Introduction
Colon cancer is a common cancer worldwide and
remains the third most common cause of cancer-associated mortality
in the United States. It accounts for >50,000 mortalities and
~137,000 cases are diagnosed each year (1). Currently, more patients with colon
cancer are diagnosed at the earlier stage, however the chemotherapy
is often ineffective in patients with colon cancer, because colon
cancer has a high recurrence rate (2). Thus, it is important to develop
novel, effective drugs to treat colon cancer.
Scutellaria barbata D. Don is a natural
medicinal herb prevalent in Korea and southern China, and used to
treat ischemia heart diseases, neurological disorders, hepatitis,
inflammation and osteomyelitis (3,4). As
a major type of active compound, Scutellarin has been reported to
produce many biological activities, including anti-oxidative,
anti-inflammatory, cardioprotective effects, and also effects
against human immunodeficiency virus (5–7). It
was previously reported that Scutellarin also exhibited a potent
ability to inhibit the growth of colon cancer, tongue carcinoma and
squamous cell carcinoma (8,9).
However, whether Scutellarin produces therapeutic effects on colon
cell carcinoma and the molecular mechanisms involved have not been
elucidated. Thus, the current study aimed to elucidate these
effects and the molecular mechanisms.
The present study reported that Scutellarin inhibits
the growth of colon cancer cells and induces apoptosis. The
molecular mechanisms may be associated with the activation of p53
and the regulation of Bcl-2 apoptosis regulator (Bcl-2) and Bcl-2
associated X apoptosis regulator (Bax). These findings suggest that
Scutellarin may be useful as a therapeutic drug for colon
cancer.
Materials and methods
Reagents
Scutellarin was purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany) and was dissolved in dimethyl
sulfoxide (DMSO; less than 0.1%, v/v, without detectable effects)
for all experiments of the current study. DMSO was used as the
control treatment. The pifithrin-α was purchased from
Sigma-Aldrich; Merck Millipore (Taufkirchen, Germany) and dissolved
in DMSO to a final concentration of 50 mM. All other reagents were
purchased from Sigma-Aldrich; Merck Millipore unless specifically
noted.
Cell culture
The HCT-116 human colon carcinoma cell line was
purchased from American Type Culture Collection (Manassas, VA,
USA), and cultured with Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck Millipore) supplemented with 10% fetal bovine
serum (Sigma-Aldrich; Merck Millipore) and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The HCT-116 cells were cultured as
monolayer in an incubator at 37°C in a humidified atmosphere of 5%
CO2 and 95% air. The culture medium was changed every
two days.
Cell viability assay
Evaluation of cellular viability of HCT-116 cells
was performed using an MTT assay. The HCT-116 cells were seeded in
96-well plates, at a density of 1,500 cells/well, and incubated
overnight. The cells were exposed to Scutellarin (10, 30 and 100
µM) for 48 h. Fresh DMEM containing 5 mg/ml MTT (Sigma-Aldrich;
Merck Millipore) were introduced to the cells at 37°C for 4 h. DMSO
(100 µM; Sigma-Aldrich; Merck Millipore) was then added to
solubilize the MTT product. The absorbance was measured at 540 nm
with a background subtraction at 650 nm using an EMax Endpoint
Microplate Reader (Molecular Devices LLC, Sunnyvale, CA, USA). This
assay was repeated five times.
Hoechst 33342 dye staining
Morphological evaluation of apoptosis of HCT-116
cells was performed using on Hoechst 33342 staining (Invitrogen;
Thermo Fisher Scientific, Inc.). HCT-116 cells (5×105
cells/well) were incubated in the absence and presence of
Scutellarin (10, 30 and 100 µM) for 48 h. The HCT-116 cells were
then fixed in 4% paraformaldehyde at room temperature for 30 min
and rinsed with phosphate-buffered saline (PBS). The fixed HCT-116
cells were exposed to Hoechst 33342 (20 µg/ml) at room temperature
for 15 min. Apoptotic morphological changes of HCT-116 cells were
observed using an inverted fluorescence microscope. This assay was
repeated five times.
Acridine orange/ethidium bromide
(AO/EB) double staining
The AO/EB (Sigma-Aldrich; Merck Millipore) stain was
used to detect the apoptosis of cancer cells. HCT-116 cells
(5×105 cells/well) were seeded in 6-well plates before
they were incubated with 10 µl prepared AO/EB working solution (100
µg/ml AO and 100 µg/ml EB in PBS) for 5 min. The nuclear
alterations and apoptotic body formation of HCT-116 cells were
visualized immediately using an inverted fluorescence microscope
(Eclipse TE300; Nikon Corporation, Tokyo, Japan). This assay was
repeated three times.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Apoptosis of HCT-116 cells was determined using a
In Situ Cell Death Detection kit (Roche Applied Science,
Penzberg, Germany) according to the manufacturer's protocol.
