Introduction
Osteoporosis is a bone disease that is characterized
by reduced bone mass and microarchitectural deterioration, which
may lead to an increased risk of bone fractures. It is estimated
that ~20% of women over 50 years old develop osteoporosis globally
(1). In addition, female bone
density decreases rapidly during perimenopause and continues to
gradually decrease in postmenopausal women (2). Osteoporosis decreases bone strength
and increases the risk of fractures (3,4).
Among aged individuals, hip fractures are associated with high
mortality rates (5). Normal bone
remodeling is achieved by a balance between bone resorption and
bone formation (6); however, in
postmenopausal osteoporosis, the rate of bone resorption by
osteoclasts is greater compared with bone formation by osteoblasts
(7). Receptor activator of nuclear
factor (NF)-κB ligand (RANKL) and macrophage colony-stimulating
factor (M-CSF) are key for the maturation of osteoclasts (8). The binding of RANKL to receptor
activator of nuclear factor κB (RANK) protein on the membrane of
osteoclast precursor cells activates the NF-κB pathway [NF-κB is
the key nuclear activator of nuclear activator of activated T-cells
1 (NFATc1) expression], which may result in fusion of mononuclear
osteoclasts (9). The main role of
M-CSF is to induce pre-osteoclasts to express RANK, which is a
receptor of RANKL (10).
At present, therapeutic strategies used to treat
osteoporosis involve interference with either the differentiation
of osteoclasts or their function. However, many of these drugs
exert unwanted side effects. For example, bisphosphonates and
denosumab have been associated with osteonecrosis of the jaw and
atypical femur fractures. In addition, bisphosphonates have been
associated with atrial fibrillation and kidney damage (11,12).
Therefore, the development of novel therapies for the treatment of
osteoporosis is required.
Fucoidan is a type of sulfated polysaccharide
isolated from seaweed (13). It
predominantly consists of fucose, and small amounts of alduronic
acid, galactose and xylose. A previous study reported that fucoidan
may exhibit antitumorigenic, antiviral, anticoagulative and
antioxidative properties (14).
Furthermore, it has been reported that low-molecular weight
fucoidan (LMWF) promotes the expression of alkaline phosphatase and
type I collagen (15). However,
the effects of LMWF on osteoclasts and osteoporosis in
ovariectomized rats remain to be elucidated. A previous study
reported that apoptosis induced by fucoidan was attenuated by
caspase inhibitors, indicating that fucoidan-induced apoptosis was
dependent on the activation of caspases (16). Fucoidan contains ≥25% organic
sulfate and 60% polysaccharide.
A previous study compared the biological activity of
LMWF and regular fucoidan, and the results indicated that LMWF had
a higher activity compared with fucoidan (17). To the best of our knowledge, the
effects of LMWF on osteoporosis have not been previously
assessed.
The present study aimed to determine the effects and
mechanisms of action of LMWF on osteoporosis, using an
ovariectomized female rat model. Furthermore, the effects of LMWF
on RANKL and M-CSF-induced RAW264.7 cell differentiation into
osteoclasts were investigated in vitro.
Materials and methods
Preparation of LMWF
Fucoidan (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was digested consecutively with glucoamylase, pectinase
and cellulose complex (Sigma-Aldrich; Merck Millipore), into three
different LMWF solutions as previously described (18). Fucoidan was dissolved in dimethyl
sulfoxide (10 mg/ml), filtered using a 0.2 mm filter and stored at
−20°C. The effect of these LMWF solutions on the growth and
viability of osteoclasts was evaluated using MTT assay as
previously described (19), in
order to select the strongest inhibitory concentration as
preparation for the subsequent experiments. The concentrations of
the three LMWF solutions tested were 50, 10, 5, 1, 0.5 and 0.1
µg/ml. It was determined that 5 µg/ml of the LMWF generated by
pectinase digestion exhibited the strongest inhibitory effect on
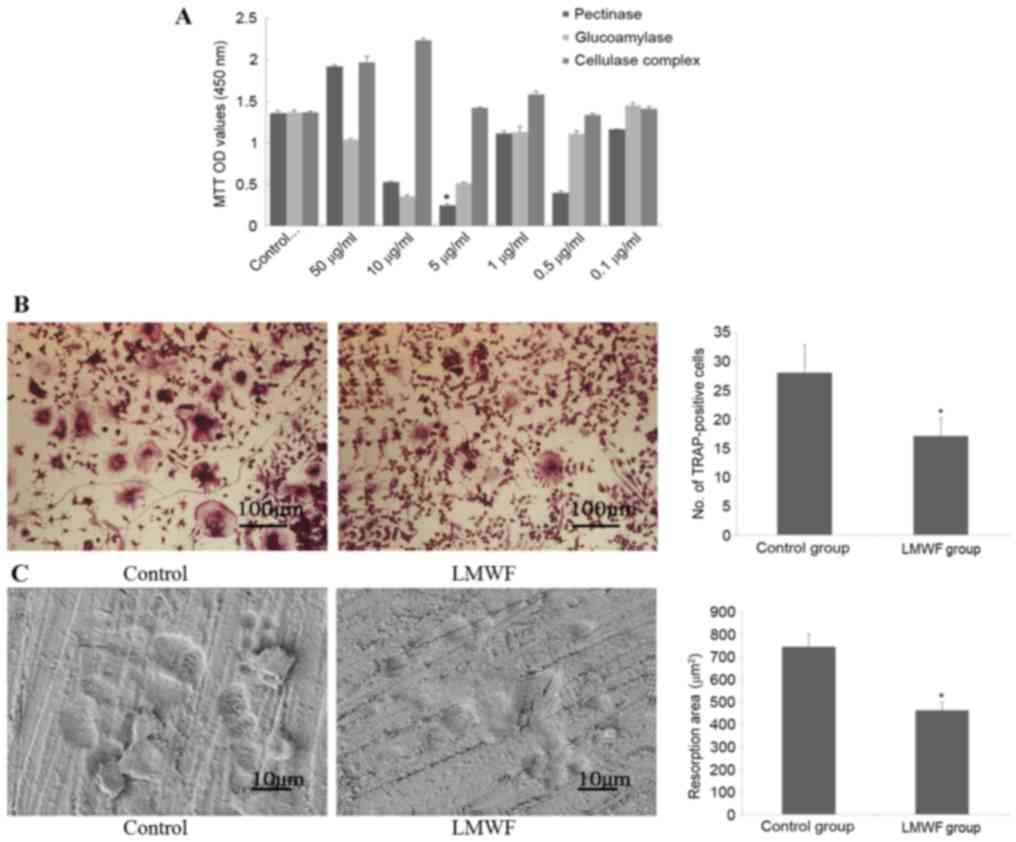
osteoclasts (Fig. 1A). Based on
these results, subsequent experiments were performed using 5 µg/ml
of the LMWF generated by pectinase digestion.
