Introduction
The increasing incidence of obesity, leading to
metabolic complications or metabolic syndrome, is now recognized as
a major public health problem (1).
Investigations have focused on the association between obesity and
various chronic diseases; however, few studies have specifically
defined its effect in acute inflammatory diseases (2). Previous studies have confirmed
obesity as a negative prognostic factor in acute pancreatitis (AP)
(3–5). Patients with AP who are obese have a
higher rate systemic inflammatory response and poorer outcomes
(4,6). Meta-analysis has suggested that
obesity is a risk factor for the development of local and systemic
complications, and mortality rates in AP (7,8).
Novel scoring systems for the severity of AP have also been
suggested, including obesity as an independent essential predictive
factor (9).
Over the past decade, fat tissue has been viewed not
only as a site of energy storage, but also as an active endocrine
organ, which secretes a variety of bioactive substances known as
adipocytokines (10).
Adipocytokines have a wide variety of endocrine, paracrine and
autocrine effects, including the regulation of energy metabolism,
immunity and inflammation (11,12).
Resistin, a 108-amino-acid peptide hormone secreted by adipocytes
and macrophages, is a member of the resistin-like molecule family
of cysteine-rich proteins (13–15).
Originally, resistin was implicated as a factor linking obesity and
diabetes by impairing insulin sensitivity and glucose tolerance in
mice (16). Elevated levels of
resistin have been used as an early marker of inflammation in
patients with AP due to its association with major local and
systemic components of the inflammatory response (17). Resistin has also been shown to
affect inflammatory cell infiltration of the pancreas and
peripancreatic visceral fat tissues, thus affecting the severity of
the clinical symptoms of AP (18,19).
Several previous studies have examined and characterized the
association between resistin and inflammatory factors (16,20–22)
and have suggested that resistin may have an effect on the severity
of AP.
It is generally considered that inflammation in AP
is crucial in the pathogenesis of local and systemic damage
(5). The activation of nuclear
factor kappa B (NF-κB), a transcription factor associated with the
activation of inflammatory genes, results in the overexpression of
inflammatory genes in pancreatic acinar cells (23). The ultimate severity of the
resulting pancreatitis may be determined by the inflammatory
responses, which occur subsequent to acinar cell injury (24). As resistin has potent
immunomodulatory and metabolic activities (15), the present study hypothesized that
the proinflammatory alterations observed in AP may be, in part,
associated with the adipocytokine resistin.
In the present study, the effects of resistin were
investigated on a cerulein-induced in vitro model of AP
using the AR42J cell line. In addition, the mechanism underlying
resistin-induced AP aggravation associated with NF-κB activation
was determined using this model.
Materials and methods
Cell cultures
Rat AR42J pancreatic acinar cells were purchased
from the China Center for Type Culture Collection (Wuhan, China).
The AR42J cells were maintained in Ham's F-12 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China), 100 U/ml penicillin and 100
µg/ml streptomycin. The cells were routinely plated at a density of
1×105 cells/ml in 6-well cluster dishes, and incubated
in a humidified incubator at 37°C with 95% air and 5%
CO2. The cells were divided into a control group, model
group (treated with cerulein) and resistin treatment group (treated
with cerulein and resistin). The drugs were dissolved in PBS and
added at the following concentrations: 100 nM cerulein
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and 8 nM
resistin (ProSpecTany TechnoGene, Ltd., Rehovot, Israel). In a
previous study, it was found that resistin exhibits maximal effects
on pancreatic acinar cells at a concentration of 8 nM (21) and this concentration is also close
to the levels of resistin in patients with AP on admission
(19). For the treatment groups,
the cells (1×105 cells/ml) were pre-treated with
resistin at 37°C for 30 min prior to the addition of cerulein.
AR42J cells treated with PBS alone were used as negative controls.
