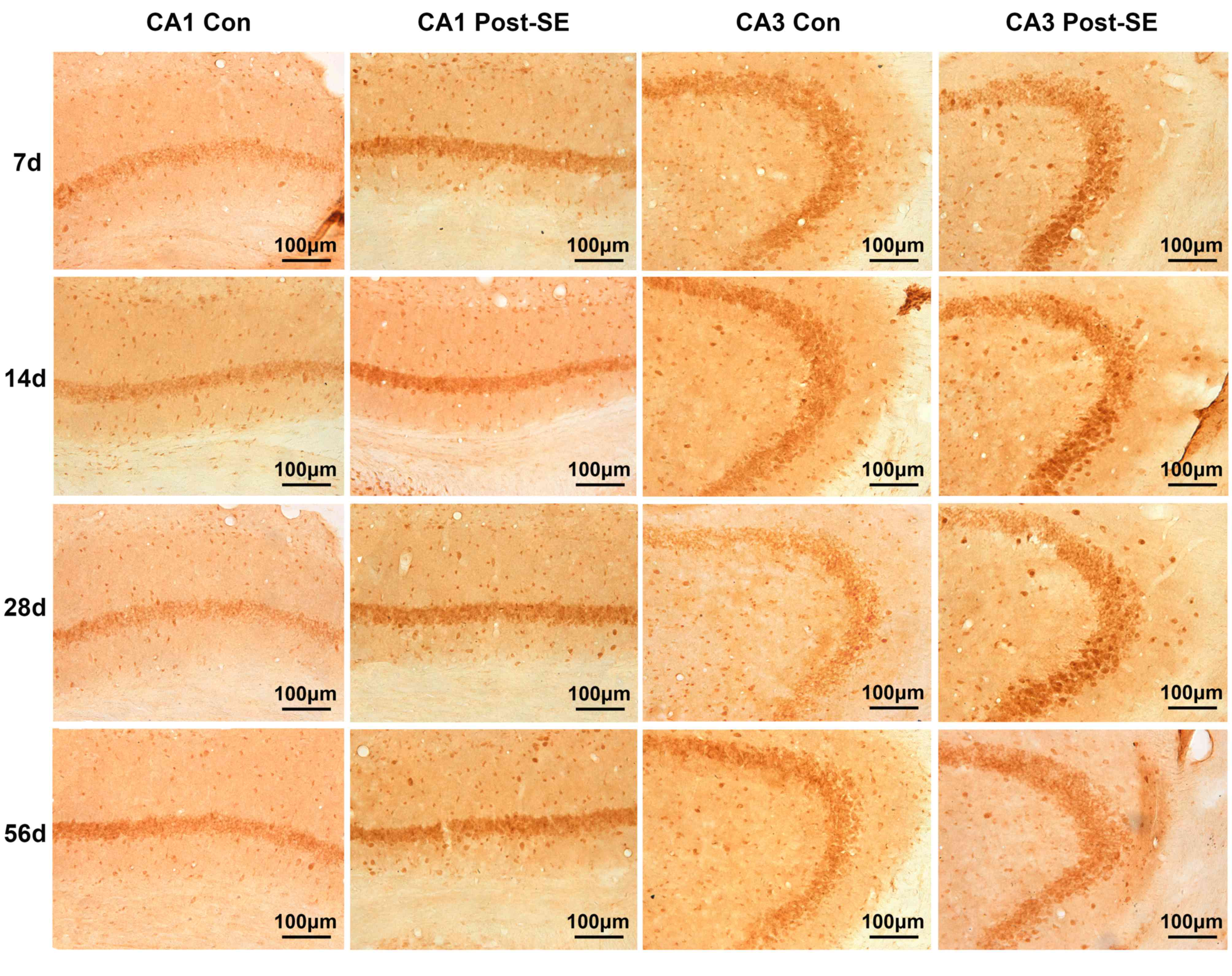
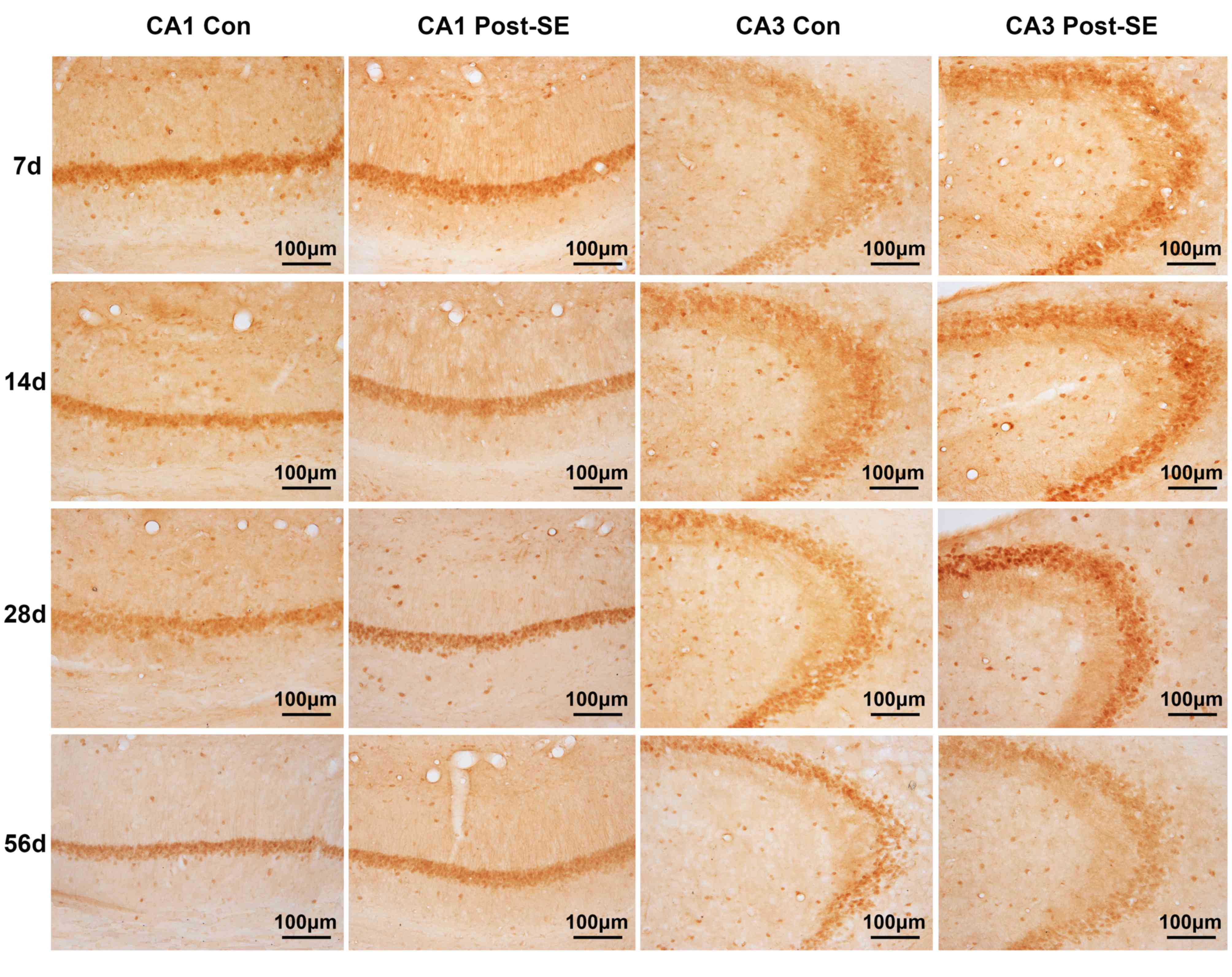
Expression of EphA4 and ephrin-A5 post
SE
To investigate the mechanism underlying the
involvement of EphA4 in hippocampal angiogenesis during
epileptogenesis, the expression and distribution of EphA4 and
ephrin-A5 in the CA1 and CA3 areas in the epileptic mice
hippocampus were initially determined. The protein expression of
EphA4 increased steadily from 7 to 28 days post-SE and subsequently
plateaued (Fig. 2E and F). In the
CA1 area the differences between the post-SE and control groups
were significant at 28 and 56 days (P<0.01) and in the CA3 area
significance was observed at 28 days (P<0.01). Similarly, the
expression of ephrin-A5 protein in CA1 area increased gradually
from 7 to 28 days post-SE compared with the corresponding control
groups with a slight decrease at 56 compared with 28 days post-SE,
although expression remained greater compared with the control
(Fig. 2G). In the CA3 area there
was no difference in the 7 day post-SE group and the control group;
however, ephrin-A5 increased from 14 to 28 days post-SE compared
with the control and declined slightly at 56 days post-SE (Fig. 2H). There was a significant
difference between the post-SE and control groups in the CA1 area
at 28 days (t=3.364; P<0.01); however, no significant
differences were observed in the CA3 area. EphA4 and ephrin-A5
staining are presented in Figs. 3
and 4, respectively.