Cell culture, induction and
analysis
The RAW264.7 murine macrophage-like cell line was
purchased from the Chinese National Platform of Experimental Cell
Resources for Sci-Tech, Institute of Basic Medicine, Chinese
Academy of Medical Sciences (Peking Union Medical College, Beijing,
China). RAW264.7 cells were grown in high glucose Dulbecco's
modified Eagle's medium (DMEM; ME100202P1; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (12676011; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin, at 37°C in a
humidified atmosphere of 95% air and 5% CO2. The cells
were cultured on sterile coverslips or osteologic slides, which
were placed into 6-well culture dishes. Cells were inoculated at a
density of 2×104/ml. The medium was changed to high-glucose DMEM
(ME100202P1; Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), with 100 ng/ml RANKL (Murine sRANK-Ligand; 31501; PeproTech,
Inc., Rocky Hill, NJ, USA) and 30 ng/ml M-CSF (Murine M-CSF, 31502;
PeproTech, Inc.) to induce differentiation 4 h after cell
attachment. Cells were incubated at 37°C, in a 5% CO2
incubator with high humidity. The medium was changed every 2 days.
After 6 days, cells were stained for tartrate-resistant acid
phosphatase (TRAP) using the TRAP staining kit (Sigma-Aldrich;
Merck Millipore) and the experiment was conducted according to the
manufacturer's protocol. A light microscope (Olympus Corporation,
Tokyo, Japan) was used to observe the number of TRAP-positive
multinucleated cells (≥3 nuclei). To visualize bone resorption
pits, osteologic slides were fixed in 2.5% glutaraldehyde, washed
by sonication in phosphate-buffered saline (PBS), dehydrated in a
graded, ascending series of ethanol, sputter-coated with gold and
palladium. Images were captured using a scanning electron
microscope.
Scoring of TRAP-positive cells and
analysis of bone resorption surface
To assess the effects of LMWF on osteoclast
differentiation, RAW264.7 cells (2×104/ml) were cultured in two
groups; A control group and an experimental, LMWF-treated group.
Differentiation was induced as aforementioned, with the exception
that the experimental group was cultured in the presence of 5 µg/ml
LMWF in addition to RANKL and M-CSF. Cells were stained for TRAP
and the number of TRAP-positive multinucleated cells (≥3 nuclei)
was scored after 6 days. Scoring was performed at a magnification
×100 and 10 fields were randomly selected and scored, with the
average calculated. Only cells that appeared pink or rose-red and
contained ≥3 nuclei were counted, as previously described (19). The experiment was repeated three
times. For the bone resorption pit assay, cells were cultured in as
aforementioned, with the exception that they were cultured on
bovine cortical bone slices made in-house. Cells were cultured on
10 bone slices in each group. Within each bone slice, 10
non-overlapping areas were randomly selected and analyzed using
Image-Pro Plus version 6.0 image analysis software (Media
Cybernetics, Inc., Rockville, MD, USA) to evaluate bone resorption
areas.
Effects of LMWF on osteoclast
proliferation
Two groups of cells, as aforementioned, were
cultured for 6 days. Subsequently, the cells (1×106/ml) were
collected, washed with PBS, and fixed with 75% ethanol for 5 min at
room temperature. Fixed cells were resuspended in 1 ml propidium
iodide/Triton X-100 staining solution (Sigma-Aldrich; Merck
Millipore) containing 0.2 mg RNase A and were incubated at 37°C for
15 min. Stained cells were subjected to flow cytometry using a
Guava Flow Cytometer (model, EPICS® Merck Millipore) and
Expo 32 software (Beckman Coulter, Inc., Brea, CA, USA) to
calculate proliferation index (PI). PI =
(S+G2/M)/(G0/G1+S+G2/M).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for gene expression
detection
After 6 days of induction, cells were harvested for
RNA extraction. Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) The quality of RNA was
assessed by agarose gel electrophoretic analysis. RANK, TRAP,
matrix metallopeptidase-9 (MMP-9), NFATc1 and osteoclast-associated
immunoglobulin-like receptor (OSCAR) expression levels were
detected by qPCR using the Absolute™ Fast qPCR Master Mixes kit
(Guangzhou Funeng Gene Co., Ltd., Guangzhou, China). The
thermocycler conditions used were as follows: 96°C pre-denaturation
for ~5 min; 94°C denaturation for ~1 min; 58°C annealing for ~45
sec; 72°C extension for ~2 min, for a total of 35 cycles. cDNA was
reverse-transcribed using the All-in-One™ First-Strand cDNA
Synthesis kit (GeneCopoeia, Inc., Rockville, MD, USA) from the
isolated RNA and GAPDH expression was used as an internal control.
Primers were designed by Fulengen Co., Ltd. (Guangzhou, China). The
primer sequences were as follows: GAPDH, forward (F)
5′-CGGAGTCAACGGATTTGGTCGTAT-3′, reverse (R)
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′, fragment amplified, 96 bp; NFATc1,
F 5′-TCCAAAGTCATTTTCGTGGA-3′, R 5′-CTTTGCTTCCATCTCCCAGA-3′; MMP-9,
F 5′-ACGACATAGACGGCATCCA-3′, R 5′-GCTGTGGTTCAGTTGTGGTG-3′; TRAP, F
5′-AAATCACTCTTTAAGACCAG-3′, R 5′-TTATTGAATAGCAGTGACAG-3′; RANK, F
5′-CCAGGGGACAACGGAATCA-3′, R 5′-GGCCGGTCCGTGTACTCATC-3′; and OSCAR,
F 5′-TGATTGGCACAGCAGGAG-3′ and R 5′-AAGGCACAGGAAGGAAATAGAG-3′. RT
and qPCR were performed per manufacturer's protocol. Amplification
and melting curves were evaluated for each qPCR analysis. Gene
expression levels were based on levels of the initial template, and
were calculated using the 2-∆∆Cq method (20).