After 24 h, the cells and culture media were harvested for further
analysis. To determine the effect of NF-κB, AR42J cells were
incubated for 2 h at 37°C with 60 µM pyrrolidine dithiocarbamate
(PDTC) (Sigma-Aldrich; Merck Millipore), an antioxidant, which acts
as a specific inhibitor of NF-κB activation (25), prior to stimulation with cerulein
or resistin.
Estimation of amylase secretion
The culture media was collected and amylase assays
were performed. The secretion of amylase was measured using the
2-chloro-4-nitrophenyl-α-maltotrioside method according to the
manufacturer's protocols (Kehua Bio, Shanghai, China). Absorbance
data were measured at 405 nm, and amylase secretion was expressed
in units per liter using the standard curve provided by the
manufacturer.
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the AR42J cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
synthesis was performed using a One Step PrimeScript miRNA cDNA
Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China).
Specific mRNA quantification was performed using qPCR analysis
using SYBR Premix Ex TaqTM II (Takara Biotechnology Co., Ltd.) in a
Lightcycler 480 Real-Time PCR system (Roche Diagnostics, Meylan,
France) as previously described (26). The tumor necrosis factor-α (TNF-α),
interleukin (IL)-6 and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) primers were designed using Primer Express 2.0 computer
software (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
gene-specific primers used were as follows: TNF-α, sense
5′-TGAACTTCGGGGTGATCG-3′ and antisense 5′-GGGCTTGTCACTCGAGTTTT-3′;
IL-6, sense 5′-TCGAGCCCACCAGGAACGAAAGT-3′ and antisense
5′-AGTAGGGAAGGCAGTGGCTGTCA-3′; GAPDH, sense
5′-CTCAACTACATGGTCTACATGTTCCA-3′ and antisense:
5′-CTTCCCATTCTCAGCCTTGACT-3′. All reactions involved initial
denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for
10 sec, 60°C for 20 sec and 79°C for 20 sec. The Cq value was
defined as the number of PCR cycles in which the fluorescence
signal exceeded the detection threshold value. First, ΔCq=Cq
Gene-Cq GAPDH. Then, ΔΔCq=ΔCq treated-ΔCq control. Lastly,
2ΔΔCq was calculated to represent the relative mRNA
expression of target genes (27).
The relative quantity of mRNA for each gene was normalized based on
that of the housekeeping gene, GAPDH.
Western blot analysis
Variations in the protein expression levels of the
NF-κB p65 subunit in the nuclei of AR42J cells were detected using
western blot analysis. Cell lysates and nuclear extracts were
prepared using a Nuclear and Cytoplasmic Protein Extraction kit
(Beyotime Intitute of Biotecnology, Nantong, China) according to
the manufacturer's protocols. The proteins were quantified using a
BCA Protein Assay kit (Beyotime Intitute of Biotecnology). For the
western blot assays, equal quantities of the protein samples (18
µl) were separated by 10% SDS-PAGE, following which they were
electrophoretically transferred onto PVDF membranes (Invitrogen;
Thermo Fisher Scientific, Inc.). The non-specific sites on each
blot were blocked for 1 h at room temperature with 5% milk powder
diluted in TBS with 0.05% Tween-20 (TBST). The membranes were then
incubated with the following antibodies: Rabbit polyclonal
anti-NF-κB p65 (diluted 1:1,000; Abcam, Cambridge, MA, USA) and
mouse monoclonal anti-lamin-B (diluted 1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Following incubation at
4°C overnight with primary antibodies, the blots were washed four
times with TBST buffer. The blots were then finally incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibodies (1:10,000; Santa Cruz Biotechnology, Inc.) and
HRP-conjugated goat anti-mouse secondary antibodies (1:1,000; Santa
Cruz Biotechnology, Inc.) at room temperature for 2 h. The proteins
were detected using an enhanced chemiluminescence reagent (Pierce;
Thermo Fisher Scientific, Inc.). Band intensity was quantified
using Bandscan 5.0 software (Glyko, Novato, CA, USA). Protein
expression was normalized to lamin B.