Following exposure to Scutellarin (10, 30 and 100 µM) for 24 h,
HCT-116 cells (1×106) were fixed with 4%
paraformaldehyde in PBS for 1 h at room temperature, and
permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2
min on ice. Then the HCT-116 cells were treated with the prepared
TUNEL reaction mixture for 1 h at 37°C in the dark, and TUNEL
staining was visualized using a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The TUNEL assay was performed three
times.
Western blot analysis
Following treatment with Scutellarin for 24 h,
HCT-116 cells (1×106) were homogenized on ice, and the
cell lysates were prepared by centrifugation at 14,000 × g for 10
min at 4°C. The protein concentration was quantified using the
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Inc.).
Equal quantities of protein (60 µg) were separated using SDS-PAGE
(10–12%) and then transferred onto a nitrocellulose membrane. The
blots were blocked with 5% non-fat milk for 1 h at room temperature
and then washed three times with PBS (Sigma-Aldrich; Merck
Millipore) supplemented with 0.1% Tween-20 (PBS-T; Sigma-Aldrich;
Merck Millipore), before they were incubated with primary
antibodies against phosphorylated p53, p53, Bcl-2, Bax, p21 and
cleaved caspase-3 for 2 h at room temperature. After washing with
three times with PBS-T, Membranes were subsequently incubated with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody [cat. no. sc-2005; dilution, 1:5,000 in 5% bovine serum
albumin (BSA); Santa Cruz Biotechnology, Inc., Dallas, TX, USA] and
anti-mouse IgG (cat. no. sc-2030; dilution, 1:5,000 in 5% BSA;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature, before
they were incubated with horseradish peroxidase conjugate (GE
Healthcare Life Sciences, Chalfont, UK). The bands were detected by
chemiluminescence using an ECL kit (GE Healthcare Life Sciences).
ImageJ software (version, 1.50; National Institutes of Health,
Bethesda, MD, USA) was used to quantify the expression of proteins
based on the intensity of the bands. The experiments were repeated
three times. The mouse monoclonal p53 (cat. no. sc-98; dilution,
1:500), mouse monoclonal phosphorylated p53 (cat. no. sc-99;
dilution, 1:200), mouse monoclonal Bcl-2 (cat. no. sc-7382;
dilution, 1:500), mouse monoclonal Bax (cat. no. sc-23959;
dilution, 1:200), mouse monoclonal p21 (cat. no. sc-6246; dilution,