Animals
A total of 60 healthy 6-month-old female
Sprague-Dawley (SD) rats weighing 220–240 g were provided by
Tianjin Hospital Center for Experimental Animals (Tianjin, China;
approval no. SCXK 2007004). All experimental procedures were
approved by the Institutional Animal Care and Use Committee at the
Tianjin Hospital Ethics Committee of China (Tianjin, China). The
methods were carried out in accordance with the approved
guidelines. Animals were housed at a room temperature of 21–24°C,
in a lighted room between 7:00 a. m. and 7:00 p.m.; animals were
allowed to engage in physical activity and received ad
libitum access to food and water.
Animal experiments
A total of 60 female SD rats were randomly assigned
to the following treatment groups (n=20/group): i) Ovariectomized
group (OVX); ii) sham surgery group (SHAM); and iii) LMWF group
(LMWF dose: 5 mg/kg). SHAM group rats were subjected to a sham
surgery (only an incision in the back was made) without ovary
removal. The other two groups were subjected to bilateral
ovary-removal surgery through an incision in the back following
anesthesia with 10% chloral hydrate (0.3 ml/100 g). Saline (for the
OVX and SHAM groups) or LMWF (for the LMWF group) was administered
by intraperitoneal injection 5 days after surgery. Rats were
euthanized with 10% chloral hydrate (1 ml/100 g) at 4 or 8 weeks
following administration of treatment. A total of 10 rats from each
group were euthanized at each time-point.
The bone mineral density (BMD) of the right femur of
the rats (n=5 per group at each time-point) was measured using
LUNAR DPXIQ bone densitometer (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA). Bones were then subjected to a micro-computed
tomography (CT) scan (Bruker microCT, Antwerp, Belgium). Scanning
was performed at a setting of 21-µm resolution, 360° rotation with
an incremental rotation of 0.4°, voltage of 80 kV, current of 80 A
and 2.960 msec exposure time. Images were captured at an average
gain of 4, pixel 1×1. For image normalization, the images were
scanned in black and white and the grids were standardized.
Trabecular bone volume fraction (BV/TV, %), trabecular thickness
(Tb.Th.) and trabecular number (Tb.N.) of the distal end of the
femur were determined for evaluation.
For the 4-week and 8-week time-points, rats were
injected with an intramuscular injection of tetracycline (25 mg/kg)
at 10 days or 3 days prior to euthanasia, as previously described
(21). Following euthanasia, the
right femurs were collected and fixed in ethanol for 1 week. The
distal 1/3 of each femur (n=5 per group at each time-point) was
dehydrated and embedded in semi-butyl methacrylate
polymerization/methyl ester. Using a heavy-duty microtome, 7 µm
undecalcified bone sections were cut. The sections were washed in
distilled water and treated with 3% sodium thiosulphate for 5 min,
and then washed in water. von Kossa and Giemsa staining were
performed for 5 min, respectively, and sections were the mounted.
Images were captured using a light microscope and analyzed using
Image-Pro Plus version 6.0 software.
Rats were anesthetized with 10% chloral hydrate (0.3
ml/100 g) and serum samples (1 ml) were collected by cardiac
puncture 8 weeks after the administration of LMWF or saline
control. Samples were centrifuged at 300 × g for 10 min at room
temperature. Serum procollagen type I N propeptide (PINP) and
C-terminal telopeptide-1 (CTX-1) levels were determined using
corresponding ELISA kits (Ra PINP kit; cat. no. 20131010 and Ra
CTX-1 kit; cat. no. 20130311; Cusabio Biotech Co., Ltd., Baltimore,
MD, USA).
Assessment of femoral mechanical
properties
The mechanical strength of the shaft and neck of the
left femur (n=10/group) was determined as previously described by
Ma and Fu (22). All testing was
performed on a Bose ElectroForce3200 electromagnetic test
instrument (Bose Corporation, Framingham, MA, USA). The midshaft
femoral strength was tested using a three-point bending test and
the femoral neck strength was tested by a compression test
(n=10).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was conducted using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). All experimental data were analyzed using
an one way analysis of variance and multiple comparisons were
performed using Duncan's test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Number of TRAP-positive cells and
analysis of the bone resorption surface
As presented in Fig.
1B, 6 days after RANKL induction of RAW264.7 cells, the number
of cells that stained positive for TRAP in the LMWF group was
significantly lower compared with the control group (P<0.05),
indicating that more osteoclasts were generated when LMWF was
absent. In addition, TRAP-positive cells from the LMWF group had
fewer nuclei in general (P<0.05; data not shown). The bone
resorption assay revealed that there were significantly more bone
resorption pits in the control group compared with the LMWF group
(P<0.05; Fig. 1C).
Effects of LMWF on osteoclast
proliferation
FACS analysis revealed that the PI of cells from the
LMWF group was significantly lower compared with cells from the
control group (26.2±3.62 vs. 37.56±3.08, respectively; P<0.05;
data not shown), thus suggesting that LMWF inhibited osteoclast
proliferation.