Statistical analysis
All statistical analyses were performed using SPSS
version 12.0 (SPSS, Inc., Chicago, CA, USA). Data are expressed as
the mean ± standard deviation of three independent experiments
performed in duplicate. Statistical significance was evaluated
using one-way analysis of variance with Student-Newman-Keuls test
for post hoc analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of resistin on amylase
secretion in cerulein-treated AR42J cells
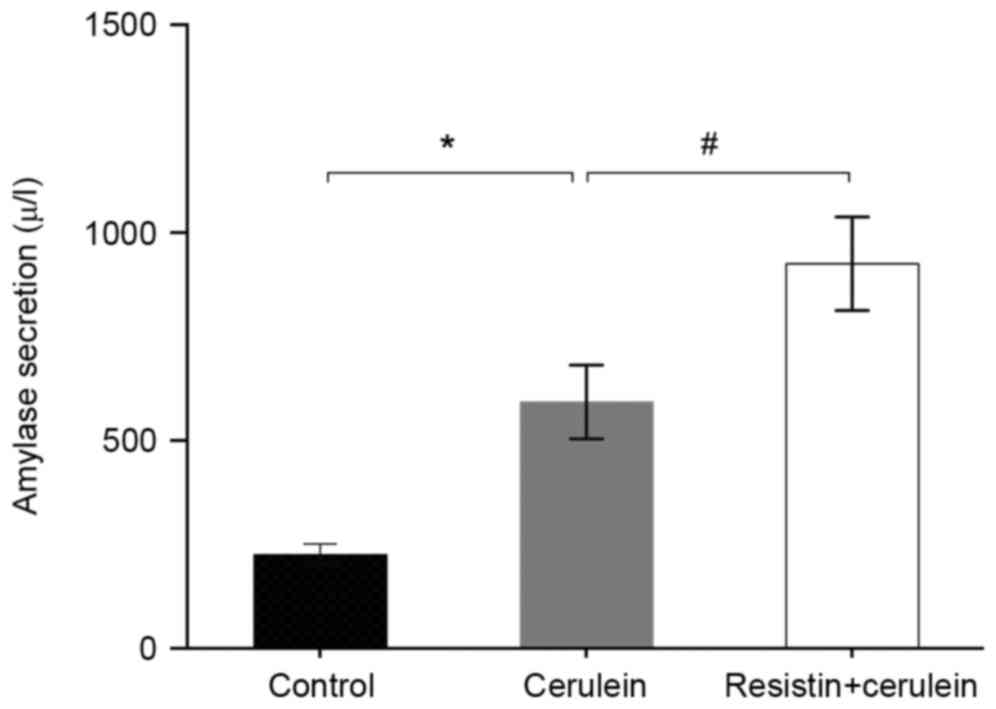
Treatment of the AR42J cells with cerulein increased
the secretion of amylase into the culture media, compared with the
untreated control cells (P<0.01; Fig. 1). In the resistin-pretreated in
vitro AP model, a significantly higher concentration of amylase
secretion was produced, compared with that in the cerulein-treated
group (P<0.05; Fig. 1).
Effects of resistin on the mRNA
expression of proinflammatory cytokines in cerulein-treated AR42J
cells
The results of the RT-qPCR analysis demonstrated
that the mRNA expression levels of TNF-α and IL-6 in the
cerulein-treated group were increased significantly, compared with
the levels observed in the untreated controls (P<0.01; Fig. 2). Resistin treatment augmented the
increased mRNA levels of TNF-α and IL-6 mRNA elicited by cerulein
(TNF-α, P<0.01; IL-6, P<0.05; Fig. 2). Notably, pretreatment of cells
with PDTC significantly attenuated the mRNA expression levels of
TNF-α and IL-6 in the resistin+cerulein-treated group (P<0.01;
Fig. 2) and the cerulein-treated
group (TNF-α, P<0.01; IL-6, P<0.05; Fig. 2).