1:200), rabbit polyclonal cleaved caspase-3 (cat. no. sc-22171;
dilution, 1:500) antibodies were obtained from Santa Cruz
Biotechnology, Inc., and mouse monoclonal β-actin (cat. no. AC-15;
dilution, 1:2,000) was purchased from Sigma-Aldrich; Merck
Millipore.
Statistical analysis
Data used for statistical analysis are expressed as
the mean ± standard error. Significant differences among groups was
determined using Bonferroni-corrected analysis of variance. All
statistical analysis was performed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistical significant difference.
Results
Scutellarin inhibits the growth of
HCT-116 cells
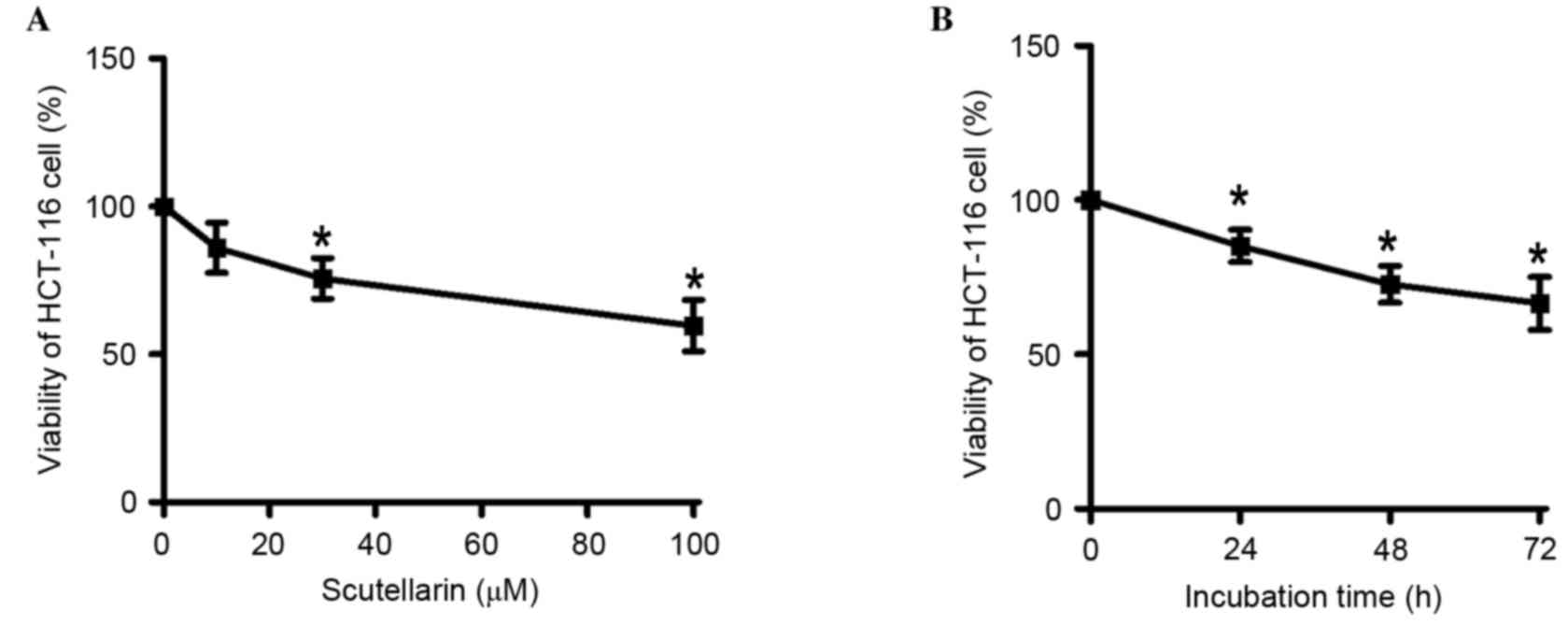
The effect of Scutellarin on the viability of
HCT-116 cells was evaluated using an MTT assay. As demonstrated in
Fig. 1A, 10, 30 and 100 µM
Scutellarin resulted in a significant reduction in the viability of
HCT-116 cells when compared with control cells (P=0.0578, P=0.0062
and P=0.0023, respectively). The reduction in HCT-116 cell
viability was concentration-dependent. The viability of HCT-116
cells was then determined following treatment with 30 µM
Scutellarin for 24, 48 and 72 h (Fig.
1B). It was demonstrated that 30 µM Scutellarin gradually
decreased the viability of HCT-116 cells and suppressed cellular
growth with increasing incubation time, when compared with the 0
h-time point (24 h, P=0.0131; 48 h, P=0.0025; 72 h, P=0.0044).
These results suggest that Scutellarin demonstrated
antiproliferative activities in HCT-116 cells.
Scutellarin induces apoptosis of
HCT-116 cells
Additionally, the current study investigated whether
the apoptosis HCT-116 cells was induced by Scutellarin treatment.
The nucleolar changes of HCT-116 cells were detected using a
fluorescent microscope following Hoechst 33342 (Fig. 2A) and AO/EB (Fig. 2B) staining. Scutellarin-treated
HCT-116 cells exhibited condensation of chromatin and pyknosis of
nuclei (Fig. 2A and B). By
contrast, untreated HCT-116 cells exhibited intact nuclear
architecture. That Scutellarin induced the apoptosis of HCT-116
cells was further confirmed using a TUNEL assay. Untreated HCT-116
cells were predominantly are negative for TUNEL fluorescence,
however Scutellarin treatment markedly increased the number of
TUNEL-positive HCT-116 cells compared with untreated cells
(Fig. 2C). These results confirmed
that Scutellarin increased apoptosis of human colon cancer
cells.