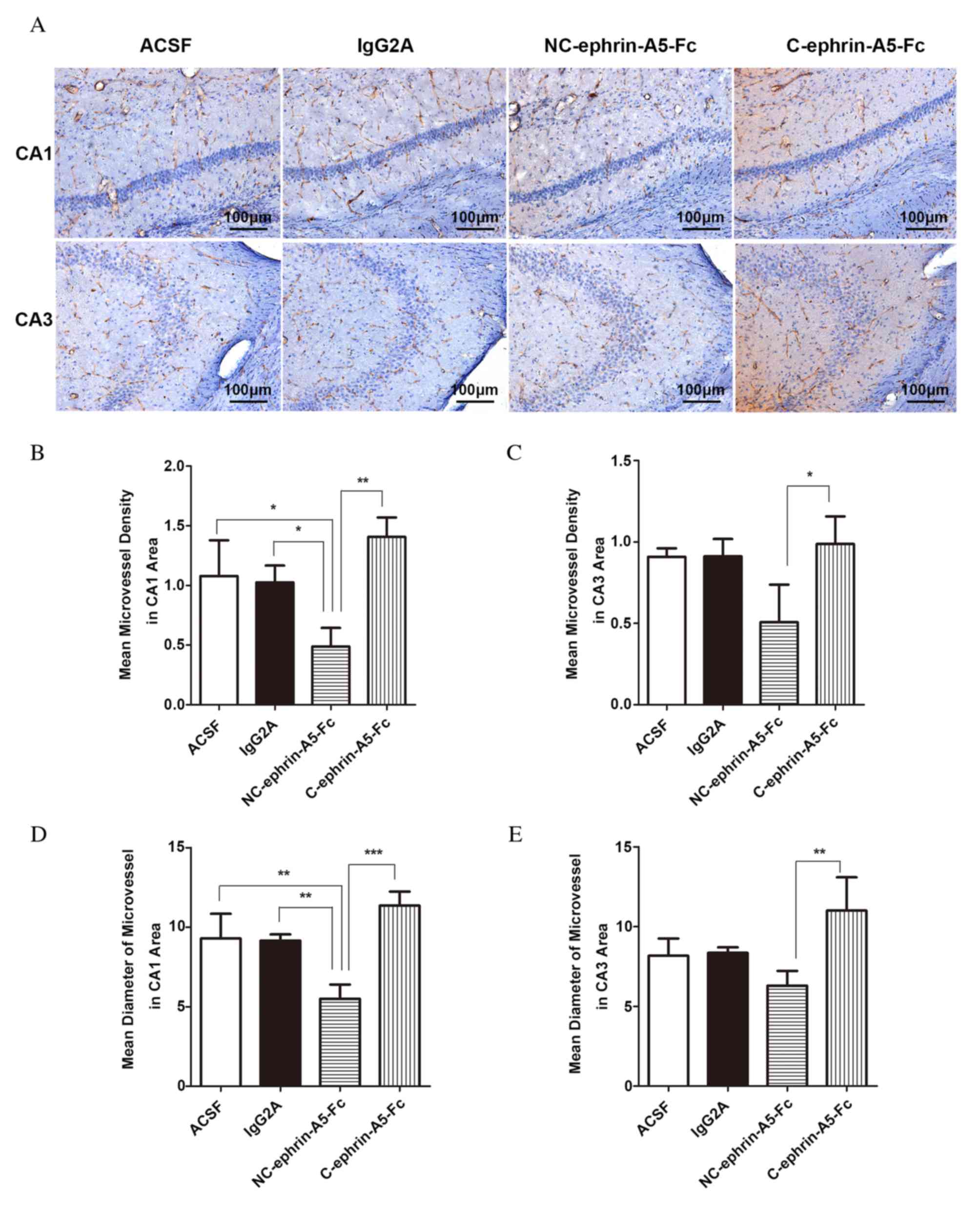
Effect of ephrin-A5-Fc infusion on
EphA4 expression
To further address whether EphA4 mediates
angiogenesis in the epileptic hippocampus, ephrin-A5-Fc
immunoadhesin was stereotactically injected into the hippocampus.