Effects of resistin on the protein
levels of NF-κB p65 in the nuclei of cerulein-treated AR42J
cells
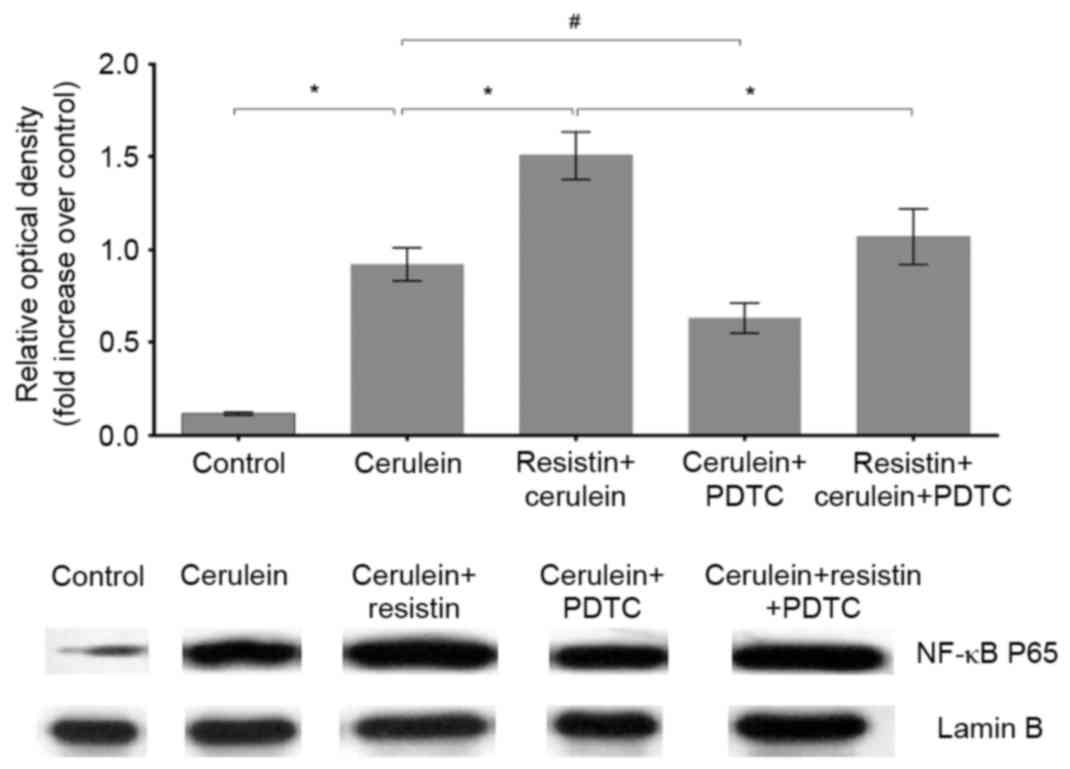
Cerulein treatment significantly increased the
protein levels of the NF-κB p65 subunit in the nuclei of the AR42J
cells, compared with the untreated controls (P<0.01; Fig. 3). Resistin treatment augmented the
increase in the protein level of NF-κB p65 elicited by cerulein
(P<0.01; Fig. 3). PDTC
pretreatment significantly recovered the protein levels of NF-κB
p65 in the cerulein+resistin-treated group (P<0.01; Fig. 3) and cerulein-treated group
(P<0.05; Fig. 3).
Discussion
The present study aimed to investigate the
functional consequences of exposing rat pancreatic acinar cells to
resistin and to determine whether it amplifies proinflammatory
signaling in an in vitro AP model. Treatment of the AR42J
cells with cerulein, which induced the secretion of amylase and
mRNA expression levels of TNF-α and IL-6, led to the successful
development of the in vitro model of AP. Pancreatic AR42J
cells are derived from acinar cells and are the only currently
available cell line that maintains normal pancreatic acinar cell
characteristics (28). The
expression of AR42J cell receptor and signal transduction
mechanisms have been demonstrated to parallel the mechanisms found
in human pancreatic acinar cells (29). Therefore, these cells are now
widely applied in investigations of cellular secretion, growth,
proliferation and apoptosis of the exocrine pancreas (30).