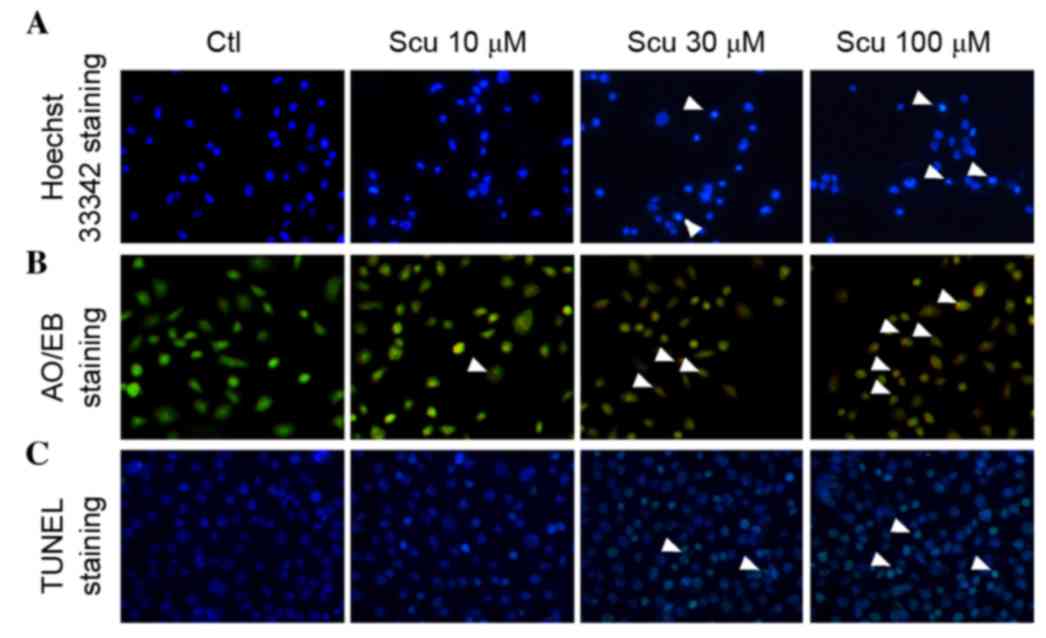 | Figure 2.Effects of Scu on the apoptosis of
HCT-116 cells. (A) Evaluation of HCT-116 apoptosis by Hoechst 33342
staining following Scu (10, 30 and 100 µM) treatment
(magnification, ×200). White arrows indicate abnormal nuclei. (B)
Apoptosis of HCT-116 cells caused by Scu (10, 30 and 100 µM) as
demonstrated by AO/EB staining (magnification, ×200). White arrows
indicate AO/EB-positive cells. (C) TUNEL staining was used to
assess the effects of Scu (10, 30 and 100 µM) on HCT-116 apoptosis
(magnification, ×200). White arrows indicate TUNEL-positive cells.
Ctl, control; Scu, Scutellarin; AO/EB, acridine orange/ethidium
bromide; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end
labeling. |
Scutellarin regulates Bcl-2 and Bax
expression in HCT-116 cells
The inactivation of Bcl-2 and the activation of Bax
commit cancer cells to undergo apoptosis (10). Thus, the effect of Scutellarin on
the expression levels of Bcl-2 and Bax in HCT-116 cells were
investigated. HCT-116 cells were exposed to 10, 30 and 100 µM
Scutellarin for 48 h, and the expression of Bcl-2 protein was
significantly decreased when compared with untreated HCT-116 cells
(P=0.3036, P=0.0890 and P=0.0093, respectively; Fig. 3A). Consistently, the expression of
Bax protein in HCT-116 cells was increased significantly in the
presence of 10 (P=0.011), 30 (P=0.0003) and 100 µM (P=0.0002)
Scutellarin when compared with the control. The changes in the
levels of Bcl-2 and Bax protein expression in HCT-116 cells were
associated with the concentration of Scutellarin used.
Additionally, the active form of caspase-3 protein was also
increased in HCT-116 treated with Scutellarin (Fig. 3B). These results suggest that
Scutellarin induces apoptosis of HCT-116 cells via regulating
Bcl-2/Bax expression and activating caspase-3.