The expression of EphA4 decreased in the NC-ephrin-A5-Fc group and
increased in C-ephrin-A5-Fc group compared with the ACSF and
IgG2A control groups in CA1 and CA3 areas; however, no
statistically significant differences were observed using Tukey's
post-hoc test (Fig. 5). Compared
with C-ephrin-A5-Fc group, EphA4 expression was significantly
reduced in the NC-ephrin-A5-Fc group in the CA1 and CA3 areas
(P<0.05). No statistically significant differences were observed
between the control groups.
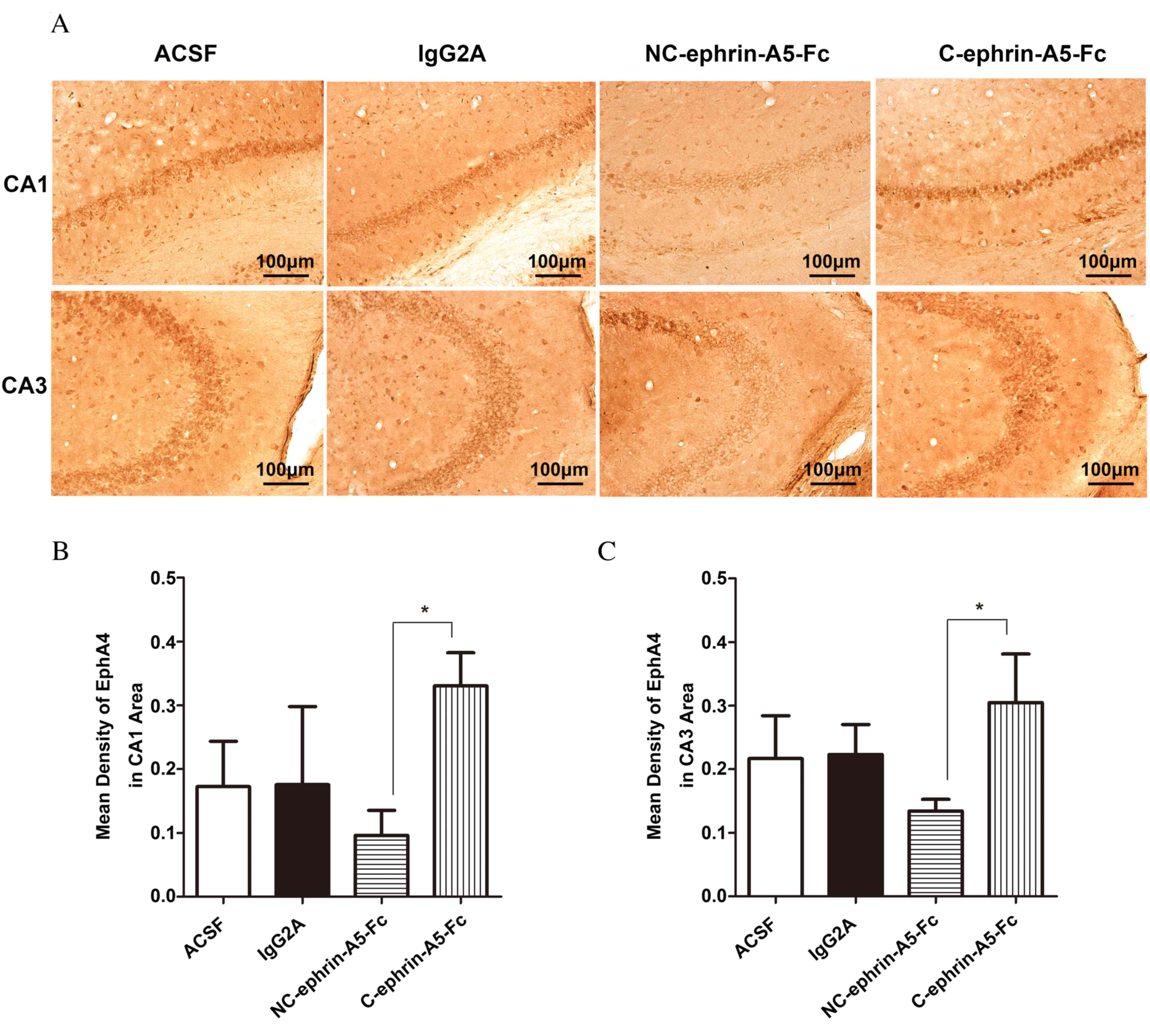 | Figure 5.Expression of EphA4 in the
hippocampal CA1 and CA3 areas following ephrin-A5-Fc
intrahippocampal infusions. Mice received intrahippocampal
infusions of ACSF, IgG2A isotype control,
NC-ephrin-A5-Fc or C-ephrin-A5-Fc via micro-osmotic pump for 7
days, beginning 4 days following pilocarpine treatment. (A)
Immunohistochemistical staining of EphA4, which appears brown. The
expression of EphA4 was analyzed in the (B) CA1 and (C) CA3 areas,
and was reduced by NC-ephrin-A5-Fc and increased by C-ephrin-A5-Fc.