In the present study, pretreatment with resistin
augmented the secretion of amylase and the expression levels of
proinflammatory molecules in the in vitro model of AP. These
findings correlate well with clinical observations suggesting the
validity of reports linking the clinical severity and outcomes of
AP with circulating levels of resistin (17,19).
Daniel et al (18) also
demonstrated that the levels of resistin may provide a useful tool
for the prognosis and monitoring of AP. Resistin is widely
considered to be an obesity-associated adipocytokine, based on
numerous studies, which have demonstrated high circulating levels
of resistin in obese rodents and are associated with several
pathological conditions (16,31,32).
The novel feature of resistin as a proinflammatory molecule has
been acknowledged. Several studies have reported that resistin can
trigger a proinflammatory state (16,22,33,34).
Silswal et al (35)
incubated macrophages with recombinant resistin and found that the
production of TNF-α and IL-12 increased. The exposure of hepatic
stellate cells to resistin has been also found to result in
increased expression levels of monocyte chemoattractant protein-1
and IL-8 (36). In our previous
study, it was demonstrated that resistin treatment was capable of
inducing the expression of TNF-α and IL-6 in pancreatic acinar
cells (21).
Inflammation is the hallmark of human and
experimental pancreatitis (37).
It is well known that proinflammatory responses occurring
subsequent to acinar cell injury can determine the ultimate
severity of the resulting pancreatitis. Elevated TNF-α and IL-6
have been reported to be correlated with disease severity in
patients with AP (38). Preventing
the expression of TNF-α attenuates the stress response and leads to
decreases in the mortality rates of patients with AP (39). The overexpression of IL-6 has been
shown to increase susceptibility to AP (40). In the present study, the augmented
expression of inflammatory cytokines induced by resistin exposure
in the AP model led to an increase in the severity of injury to
pancreatic acinar cells, which contributed evidence to the
previously reported association between obesity and increased
severity in patients with AP (39).
NF-κB has the ability to upregulate the expression
of inflammatory molecules induced in experimental pancreatitis
models (41). The present study
further investigated the role of NF-κB inflammatory response
augmentation induced by resistin in an in vitro model of AP.
It was shown that the increasing production of proinflammatory
cytokines was accompanied by NF-κB activation. Pretreatment with
the NF-κB inhibitor, PDTC, completely attenuated the production of
proinflammatory cytokines induced by resistin in the in
vitro AP model. This suggested that the effect of resistin on
augmenting the inflammatory response in the AP model was
specifically dependent on NF-κB activation. This finding is
consistent with previous studies, demonstrating that NF-κB
activation is a key mediator of the inflammatory response in
patients with AP (24,41,42).
In conclusion, the results of the present study
showed that resistin amplified the expression of proinflammatory
cytokines via the NF-κB pathway in the cerulein-induced in
vitro AP model. These preliminary indications suggested that
the overproduction of obesity-associated resistin and associated
inflammatory response may result in aggravation of the severity of
AP. However, further investigations are required for future
application of these findings in humans.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
30901433).
References
|
1
|
Ogden CL, Carroll MD, Kit BK and Flegal
KM: Prevalence of childhood and adult obesity in the United States,
2011–2012. Jama. 311:806–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abu Hilal M and Armstrong T: The impact of
obesity on the course and outcome of acute pancreatitis. Obes Surg.
18:326–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen SM, Xiong GS and Wu SM: Is obesity an
indicator of complications and mortality in acute pancreatitis? An
updated meta-analysis. J Dig Dis. 13:244–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sadr-Azodi O, Orsini N, Andrén-Sandberg A
and Wolk A: Abdominal and total adiposity and the risk of acute
pancreatitis: A population-based prospective cohort study. Am J
Gastroenterol. 108:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Premkumar R, Phillips AR, Petrov MS and
Windsor JA: The clinical relevance of obesity in acute
pancreatitis: Targeted systematic reviews. Pancreatology. 15:25–33.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Acharya C, Navina S and Singh VP: Role of
pancreatic fat in the outcomes of pancreatitis. Pancreatology.