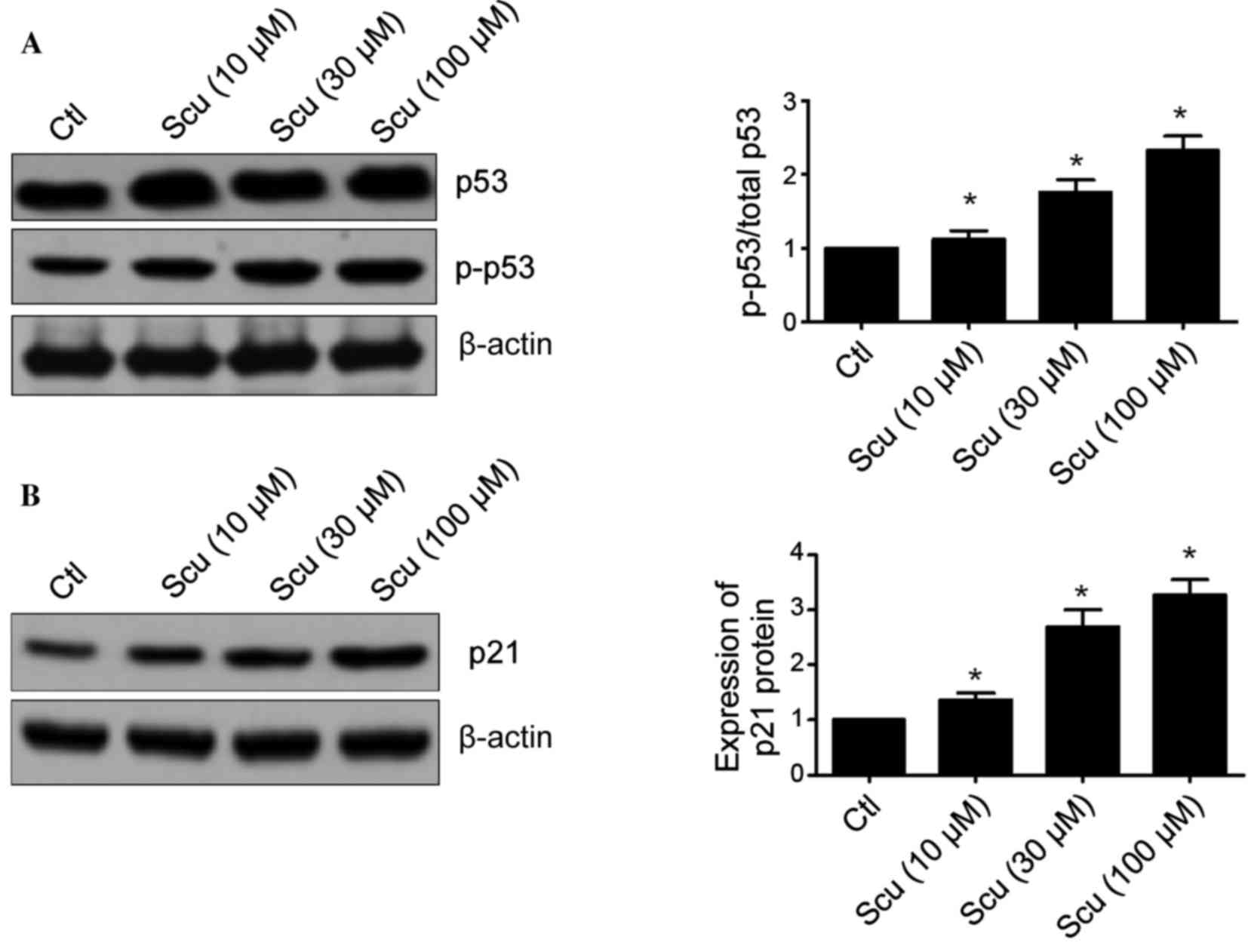
Scutellarin affects the expression of
p53 and p21 in HCT-116 cells
Lots of studies have demonstrated that p53 is an
important tumor suppressor gene and its inactivation is involved in
tumorigenesis and chemotherapy resistance (11–13).
The effects of Scutellarin on the expression of p53 and p21
proteins were investigated. Fig.
4A demonstrated that Scutellarin significantly increased
phosphorylation of p53 in HCT-116 cells when compared with the
control (10 µM, P=0.0035; 30 µM, P=0.0005; and 100 µM, P=0.0002).
p21 is a downstream target of the p53 pathway (12). Scutellarin-treated HCT-116 cells
exhibited a significantly decreased level of p21 protein expression
when compared with the control (10 µM, P=0.0096; 30 µM, P=0.0011;
and 100 µM, P=0.0002; Fig. 4B).
These results suggest that the p53 pathway may be involved in the
Scutellarin-induced apoptosis of HCT-116 cells.