Data are expressed as the mean ± standard deviation (n=3).
*P<0.05. Scale bars represent 100 µm. EphA4, ephrin receptor A4;
CA, Cornu Ammonis; ACSF, artificial cerebrospinal fluid; NC,
non-clustered; C, clustered. |
Effect of ephrin-A5-Fc infusion on
density and diameter of microvessels
The MVDs and mean diameters of microvessels in the
CA1 and CA3 areas of the slices were calculated following PECAM-1
staining (Fig. 6A). Compared with
the ACSF and IgG2A control groups, the MVD of
microvessels in the CA1 area decreased in the NC-ephrin-A5-Fc group
(P<0.05) and increased in the C-ephrin-A5-Fc group (P>0.05;
Fig. 6B). MVD in the CA3 area
revealed a similar pattern, but with no significant differences
compared with the ACSF and IgG2A groups (Fig. 6C). However, the MVD in the
NC-ephrin-A5-Fc group was 34.71% of that in the C-ephrin-A5-Fc
group in the CA1 area (P<0.01), and 51.29% in the CA3 area
(P<0.05). The microvessels in the NC-ephrin-A5-Fc group were
narrower compared with the ACSF and IgG2A groups
(P<0.01; Fig. 6D). The mean
diameter in the CA3 area revealed a similar pattern, but without
significant differences compared with the ACSF and IgG2A
groups (Fig. 6E). The mean
diameter in the NC-ephrin-A5-Fc group was 48.35% of that in the
C-ephrin-A5-Fc group in the CA1 area (P<0.001), and 57.21% in
the CA3 area (P<0.01). No statistically significant differences
were observed between the control groups.
Discussion
The present study demonstrated that microvessels
labeled with PECAM-1 in adult hippocampal CA1 and CA3 areas of mice
were influenced temporally by epileptogenesis. Expression of the
receptor EphA4, and its ligand ephrin-A5, increased in the CA1 and
CA3 areas during epileptogenesis. Upon removal of its ligand using
NC-ephrin-A5-Fc, EphA4 expression was downregulated and the
microvessels density and diameter decreased; however, when
ephrin-A5 was initiated with C-ephrin-A5-Fc, EphA4 expression
increased and the microvessels demonstrated greater
disorganization.
Recombinant soluble ligand-type immunoadhesins
appear to act as antagonists and downregulate EphA4 receptor
expression; these may be converted to an agonistic reagent via
artificial clustering. In the present study, EphA4 affected the
morphology of microvessels and was inhibited by NC-ephrin-A5-Fc;
C-ephrin-A5-Fc had the opposite function. Previous studies have
revealed that EphA4 may be blocked by unclustered ephrin-A5-Fc to
promote axonal regeneration (23);
however, clustered ephrin-A5-Fc facilitated EphA4 expression and
activated astrocyte proliferation (27). These studies have primarily been
performed in vivo. Further studies are required to
investigate the distinct underlying mechanisms of EphA4 and
ephrin-A5 interaction in the mediation of endothelial
proliferation, migration and vessel remodeling in vitro.
In addition to its role in angiogenesis, EphA4
contributes to other hippocampal remodeling processes that are
relevant to epileptogenesis. The traditional neurogenesis pool in
the subgranular zone of dentate gyrus is triggered by SE and SRSs
(26). EphA4 is associated with
apoptosis of neural cells in the hippocampus, and maintains neural
stem/progenitor cells (NSC/NPC) in an undifferentiated state; its
downregulation may therefore damage NPC proliferation and
accelerate premature differentiation (18,28).