Effects of LMWF on expression of genes
involved in osteoclast differentiation
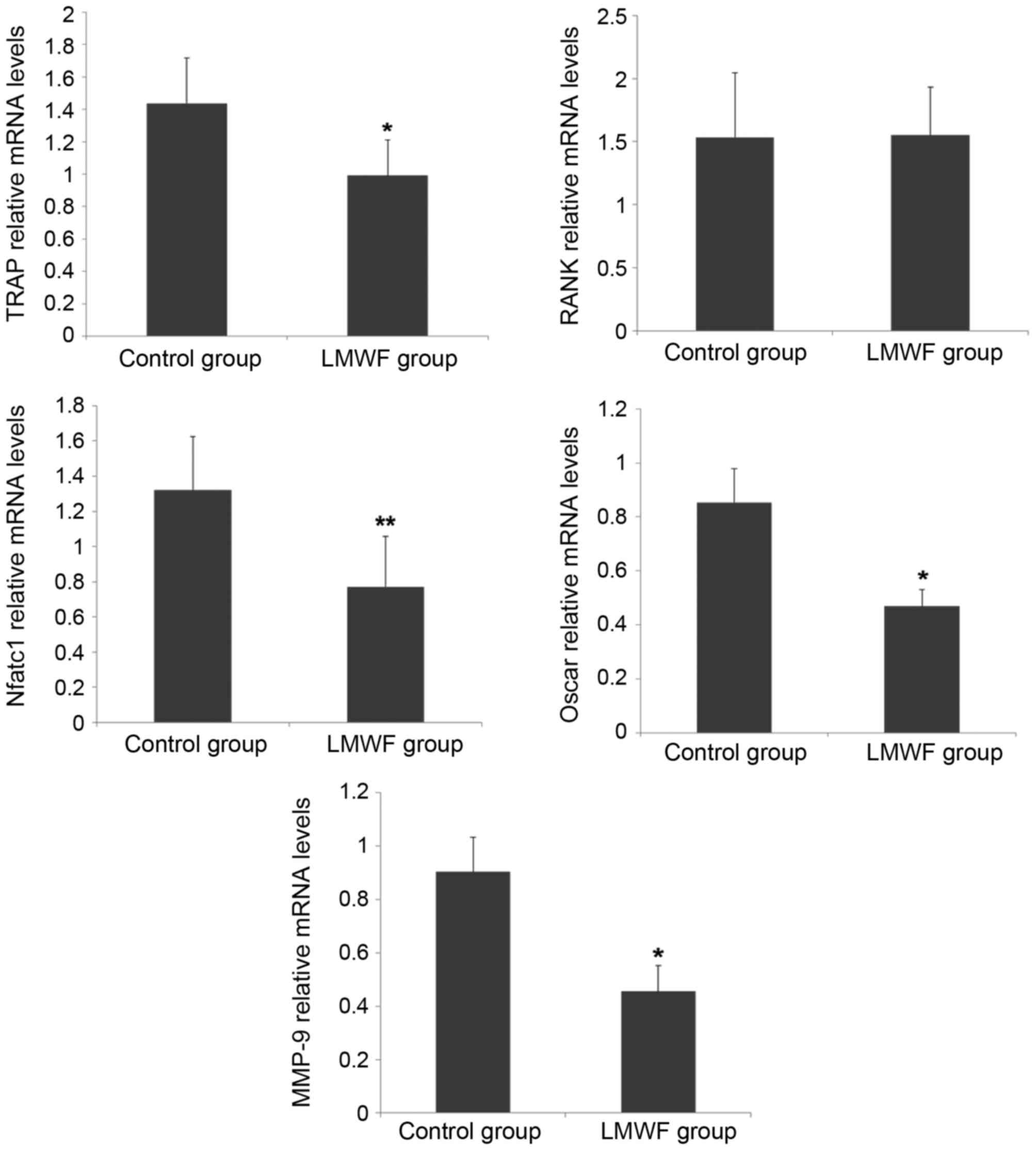
RT-qPCR was performed to assess expression levels of
the genes encoding TRAP, RANK, NFATc1, OSCAR and MMP-9. As
presented in Fig. 2, LMWF
significantly inhibited the expression levels of osteoclast marker
genes TRAP (P<0.05), NFATc1 (P<0.01) and OSCAR (P<0.05)
when compared with the control group. However, LMWF had no effect
on RANK gene expression (P>0.05). LMWF also exhibited an
inhibitory effect on MMP-9 expression (P<0.05).
BMD and micro-CT analysis in OVX
rats
As presented in Fig.
3A, 4 weeks after the ovariectomy the distal femur of the OVX
group exhibited trabecular bone loss, which progressively worsened
8 weeks after surgery when compared with the SHAM group. As
presented in Fig. 3B, the BMD of
rats from the OVX group was significantly lower for both
time-points investigated compared with that of rats from the SHAM
group (P<0.05), thus suggesting that ovariectomy may lead to
bone loss. The ovariectomized rats receiving LMWF had a
significantly higher BMD compared with the OVX group (P<0.05;
Fig. 3B), indicating that LMWF may
minimize bone loss. In addition, the BMD increase at the 8-week
time-point was higher than that at the 4-week time-point; however,
still lower when compared with the SHAM group.
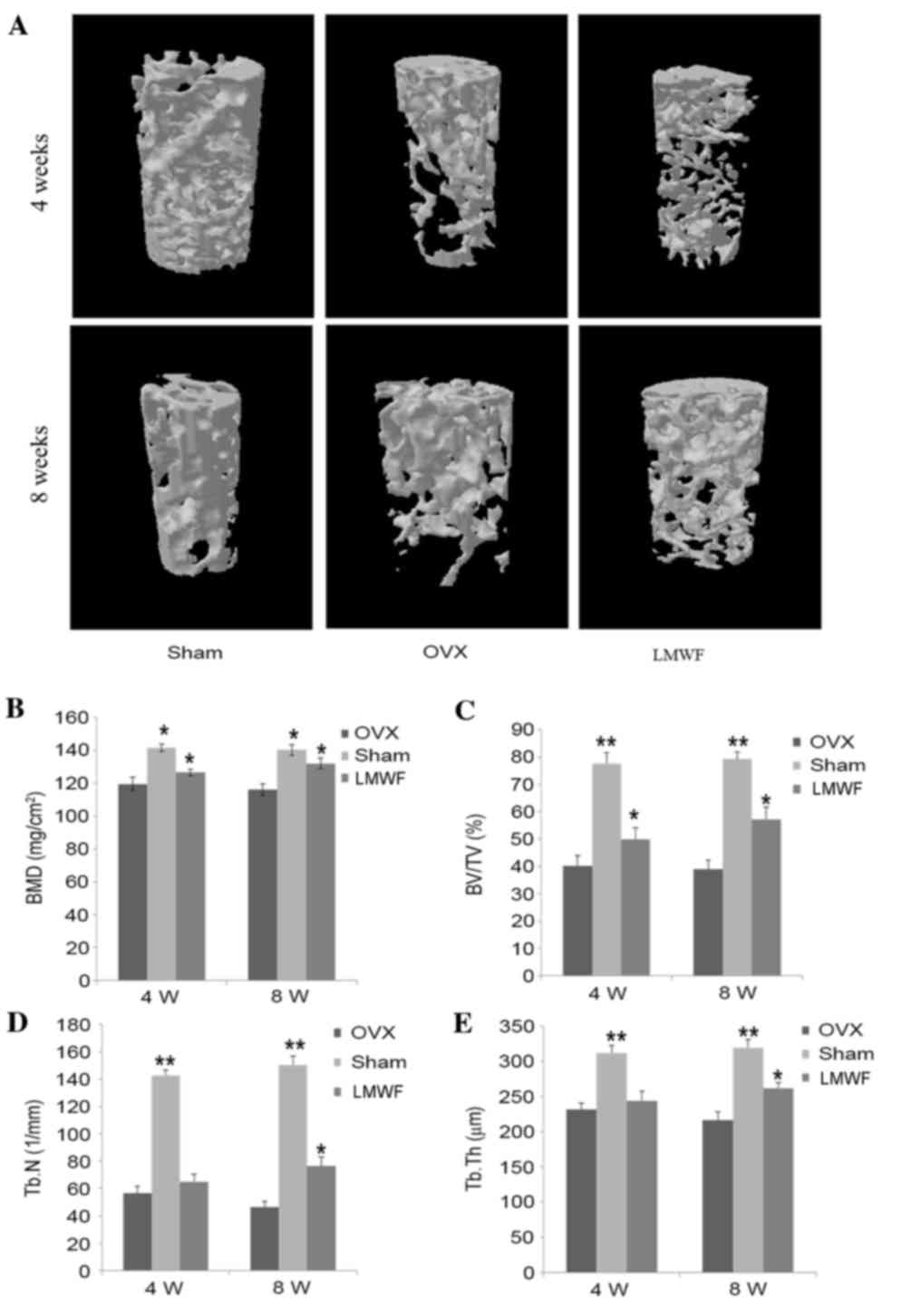 | Figure 3.(A) Representative samples of 3D
architecture of trabecular bone within the distal femoral
metaphyseal region from the different treatment groups. (B) BMD
quantification using dual energy X-ray absorptiometry. Effects of
low-weight molecular fucoidan on the (C) BV/TV, (D) Tb.n and (E)
Tb.Th of the distal femoral metaphysic in OVX rats by
micro-computed tomography analysis. Data are presented as the mean
± standard deviation, n=5. *P<0.05, **P<0.01 vs. OVX group.