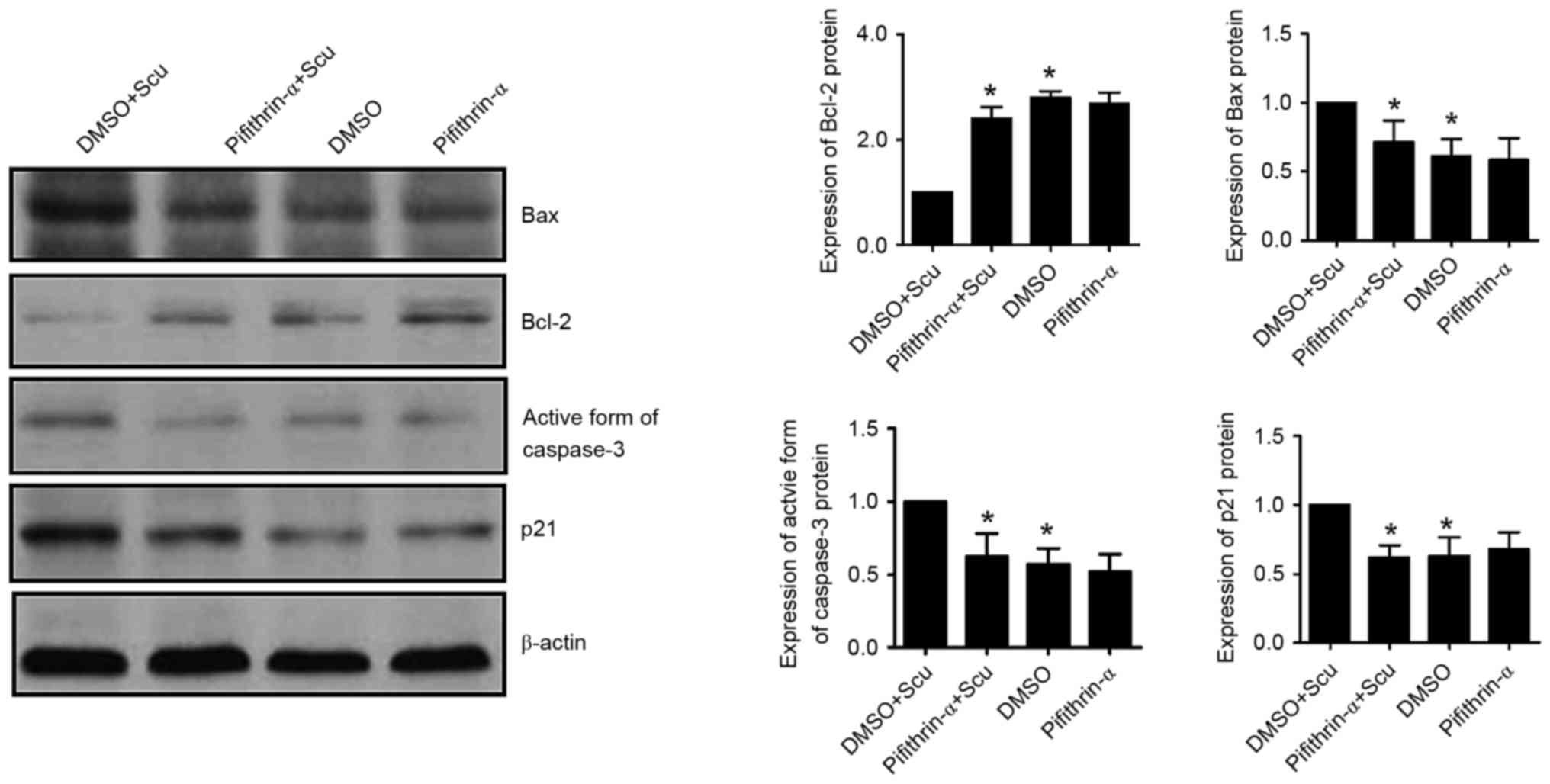
Scutellarin-induced apoptosis of
HCT-116 was abrogated by suppressing p53
To further investigate the role of p53 in
Scutellarin-induced apoptosis of HCT-116, the effects of a p53
inhibitor, pifithrin-α, on Scutellarin-induced apoptosis of HCT-116
was determined in the current study. Fig. 5 demonstrated that the p53
inhibitor, pifithrin-α (50 µM), abrogated the increase in Bax
protein (P=0.0434) and decrease of Bcl-2 protein (P=0.0007) induced
by 30 µM Scutellarin. In addition, the increase in the active form
of caspase-3 induced by Scutellarin treatment was significantly
abrogated by pifithrin-α in HCT-116 cells (P=0.0212). Furthermore,
p21 protein expression was reduced by pifithrin-α treatment in
HCT-116 cells when compared with the levels in Scutellarin-treated
cells (P=0.0032).
Discussion
Colon cancer is the third most common type of
cancer, and >149,000 patients are diagnosed every year worldwide
(14). In recent years, the
occurrence of colon cancer has gradually increased. Currently, the
therapeutics used to treat colon cancer are based on surgery
combined with adjuvant chemotherapy (15). However, often these treatments fail
to control the recurrence of colon cancer, as colon tumor cells
develop resistance to the majority chemotherapy drugs (13). Thus, it is necessary to elucidate
novel drugs to treat colon cancer.
Scutellarin is a major active compound extracted
from Scutellaria barbata D. Don, an herbal plant that has
been used for centuries to treat various aliments (4,8).