Recently, it has been discovered that neurogenesis may exist in the
CA fields serving as a vascular stem cell niche post-SE (6). Although in the present study EphA4
dynamic expression was associated with angiogenesis affected by
seizures in the CA1 and CA3 areas, whether EphA4 is involved in
this novel niche requires further investigation. Additionally,
EphA4 is critical for rewiring dendritic synaptic circuits
(29) and regulating repulsive
axon guidance (30). Electron
microscopic immunocytochemistry analysis has revealed that EphA4 is
enriched in the myelinated/unmyelinated axons, axon terminals and
dendritic spines of the CA1 and CA3 fields (31). Activated EphA4 induces spine
retraction, whereas blocked EphA4 disrupts synaptogenesis in the
hippocampal pyramidal cells (29).
Furthermore, EphA4 may be involved in epileptic network
establishment. Glutamate transporter subtype-1 (GLT-1) is essential
for maintaining the balance between excitatory and suppressive
networks, and the long-term potential (LTP) is critical for memory
and other cognitive impairments in TLE. EphA4 affects GLT-1 levels
in CA1 regions and gene deletion of EphA4 reduced the LTP in
post-synaptic CA1 pyramidal cells (32).
SE and SRSs may damage neuron survival and stimulate
tissue remodeling, including neurogenesis and astrogliosis. These
processes require microvessels to supply abundant oxygen and
nutrients to the damaged regions, which serves as a compensation
mechanism. Epileptogenic brain injuries and seizures themselves may
disturb blood-brain barrier permeability, increase the brain blood
flow and trigger an increase in brain vascularity, which is
associated with seizure frequency (33–35).
Inhibition of tissue-type plasminogen activator and vascular
endothelial growth factor (VEGF), or activation of erythropoietin
and VEGFR2, are potential strategies for the treatment of
epileptogenesis (36). In the
present study, the vascular damage in the CA1 and CA3 areas began
in the acute period following pilocarpine-induced SE. As a
consequence, angiogenesis continued from the latency period to the
chronic period in the areas where oxygen and glucose were required
for tissue repair. Microvessel density increased compared to the
control groups; these results are consistent with those of Rigau
et al (35). However,
Ndode-Ekane et al (10)
demonstrated that the hippocampal vascular density decreases
post-SE. These differences may be due to differences in animal
species, immunopositive vascular markers and estimation methods.
Notably, previous studies have suggested that the vascular
plasticity in human TLE hippocampi is not true angiogenesis, and
that the microvessels are actually atrophic, primarily with
spine-like protrusions and a reduced lumen containing reactive
astrocytes (37,38). As the objectives and methods were
different from those in the present study, and the range of
microvessel diameters is not defined, further studies are required
to investigate the structural and functional features of the
microvessels in the injured hippocampal regions during
epileptogenesis.
In conclusion, the results of the present study
demonstrated that angiogenesis occurs, and that the molecules EphA4
and ephrin-A5 are expressed in the hippocampal CA1 and CA3 areas
throughout epileptogenesis. PECAM-1 may detect epileptic
microvessel patterns in the hippocampi of mice and EphA4 may
contribute to the microvessel plasticity via the ephrin-A5
signaling pathway. EphA4 may therefore be a potential target for
clinical therapy.
Acknowledgements
The authors thank Miss. Jinghui Liang (Department of
Neurology, Xiangya Hospital, Central South University, Changsha,
China) for tissue preparation and Miss. Jinghui Liang, Dr Zhaohui
Luo and Dr Zhiguo Wu (Department of Neurology, Xiangya Hospital,
Central South University) for outstanding technical assistance. The
present study was supported by the National Natural Science
Foundation of China (grant nos. 81100967, 81371435 and 81401078)
and the Specialized Research Fund for the Doctoral Program of
Higher Education (grant no. 20110162120002).