OVX, ovariectomized; BMD, bone mineral density; BV/TV, bone
volume/total volume; Tb.n, trabecular number; Tb.Th, trabecular
thickness; 4 W, 4 weeks; 8 W, 8 weeks. |
Quantification of micro-CT scan results are
presented in Fig. 3C-E. Compared
with the OVX group, the LMWF group demonstrated a significantly
higher BV/TV, Tb.Th. and Tb.N at 8 weeks (P<0.05 for all
measurements), whereas these measurement were lower compared with
in the SHAM group. In addition, the rats of the LMWF group
demonstrated greater Tb.N in the distal femur compared with the OVX
group.
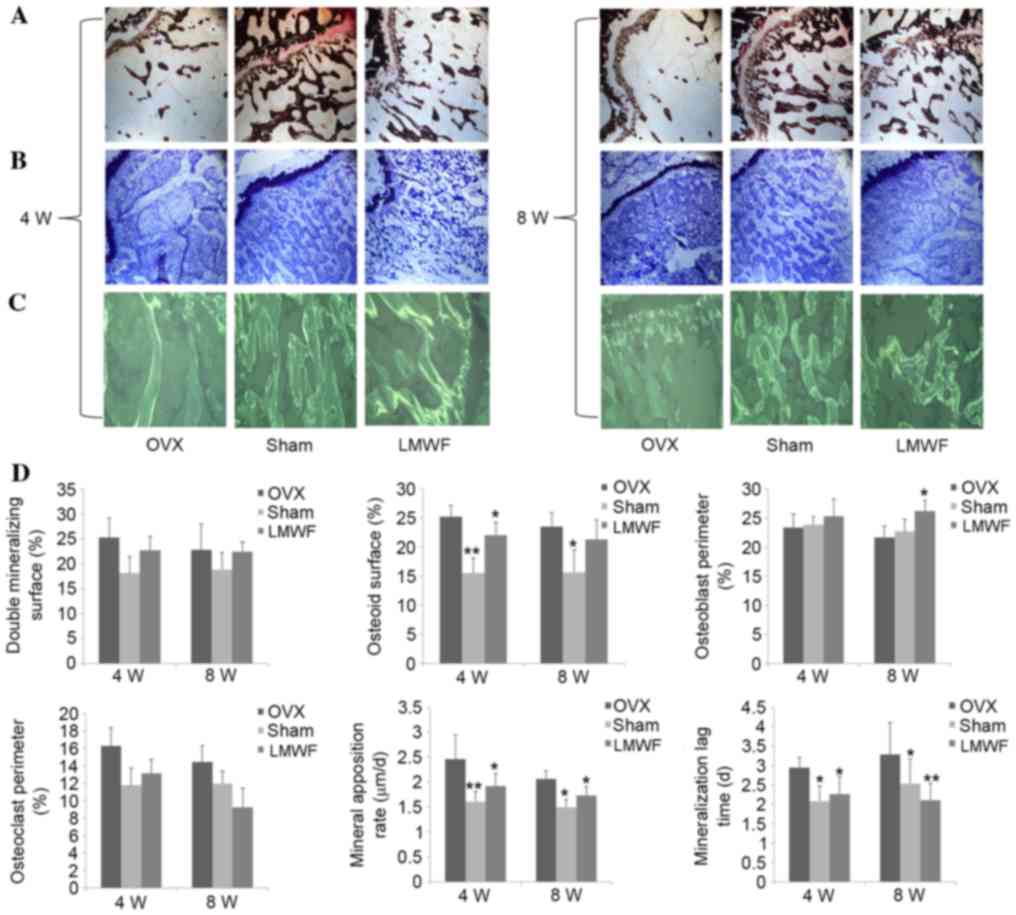
Histomorphometric analysis
Fig. 4 presents the
quantitative histomorphometric analyses of the undecalcified
histological sections stained with (A) von Kossa and (B) Giemsa.
The OVX group exhibited higher tetracycline labeling (Fig. 4C), thicker osteoid seams, and a
higher rate of mineralization on osteoblast surfaces and osteoclast
surfaces when compared with the SHAM group (Fig. 4D; P<0.05), which was consistent
with the high bone turnover usually observed in osteoporosis. LMWF
administration reduced bone turnover rate in OVX rats; however, the
surface area for bone formation and the mineralization time was
increased compared with the OVX group (Fig. 4D; P<0.05).