14:403–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martínez J, Johnson CD, Sánchez-Paya J, de
Madaria E, Robles-Diaz G and Perez-Mateo M: Obesity is a definitive
risk factor of severity and mortality in acute pancreatitis: An
updated meta-analysis. Pancreatology. 6:206–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong S, Qiwen B, Ying J, Wei A and
Chaoyang T: Body mass index and the risk and prognosis of acute
pancreatitis: A meta-analysis. Eur J Gastroenterol Hepatol.
23:1136–1143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson CD, Toh SK and Campbell MJ:
Combination of APACHE-II score and an obesity score (APACHE-O) for
the prediction of severe acute pancreatitis. Pancreatology. 4:1–6.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grundy SM: Adipose tissue and metabolic
syndrome: too much, too little or neither. Eur J Clin Invest.
45:1209–1217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vazquez-Vela ME, Torres N and Tovar AR:
White adipose tissue as endocrine organ and its role in obesity.
Arch Med Res. 39:715–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao H: Adipocytokines in obesity and
metabolic disease. J Endocrinol. 220:T47–T59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steppan CM, Bailey ST, Bhat S, Brown EJ,
Banerjee RR, Wright CM, Patel HR, Ahima RS and Lazar MA: The
hormone resistin links obesity to diabetes. Nature. 409:307–312.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bokarewa M, Nagaev I, Dahlberg L, Smith U
and Tarkowski A: Resistin, an adipokine with potent proinflammatory
properties. J Immunol. 174:5789–5795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qatanani M, Szwergold NR, Greaves DR,
Ahima RS and Lazar MA: Macrophage-derived human resistin
exacerbates adipose tissue inflammation and insulin resistance in
mice. J Clin Invest. 119:531–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jamaluddin MS, Weakley SM, Yao Q and Chen
C: Resistin: functional roles and therapeutic considerations for
cardiovascular disease. Br J Pharmacol. 165:622–632. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Daniel P, Leśniowski B, Jasińska A,
Pietruczuk M and Malecka-Panas E: Usefulness of assessing
circulating levels of resistin, ghrelin, and IL-18 in alcoholic
acute pancreatitis. Dig Dis Sci. 55:2982–2987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daniel P, Leśniowski B, Mokrowiecka A,
Jasińska A, Pietruczuk M and Małecka-Panas E: Circulating levels of
visfatin, resistin and pro-inflammatory cytokine interleukin-8 in
acute pancreatitis. Pancreatology. 10:477–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schäffler A, Hamer O, Dickopf J, Goetz A,
Landfried K, Voelk M, Herfarth H, Kopp A, Büchler C, Schölmerich J
and Brünnler T: Admission resistin levels predict peripancreatic
necrosis and clinical severity in acute pancreatitis. Am J
Gastroenterol. 105:2474–2484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Filková M, Haluzik M, Gay S and Senolt L:
The role of resistin as a regulator of inflammation: Implications
for various human pathologies. Clin Immunol. 133:157–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang CY, Wang W, Tang JX and Yuan ZR: The
adipocytokine resistin stimulates the production of proinflammatory
cytokines TNF-alpha and IL-6 in pancreatic acinar cells via
NF-kappaB activation. J Endocrinol Invest. 36:986–992.
2013.PubMed/NCBI
|
|
22
|
Song YZ, Guan J, Wang HJ, Ma W, Li F, Xu
F, Ding LB, Xie L, Liu B, Liu K and Lv Z: Possible involvement of
serum and synovial fluid resistin in knee osteoarthritis: Cartilage
damage, clinical, and radiological links. J Clin Lab Anal.
30:437–443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gukovsky I, Li N, Todoric J, Gukovskaya A
and Karin M: Inflammation, autophagy, and obesity: Common features
in the pathogenesis of pancreatitis and pancreatic cancer.