References
|
1
|
Perry MS and Duchowny M: Surgical versus
medical treatment for refractory epilepsy: Outcomes beyond seizure
control. Epilepsia. 54:2060–2070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turski WA, Cavalheiro EA, Schwarz M,
Czuczwar SJ, Kleinrok Z and Turski L: Limbic seizures produced by
pilocarpine in rats: Behavioural, electroencephalographic and
neuropathological study. Behav Brain Res. 9:315–335. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curia G, Longo D, Biagini G, Jones RS and
Avoli M: The pilocarpine model of temporal lobe epilepsy. J
Neurosci Methods. 172:143–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gröticke I, Hoffmann K and Löscher W:
Behavioral alterations in the pilocarpine model of temporal lobe
epilepsy in mice. Exp Neurol. 207:329–349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu K, Li SY, Xiao B, Bi FF, Lu XQ and Wu
XM: Protective effects of quercetin against status epilepticus
induced hippocampal neuronal injury in rats: Involvement of
X-linked inhibitor of apoptosis protein. Acta Neurol Belg.
111:205–212. 2011.PubMed/NCBI
|
|
6
|
Zhang L, Hernández VS, Estrada FS and
Luján R: Hippocampal CA field neurogenesis after pilocarpine
insult: The hippocampal fissure as a neurogenic niche. J Chem
Neuroanat. 56:45–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noebels JL, Avoli M, Rogawski MA, Olsen RW
and Delgado-Escueta AV: Jasper's Basic Mechanisms of the Epilepsies
(Internet). 4th. Bethesda (MD): National Center for Biotechnology
Information (US); pp. 1–102. 2012
|
|
8
|
Zha XM, Dailey ME and Green SH: Role of
Ca2+/calmodulin-dependent protein kinase II in dendritic
spine remodeling during epileptiform activity in vitro. J Neurosci
Res. 87:1969–1979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang FR and Loke WK: Cyto-, axo- and
dendro-architectonic changes of neurons in the limbic system in the
mouse pilocarpine model of temporal lobe epilepsy. Epilepsy Res.
89:43–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ndode-Ekane XE, Hayward N, Gröhn O and
Pitkänen A: Vascular changes in epilepsy: Functional consequences
and association with network plasticity in pilocarpine-induced
experimental epilepsy. Neuroscience. 166:312–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mosch B, Reissenweber B, Neuber C and
Pietzsch J: Eph receptors and ephrin ligands: Important players in
angiogenesis and tumor angiogenesis. J Oncol.
2010:1352852010.PubMed/NCBI
|
|
12
|
Himanen JP, Rajashankar KR, Lackmann M,
Cowan CA, Henkemeyer M and Nikolov DB: Crystal structure of an Eph
receptor-ephrin complex. Nature. 414:933–938. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith FM, Vearing C, Lackmann M, Treutlein
H, Himanen J, Chen K, Saul A, Nikolov D and Boyd AW: Dissecting the
EphA3/Ephrin-A5 interactions using a novel functional mutagenesis
screen. J Biol Chem. 279:9522–9531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greferath U, Canty AJ, Messenger J and
Murphy M: Developmental expression of EphA4-tyrosine kinase
receptor in the mouse brain and spinal cord. Mech Dev. 119:(Suppl
1). S231–S238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jing X, Miwa H, Sawada T, Nakanishi I,
Kondo T, Miyajima M and Sakaguchi K: Ephrin-A1-mediated
dopaminergic neurogenesis and angiogenesis in a rat model of
Parkinson's disease. PLoS One. 7:e320192012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omoto S, Ueno M, Mochio S and Yamashita T:
Corticospinal tract fibers cross the ephrin-B3-negative part of the
midline of the spinal cord after brain injury. Neurosci Res.
69:187–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toyoda Y, Shinohara R, Thumkeo D, Kamijo
H, Nishimaru H, Hioki H, Kaneko T, Ishizaki T, Furuyashiki T and
Narumiya S: EphA4-dependent axon retraction and midline
localization of Ephrin-B3 are disrupted in the spinal cord of mice
lacking mDia1 and mDia3 in combination. Genes Cells. 18:873–885.
2013.PubMed/NCBI
|
|
18
|
Khodosevich K, Watanabe Y and Monyer H:
EphA4 preserves postnatal and adult neural stem cells in an
undifferentiated state in vivo. J Cell Sci. 124:1268–1279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hara Y, Nomura T, Yoshizaki K, Frisén J
and Osumi N: Impaired hippocampal neurogenesis and vascular
formation in ephrin-A5-deficient mice. Stem Cells. 28:974–983.
2010.PubMed/NCBI
|
|
20
|
Ogita H, Kunimoto S, Kamioka Y, Sawa H,
Masuda M and Mochizuki N: EphA4-mediated Rho activation via
Vsm-RhoGEF expressed specifically in vascular smooth muscle cells.