Mechanical testing
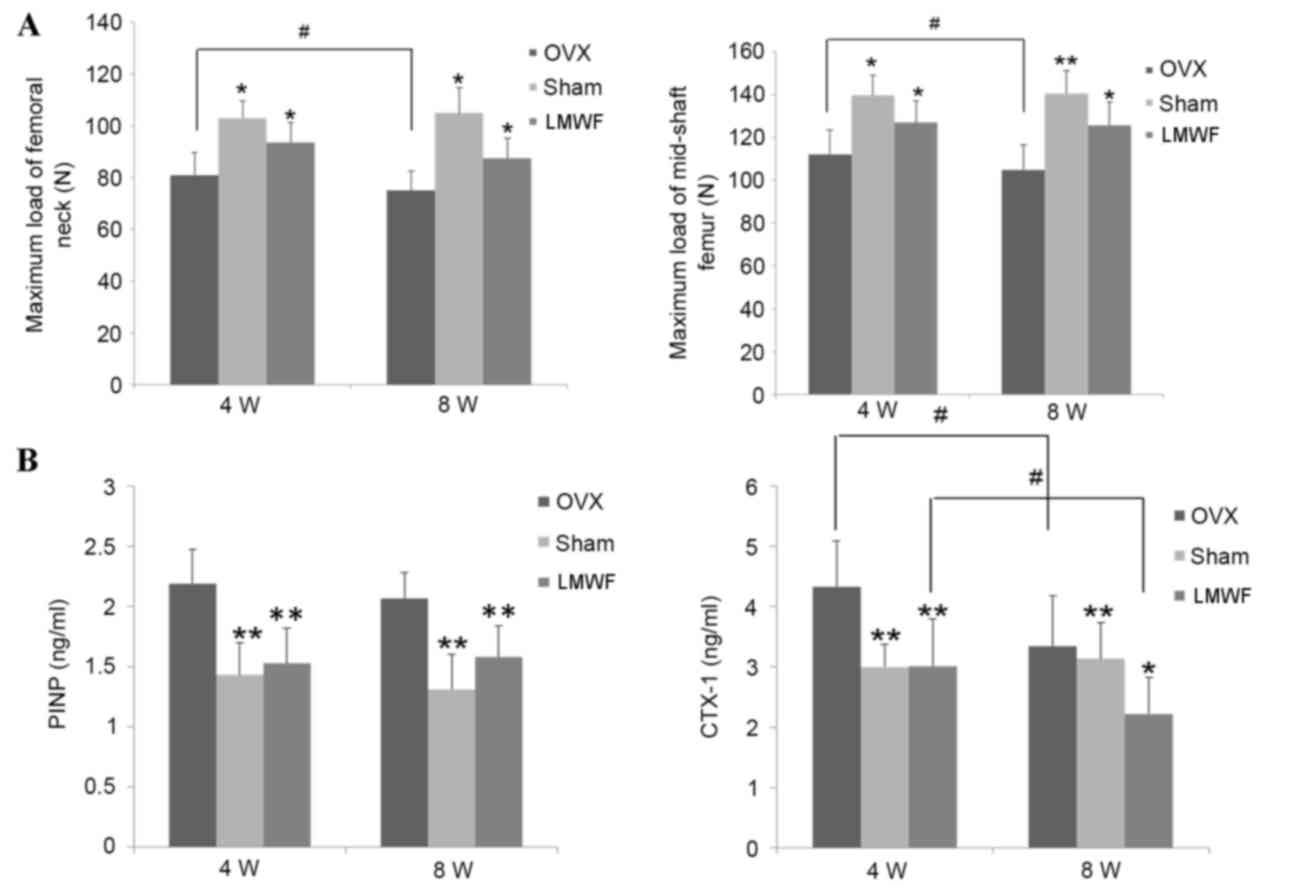
As presented in Fig.
5A, the maximal strength of the femur as assessed by the
three-point bending test and compression test was significantly
lower in the OVX group when compared with the SHAM group rats
(P<0.05). The maximum load of femoral neck in the OVX group was
significantly reduced 8 weeks after surgery when compared with 4
weeks after surgery (P<0.05). Mechanical strength of femoral
neck and mid-shaft femoral in OVX rats was lower compared with the
SHAM group (P<0.05; Fig. 5A).
The LMWF group demonstrated higher femoral mechanical strength of
femoral neck and mid-shaft femoral in rats 4 and 8 weeks after
ovariectomy when compared with the OVX group (P<0.05; Fig. 5A).
Serum analysis
The OVX group had significantly increased levels of
CTX-1 and PINP in serum compared with the SHAM group rats at 4 and
8 weeks after the operation. As presented in Fig. 5B LMWF treatment significantly
decreased the levels of serum PINP (P<0.01 for both time-points)
and CTX-1 (P<0.01 at 4 weeks and P<0.05 at 8 weeks) compared
with the OVX group rats.
Discussion
The present study determined that LMWF inhibits the
induction of RAW264.7 cells into mature osteoclasts by RANKL and
M-CSF in vitro. The in vivo experiments using an
osteoporosis animal model confirmed that LMWF inhibited bone loss
and prevented microarchitectural deterioration, and increased
femoral mechanical strength following ovariectomy. To the best of
our knowledge, the present study is the first to determine the
potential use of LMWF for the treatment of osteoporosis.
M-CSF and RANKL are considered the key signaling
factors in the process of osteoclast differentiation (9). RANKL is essential for
osteoclastogenesis and the subsequent bone resorption (8), whereas M-CSF may stimulate the
movement and expansion of osteoclasts, inhibit their apoptosis and
enhance RANKL-induced osteoclast formation (7,23).
TRAP staining is a commonly used method to identify osteoclasts
(24) and the present study
revealed that following RANKL and M-CSF administration,
TRAP-positive osteoclast-like cells were observed 2 days after the
induction, and a greater number was observed 6 days after the
induction. Examination of TRAP-stained slides and thin osteologic
slides for bone resorption assay revealed that the presence of LMWF
in addition to RANKL and M-CSF resulted in fewer mature osteoclasts
and reduced bone resorption pits compared with the untreated
control group.
MMP-9 is secreted by osteoclasts, and in addition to
degradation of the matrix, aids in osteoclast movement (25,26).
The present study determined that LMWF reduced the expression
levels of TRAP and MMP-9. NFATc1 is an important transcription
factor for osteoclastogenesis, and is involved in the regulation of
the expression of numerous osteoclast-specific genes; therefore, it
has an impact on osteoclast cell formation and function (27,28).
Cytoplasmic NF-κB is activated during osteoclast differentiation
following RANKL stimulation and may result in activation of
intracellular signaling cascades. The activated NF-κB is
subsequently translocated to the nucleus with NFATc2, to activate
expression and auto-amplification of NFATc1 (29,30).
The present study revealed that LMWF may significantly inhibit
NFATc1 expression in osteoclasts. RANK is a type I membrane
receptor protein on osteoclasts and their precursor cells; the
binding of RANK and RANKL subsequently activates the signal
transduction pathway in precursor cells, resulting in osteoclast
maturation. In mature osteoclasts, this activated pathway serves to
maintain bone resorption activity and survival of osteoclasts
(31). In the present study, LMWF
had no effect on RANK mRNA expression. Therefore, it is also
possible that it does not affect osteoclast cell membrane RANK
receptors. OSCAR is an osteoclast-specific receptor that was
recently identified (32) and is a
type of leukocyte receptor that may be an important factor in
inducing osteoclastogenesis (32,33).