Scutellarin has various biological activates, including
anti-oxidant, anti-inflammatory and antibacterial effects, and
thus, has been used in the clinical treatment of coronary heart
diseases, cerebral thrombosis, cerebral infarction and hypertension
(6–9). It was previously reported that
Scutellarin induced the apoptosis of colon cancer, lymphoma and
tongue cancer cells, with promising potential application in the
clinic (8,9). For instance, Scutellarin suppresses
the growth and induces apoptosis of human U937 leukemia cells via
the mitochondrial apoptosis pathway (16). In addition, Scutellarin was
demonstrated to effectively sensitize resveratrol and
5-fluorouracil-stimulated colon cancer cell apoptosis via enhancing
caspase-6 activation (9). In
vivo studies also confirmed that Scutellarin combined with
ultrasound significantly delayed tumor growth, inhibited tumor
angiogenesis and caused cancer-cell apoptosis of human tongue
carcinoma xenografts via decreasing the expression of matrix
metalloproteinase 2 and 9 (17).
To the best of our knowledge, the current study was
the first to demonstrated the antiproliferative and pro-apoptotic
effects of Scutellarin on human colon cancer cells. The present
study demonstrated that Scutellarin significantly reduced the
viability of HCT-116 cells in a time- and dose-dependent manner.
Reduced cell viability was observed even at the lowest
concentration of Scutellarin used (10 µM). Tumor cells with
inhibited growth are usually prone to undergo apoptosis (18). Thus, the current study determined
whether Scutellarin treatment induced apoptosis in HCT-116 cells,
which may explain the observed loss of viability. Hoechst 33342
staining and AO/EB staining demonstrated nuclear changes and
apoptotic body formation in HCT-116 cells. Treatment with
Scutellarin also increased the number of apoptotic cells, as
assessed by TUNEL staining. These findings confirmed that
Scutellarin was able to cause apoptosis of human colon cancer
cells.
Subsequently, the further analsyis was performed to
understand the molecular mechanism underlying the anti-tumor effect
of Scutellarin on human colon cancer. Carcinogenesis or
tumorigenesis is closely associated to the uncontrolled growth of
tumor cells and the loss of tumor cells apoptosis (19). Upregulated expression of the
anti-apoptotic protein, Bcl-2, and the downregulation of
pro-apoptotic protein, Bax, are involved in the initiation and
aggression of tumors (10,20). Conversely, inhibiting Bcl-2 or
increasing Bax expression was suggested as an important approach
for treating cancer (10). The
current study demonstrated that Scutellarin treatment reduced the
expression of Bcl-2 protein and increased the expression of Bax
protein in colon cancer cells. Additionally, the protein level of
the active form of caspase-3, a downstream target of Bcl-2 and Bax
the apoptotic pathway was also demonstrated to be increased by
Scutellarin treatment (21). These
results suggested that the regulation of Bcl-2/Bax expression and
the activation of caspase-3 protein were a molecular mechanism
underlying the Scutellarin-induced apoptosis of human colon cancer
cells.
Various studies have demonstrated that p53 is an
important tumor suppressor gene, and regulates cell cycle,
apoptosis, metastasis and senescence (11,12).
Inactivation or mutations of p53 have been well documented in human
tumors (12). By contrast,
overexpression or activation of p53 can induce cell apoptosis, and
attenuate cancer cell migration and invasion through regulating
numerous targets, including Bcl-2 and Bax (22). Also, p53 induces the apoptosis of
tumor cells via regulating the transcription of p21 (19). The current study demonstrated that
Scutellarin induced an increase in the ratio of phosphorylated p53
to total p53, which was accompanied by an upregulation in p21
protein expression. Further investigation demonstrated that
inhibition of p53 led to a reduction in the expression of
pro-apoptotic proteins (Bax and caspase-3) and an increase in the
expression of anti-apoptotic proteins (Bcl-2) in HCT-116 cells.
This indicated that Scutellarin induced apoptotic cell death in
colon cancer and inhibited cell growth in vitro via
regulation of the p53/p21 pathway. This result is consistent with a
previous report (9).
In conclusion, Scutellarin, a bioactive flavonoid
extracted from Scutellaria barbata D. Don induces apoptosis
in HCT-116 human colon carcinoma cells via activating p53 and
regulating Bcl-2/Bax expression. The results of the current study
study suggested that Scutellarin may be useful as a novel
therapeutic agent against colon cancer.
Acknowledgements
The present study was supported by The National High
Technology Research and Development Program of China and National
Natural Science Foundation of China (grant no. 81201875).
References
|
1
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sadahiro S, Suzuki T, Ishikawa K, Nakamura
T, Tanaka Y, Masuda T, Mukoyama S, Yasuda S, Tajima T, Makuuchi H
and Murayama C: Recurrence patterns after curative resection of
colorectal cancer in patients followed for a minimum of ten years.