Circ Res. 93:23–31. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shu Y, Xiao B, Wu Q, Liu T, Du Y, Tang H,
Chen S, Feng L, Long L and Li Y: The Ephrin-A5/EphA4 interaction
modulates neurogenesis and angiogenesis by the p-Akt and p-ERK
pathways in a mouse model of TLE. Mol Neurobiol. 53:561–576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerlai R and McNamara A: Anesthesia
induced retrograde amnesia is ameliorated by ephrinA5-IgG in mice:
EphA receptor tyrosine kinases are involved in mammalian memory.
Behav Brain Res. 108:133–143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldshmit Y, Spanevello MD, Tajouri S, Li
L, Rogers F, Pearse M, Galea M, Bartlett PF, Boyd AW and Turnley
AM: EphA4 blockers promote axonal regeneration and functional
recovery following spinal cord injury in mice. PLoS One.
6:e246362011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Overman JJ, Clarkson AN, Wanner IB,
Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW and
Carmichael ST: A role for ephrin-A5 in axonal sprouting, recovery,
and activity-dependent plasticity after stroke. Proc Natl Acad Sci
USA. 109:E2230–E2239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ting MJ, Day BW, Spanevello MD and Boyd
AW: Activation of ephrin A proteins influences hematopoietic stem
cell adhesion and trafficking patterns. Exp Hematol. 38:1087–1098.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Peng Z, Xiao B and Houser CR:
Activation of ERK by spontaneous seizures in neural progenitors of
the dentate gyrus in a mouse model of epilepsy. Exp Neurol.
224:133–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldshmit Y and Bourne J: Upregulation of
EphA4 on astrocytes potentially mediates astrocytic gliosis after
cortical lesion in the marmoset monkey. J Neurotrauma.
27:1321–1332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Liu N, Wang Y, Wang R, Guo D and
Zhang C: Inhibition of EphA4 signaling after ischemia-reperfusion
reduces apoptosis of CA1 pyramidal neurons. Neurosci Lett.
518:92–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murai KK, Nguyen LN, Irie F, Yamaguchi Y
and Pasquale EB: Control of hippocampal dendritic spine morphology
through ephrin-A3/EphA4 signaling. Nat Neurosci. 6:153–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dudanova I, Kao TJ, Herrmann JE, Zheng B,
Kania A and Klein R: Genetic evidence for a contribution of
EphA:EphrinA reverse signaling to motor axon guidance. J Neurosci.
32:5209–5215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tremblay ME, Riad M, Bouvier D, Murai KK,
Pasquale EB, Descarries L and Doucet G: Localization of EphA4 in
axon terminals and dendritic spines of adult rat hippocampus. J
Comp Neurol. 501:691–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Filosa A, Paixão S, Honsek SD, Carmona MA,
Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R,
Klopstock T, et al: Neuron-glia communication via EphA4/ephrin-A3
modulates LTP through glial glutamate transport. Nat Neurosci.
12:1285–1292. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morin-Brureau M, Rigau V and Lerner-Natoli
M: Why and how to target angiogenesis in focal epilepsies.
Epilepsia. 53:(Suppl 6). 64–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Romariz SA, Kde O Garcia, Dde S Paiva,
Bittencourt S, Covolan L, Mello LE and Longo BM: Participation of
bone marrow-derived cells in hippocampal vascularization after
status epilepticus. Seizure. 23:386–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rigau V, Morin M, Rousset MC, de Bock F,
Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J,
Crespel A and Lerner-Natoli M: Angiogenesis is associated with
blood-brain barrier permeability in temporal lobe epilepsy. Brain.
130:1942–1956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaminski RM, Rogawski MA and Klitgaard H:
The potential of antiseizure drugs and agents that act on novel
molecular targets as antiepileptogenic treatments.
Neurotherapeutics. 11:385–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kastanauskaite A, Alonso-Nanclares L,
Blazquez-Llorca L, Pastor J, Sola RG and DeFelipe J: Alterations of
the microvascular network in sclerotic hippocampi from patients
with epilepsy. J Neuropathol Exp Neurol. 68:939–950. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alonso-Nanclares L and Defelipe J:
Alterations of the microvascular network in the sclerotic
hippocampus of patients with temporal lobe epilepsy. Epilepsy
Behav. 38:48–52. 2014. View Article : Google Scholar : PubMed/NCBI
|