The present study determined that LMWF inhibited OSCAR mRNA
expression; therefore, it may also inhibit RANKL- and M-CSF-induced
differentiation of RAW264.7 cells to osteoclasts, thus reducing the
number of osteoclasts and the rate of bone resorption.
RANKL-induced activation of the NF-κB pathway is a
key factor in osteoclast differentiation (34). NFATc1 is likely to be the key
factor regulating RANKL-induced osteoclast differentiation, cell
fusion and activation (28). NF-κB
induction of NFATc1 is important and may occur via NF-κB binding to
the promoter region of NFATc1, leading to increased NFATc1
expression and subsequently, RANKL-induced osteoclast
differentiation (35). The present
study revealed that LMWF inhibited RANKL-induced osteoclast
formation.
In the present study, the in vivo
consequences of the in vitro observations were investigated
in an ovariectomized rat osteoporosis model. The effects of LMWF on
BMD, bone microarchitecture, remodeling and mechanical competence
were investigated. The results demonstrated that 4 weeks after
treatment with LMWF, BMD was higher in the LMWF group when compared
with the OVX group, and 8 weeks after surgery BMD increased
further.
BMD has been identified as an important factor for
the evaluation of osteoporosis-related fractures; a previous study
revealed that in accordance with BMD criteria, only 10–53% of
patients are diagnosed with osteoporosis following an osteoporotic
fracture (36). Micro-CT
voxel-based test unit may be used for the early detection of
lesions and bone structure deterioration (37,38).
The micro-CT scans performed in the present study revealed that the
trabecular microarchitecture in the distal femur of ovariectomized
rats was deteriorated 4 weeks after surgery, whereas the SHAM group
exhibited no significant changes. However, the parameters of
trabecular microarchitecture were all improved in the LMWF group
compared with the OVX group. When the treatment time was extended
to 8 weeks following surgery, BV/TV and Tb.N. exhibited further
improvement. This may suggest that LMWF prevented bone loss due to
ovariectomy and may also promote bone regeneration, and that bone
microarchitectural recovery may be directly associated with
treatment duration. The 3D reconstituted distal femur revealed that
in the OVX group, the quantity of trabecular bone and connectivity
were decreased and trabecular bone separation increased when
compared with the SHAM group. In the LMWF group, the quantity of
trabecular bone increased, spacing narrowed and connectivity
recovered. Histophotometry was used in order to evaluate bone
structure further.
LMWF it inhibited high bone turnover rate, which is
usually the result of an ovariectomy, increased the osteoid and
bone formation surface areas (osteoblast perimeter), decreased the
surface for bone resorption (osteoclast perimeter), inhibited
mineralization, and increased mineralization time after
ovariectomy. Collectively, these results suggested that LMWF
inhibited the high bone turnover rate and bone loss associated with
ovariectomy.
Consistent with the histomorphometry results, it was
demonstrated that the OVX group had increased bone turnover, since
the levels of PINP and CTX-1 were increased, this was also in line
with a previous study (39). LMWF
attenuated the increase in PINP and CTX-1 serum levels, thus
suggesting that LMWF exerts its effects on bone metabolism by
inhibiting bone turnover.
The three-point bending test and the femoral neck
compression test were used to evaluate the effects of LMWF on the
mechanical strength of the femoral shaft and femoral neck. The
present study determined that 4 weeks after ovariectomy, the
mechanical strength of femurs in the OVX group was significantly
reduced. The mechanical strength in the OVX group at the 8-week
time-point was lower than that at 4 weeks, which may be a result of
weight gain 8 weeks after surgery, this phenomenon is itself likely
a self-protective mechanism in which bone mass is augmented
(40). The mechanical strength of
the bones from the LMWF group was significantly increased 4 weeks
after the surgery when compared with the OVX group. The 8-week
time-point had a further increase in mechanical strength.
Therefore, it is evident that LMWF may increase the mechanical
strength of the femoral shaft and neck of ovariectomized rats.
In conclusion, LMWF may inhibit osteoclast precursor
differentiation, osteoclast maturation and bone resorption,
ameliorate loss of BMD and trabecular deterioration, and prevent
loss of mechanical competence. These findings suggested that LMWF
may be a novel therapeutic agent for the treatment of
postmenopausal osteoporosis. Additional research is required to
determine the optimal dosage, length of treatment and expected
duration of effect for each dose administered.
References
|
1
|
Melton LJ III, Chrischilles EA, Cooper C,
Lane AW and Riggs BL: How many women have osteoporosis? JBMR
Anniversary Classic. JBMR. 7(9)1992.J Bone Miner Res. 20. 886–892.
2005.
|
|
2
|
Sowers MR, Zheng H, Jannausch ML,
McConnell D, Nan B, Harlow S and Randolph JF Jr: Amount of bone
loss in relation to time around the final menstrual period and
follicle-stimulating hormone staging of the transmenopause. J Clin
Endocrinol Metab. 95:2155–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ritchie RO, Buehler MJ and Hansma P:
Plasticity and toughness in bone. Phys Today. 62:41–47. 2009.
View Article : Google Scholar
|
|
4
|
Zimmermann EA, Schaible E, Bale H, Barth
HD, Tang SY, Reichert P, Busse B, Alliston T, Ager JW III and
Ritchie RO: Age-related changes in the plasticity and toughness of
human cortical bone at multiple length scales. Proc Natl Acad Sci
USA. 108:14416–14421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fitzpatrick P, Kirke PN, Daly L, Van Rooij
I, Dinn E, Burke H, Heneghan J, Bourke G and Masterson J:
Predictors of first hip fracture and mortality post fracture in
older women. Ir J Med Sci. 170:49–53. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuo K: Cross-talk among bone cells.
Curr Opin Nephrol Hypertens. 18:292–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amano H, Yamada S and Felix R:
Colony-stimulating factor-1 stimulates the fusion process in
osteoclasts. J Bone Miner Res. 13:846–853. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boonen S, Reginster JY, Kaufman JM,
Lippuner K, Zanchetta J, Langdahl B, Rizzoli R, Lipschitz S, Dimai
HP, Witvrouw R, et al: Fracture risk and zoledronic acid therapy in
men with osteoporosis. N Engl J Med. 367:1714–1723. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong HS, Venkatesan J and Kim SK:
Hydroxyapatite-fucoidan nanocomposites for bone tissue engineering.