Gastroenterology. 144:1199–1209, e1194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schreck RMB, Männel DN, Dröge W and
Baeuerle PA: Dithiocarbamates as potent inhibitors of nuclear
factor kappa B activation in intact cells. J Exp Med.
175:1181–1194. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang ZY, Jiang CY, Wang L, Wang JC, Zhang
SD, Einarsson C, Eriksson M, Han TQ, Parini P and Eggertsen G:
Increased NPC1L1 and ACAT2 expression in the jejunal mucosa from
Chinese gallstone patients. Biochem Biophy Res Com. 379:49–54.
2009. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Logsdon CD, Guthrie J, Alves F and
Rosewicz S: Glucocorticoids have opposite effects on ornithine
decarboxylase and cell growth in pancreatic acinar AR42J cells.
Yale J Biol Med. 65:449–456, 465-449. 1992.PubMed/NCBI
|
|
29
|
Gonzalez A, Santofimia-Castaño P and
Salido GM: Culture of pancreatic AR42J cell for use as a model for
acinar cell function. Pancreapedia: Exocrine Pancreas Knowledge
Base; 2011
|
|
30
|
Masamune A, Sakai Y, Satoh A, Fujita M,
Yoshida M and Shimosegawa T: Lysophosphatidylcholine induces
apoptosis in AR42J cells. Pancreas. 22:75–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye H, Zhang HJ, Xu A and Hoo RL: Resistin
production from adipose tissue is decreased in db/db obese mice and
is reversed by rosiglitazone. PLoS one. 8:e655432013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elsayed EY, Mosalam NA and Mohamed NR:
Resistin and insulin resistance: A link between inflammation and
hepatocarcinogenesis. Asian Pac J Cancer Prev. 16:7139–7142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al-Daghri N, Chetty R, McTernan PG,
Al-Rubean K, Al-Attas O, Jones AF and Kumar S: Serum resistin is
associated with C-reactive protein & LDL cholesterol in type 2
diabetes and coronary artery disease in a Saudi population.
Cardiovasc Diabetol. 4:102005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al Hannan F and Culligan KG: Human
resistin and the RELM of Inflammation in diabesity. Diabetol Metab
Syndr. 7:542015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Silswal N, Singh AK, Aruna B, Mukhopadhyay
S, Ghosh S and Ehtesham NZ: Human resistin stimulates the
pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by
NF-kappaB-dependent pathway. Biochem Biophys Res Commun.
334:1092–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bertolani C, Sancho-Bru P, Failli P,
Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani
S, Ginés P, et al: Resistin as an intrahepatic cytokine:
Overexpression during chronic injury and induction of
proinflammatory actions in hepatic stellate cells. Am J Pathol.
169:2042–2053. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cohen MC and Cohen S: Cytokine function: A
study in biologic diversity. Am J Clin Pathol. 105:589–598. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhatia M: Inflammatory response on the
pancreatic acinar cell injury. Scand J Surg. 94:97–102.
2005.PubMed/NCBI
|
|
39
|
Sunden-Cullberg J, Nyström T, Lee ML,
Mullins GE, Tokics L, Andersson J, Norrby-Teglund A and Treutiger
CJ: Pronounced elevation of resistin correlates with severity of
disease in severe sepsis and septic shock. Crit Care Med.
35:1536–1542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzuki S, Miyasaka K, Jimi A and Funakoshi
A: Induction of acute pancreatitis by cerulein in human IL-6 gene
transgenic mice. Pancreas. 21:86–92. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rakonczay Z Jr, Hegyi P, Takács T,
McCarroll J and Saluja AK: The role of NF-kappaB activation in the
pathogenesis of acute pancreatitis. Gut. 57:259–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X, Ji B, Han B, Ernst SA, Simeone D
and Logsdon CD: NF-kappaB activation in pancreas induces pancreatic
and systemic inflammatory response. Gastroenterology. 122:448–457.
2002. View Article : Google Scholar : PubMed/NCBI
|