Hepatogastroenterology. 50:1362–1366. 2003.PubMed/NCBI
|
|
3
|
Kim DI, Lee TK, Lim IS, Kim H, Lee YC and
Kim CH: Regulation of IGF-I production and proliferation of human
leiomyomal smooth muscle cells by Scutellaria barbata D. Don in
vitro: Isolation of flavonoids of apigenin and luteolin as acting
compounds. Toxicol Appl Pharmacol. 205:213–224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai ZJ, Wang XJ, Li ZF, Ji ZZ, Ren HT,
Tang W, Liu XX, Kang HF, Guan HT and Song LQ: Scutellaria barbate
extract induces apoptosis of hepatoma H22 cells via the
mitochondrial pathway involving caspase-3. World J Gastroenterol.
14:7321–7328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong H and Liu GQ: Protection against
hydrogen peroxide-induced cytotoxicity in PC12 cells by
scutellarin. Life Sci. 74:2959–2973. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang GH, Wang Q, Chen JJ, Zhang XM, Tam
SC and Zheng YT: The anti-HIV-1 effect of scutellarin. Biochem
Biophys Res Commun. 334:812–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan Z, Zhao W, Zhang X, Wang B, Wang J,
Sun X, Liu X, Feng S, Yang B and Lu Y: Scutellarin alleviates
interstitial fibrosis and cardiac dysfunction of infarct rats by
inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2.
Br J Pharmacol. 162:688–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Huang D, Gao Z, Lv Y, Zhang L, Cui H
and Zheng J: Scutellarin inhibits cell migration by regulating
production of αvβ6 integrin and E-cadherin in human tongue cancer
cells. Oncol Rep. 24:1153–1160. 2010.PubMed/NCBI
|
|
9
|
Chan JY, Tan BK and Lee SC: Scutellarin
sensitizes drug-evoked colon cancer cell apoptosis through enhanced
caspase-6 activation. Anticancer Res. 29:3043–3047. 2009.PubMed/NCBI
|
|
10
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59 Suppl
7:1693S–1700S. 1999.PubMed/NCBI
|
|
11
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
12
|
Harris SL and Levine AJ: The p53 pathway:
Positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Müller M, Wilder S, Bannasch D, Israeli D,
Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M
and Krammer PH: p53 activates the CD95 (APO-1/Fas) gene in response
to DNA damage by anticancer drugs. J Exp Med. 188:2033–2045. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyle P and Ferlay J: Cancer incidence and
mortality in Europe, 2004. Ann Oncol. 16:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schrag D, Cramer LD, Bach PB and Begg CB:
Age and adjuvant chemotherapy use after surgery for stage III colon
cancer. J Natl Cancer Inst. 93:850–857. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng Y, Zhang S, Tu J, Cao Z, Pan Y, Shang
B, Liu R, Bao M, Guo P and Zhou Q: Novel function of scutellarin in
inhibiting cell proliferation and inducing cell apoptosis of human
Burkitt lymphoma Namalwa cells. Leuk Lymphoma. 53:2456–2464. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Fan H, Wang Z, Zheng J and Cao W:
Potentiation of scutellarin on human tongue carcinoma xenograft by
low-intensity ultrasound. PLoS One. 8:e594732013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ling YH, Liebes L, Jiang JD, Holland JF,
Elliott PJ, Adams J, Muggia FM and Perez-Soler R: Mechanisms of
proteasome inhibitor PS-341-induced G(2)-M-phase arrest and
apoptosis in human non-small cell lung cancer cell lines. Clin
Cancer Res. 9:1145–1154. 2003.PubMed/NCBI
|
|
19
|
Barlev NA, Liu L, Chehab NH, Mansfield K,
Harris KG, Halazonetis TD and Berger SL: Acetylation of p53
activates transcription through recruitment of coactivators/histone
acetyltransferases. Mol Cell. 8:1243–1254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin XM, Oltvai ZN, Veis-Novack DJ, Linette
GP and Korsmeyer SJ: Bcl-2 gene family and the regulation of
programmed cell death. Cold Spring Harb Symp Quant Biol.
59:387–393. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swanton E, Savory P, Cosulich S, Clarke P
and Woodman P: Bcl-2 regulates a caspase-3/caspase-2 apoptotic
cascade in cytosolic extracts. Oncogene. 18:1781–1787. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Drygin D, O'Brien SE, Hannan RD, McArthur
GA and Von Hoff DD: Targeting the nucleolus for cancer-specific
activation of p53. Drug Discov Today. 19:259–265. 2014. View Article : Google Scholar : PubMed/NCBI
|