Int J Biol Macromol. 57:138–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thinh PD, Menshova RV, Ermakova SP,
Anastyuk SD, Ly BM and Zvyagintseva TN: Structural characteristics
and anticancer activity of fucoidan from the brown alga sargassum
mcclurei. Mar Drugs. 11:1456–1476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Changotade S, Korb G, Bassil J, Barroukh
B, Willig C, Colliec-Jouault S, Durand P, Godeau G and Senni K:
Potential effects of a low-molecular-weight fucoidan extracted from
brown algae on bone biomaterial osteoconductive properties. J
Biomed Mater Res A. 87:666–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park HS, Hwang HJ, Kim GY, Cha HJ, Kim WJ,
Kim ND, Yoo YH and Choi YH: Induction of apoptosis by fucoidan in
human leukemia U937 cells through activation of p38 MAPK and
modulation of Bcl-2 family. Mar Drugs. 11:2347–2364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SB, Chun KR, Kim JK, Suk K, Jung YM
and Lee WH: The differential effect of high and low molecular
weight fucoidans on the severity of collagen-induced arthritis in
mice. Phytother Res. 24:1384–1391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan-li C, Pei-pei W, Jun-ming Z and
Song-song Z: Jian-wei: Extraction of fucoidan using multienzyme
enzymolysis and their structural analysis, antioxidation research.
Journal of Zhejiang University (Science Edition). 38:536–540.
2011.(In Chinese).
|
|
19
|
Mar Arriero M, Ramis JM, Perelló J and
Monjo M: Inositol Hexakisphosphate inhibits osteoclastogenesis on
RAW 264.7 cells and human primary osteoclasts. PloS One.
7:e431872012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carbonare L Dalle, Valenti MT, Bertoldo F,
Zanatta M, Zenari S, Realdi G, Lo Cascio V and Giannini S: Bone
microarchitecture evaluated by histomorphometry. Micron.
36:609–616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Z and Fu Q: Comparison of the
therapeutic effects of yeast-incorporated gallium with those of
inorganic gallium on ovariectomized osteopenic rats. Biol Trace
Elem Res. 134:280–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lum L, Wong BR, Josien R, Becherer JD,
Erdjument-Bromage H, Schlöndorff J, Tempst P, Choi Y and Blobel CP:
Evidence for a role of a tumor necrosis factor-alpha
(TNF-alpha)-converting enzyme-like protease in shedding of TRANCE a
TNF family member involved in osteoclastogenesis and dendritic cell
survival. J Biol Chem. 274:13613–13618. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cole AA and Walters LM: Tartrate-resistant
acid phosphatase in bone and cartilage following decalcification
and cold-embedding in plastic. J Histochem Cytochem. 35:203–206.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakrabarti S and Patel KD: Matrix
metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp
Lung Res. 31:599–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delaissé JM, Engsig MT, Everts V, del
Carmen Ovejero M, Ferreras M, Lund L, Vu TH, Werb Z, Winding B,
Lochter A, et al: Proteinases in bone resorption: Obvious and less
obvious roles. Clinica Chimica Acta. 291:223–234. 2000. View Article : Google Scholar
|
|
27
|
Ishida N, Hayashi K, Hoshijima M, Ogawa T,
Koga S, Miyatake Y, Kumegawa M, Kimura T and Takeya T: Large scale
gene expression analysis of osteoclastogenesis in vitro and
elucidation of NFAT2 as a key regulator. J Biol Chem.
277:41147–41156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asagiri M, Sato K, Usami T, Ochi S,
Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E and
Takayanagi H: Autoamplification of NFATc1 expression determines its
essential role in bone homeostasis. J Exp Med. 202:1261–1269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsuo K, Galson DL, Zhao C, Peng L,
Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H
and Wagner EF: Nuclear factor of activated T-cells (NFAT) rescues
osteoclastogenesis in precursors lacking c-Fos. J Biol Chem.
279:26475–26480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Q, Wang X, Liu Y, He A and Jia R:
NFATc1: Functions in osteoclasts. Int J Biochem Cell Biol.
42:576–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nemeth K, Schoppet M, Al-Fakhri N, Helas
S, Jessberger R, Hofbauer LC and Goettsch C: The role of
osteoclast-associated receptor in osteoimmunology. J Immunol.
186:13–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barrow AD, Raynal N, Andersen TL, Slatter
DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, et al:
OSCAR is a collagen receptor that costimulates osteoclastogenesis
in DAP12-deficient humans and mice. J Clin Invest. 121:3505–3516.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu M, Qi X, Moreno JL, Farber DL and
Keegan AD: NF-κB signaling participates in both RANKL-and
IL-4-induced macrophage fusion: Receptor cross-talk leads to
alterations in NF-κB pathways. J Immunol. 187:1797–1806. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liou SF, Hsu JH, Lin IL, Ho ML, Hsu PC,
Chen LW, Chen IJ and Yeh JL: KMUP-1 suppresses RANKL-induced
osteoclastogenesis and prevents ovariectomy-induced bone loss:
Roles of MAPKs, Akt, NF-κB and calcium/calcineurin/NFATc1 pathways.
PloS One. 8:e694682013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McNamara LM: Perspective on
post-menopausal osteoporosis: Establishing an interdisciplinary
understanding of the sequence of events from the molecular level to
whole bone fractures. J R Soc Interface. 7:353–372. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brouwers JEM, Lambers FM, Gasser JA, van
Rietbergen B and Huiskes R: Bone degeneration and recovery after
early and late bisphosphonate treatment of ovariectomized wistar
rats assessed by in vivo micro-computed tomography. Calcif Tissue
Int. 82:202–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boyd SK, Davison P, Müller R and Gasser
JA: Monitoring individual morphological changes over time in
ovariectomized rats by in vivo micro-computed tomography. Bone.
39:854–862. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoon KH, Cho DC, Yu SH, Kim KT, Jeon Y and
Sung JK: The change of bone metabolism in ovariectomized rats:
Analyses of microCT scan and biochemical markers of bone turnover.
J Korean Neurosurg Soc. 51:323–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wronski TJ, Schenk PA, Cintrón M and Walsh
CC: Effect of body weight on osteopenia in ovariectomized rats.
Calcif Tissue Int. 40:155–159. 1987. View Article : Google Scholar : PubMed/NCBI
|