Wounds are physical injuries that can be classified
as ‘open’ or ‘closed’, based on the underlying cause, and ‘acute’
or ‘chronic’, based on the physiology of wound healing (1–3).
Wound healing is essential for the restoration of disrupted
anatomical integrity and disturbed function of the affected area
(4,5). Healing is a complex and intricate
process, initiated in response to an injury, that serves to restore
the function and integrity of the damaged tissues (6). Chronic wounds are a major concern for
patients and clinicians, affecting a large number of patients and
leading to a serious reduction in their quality of life (7). Recent estimates indicate that ~6
million people suffer from chronic wounds worldwide (7,8),
while in the US, traumatic wounds result in >300,000
hospitalizations annually (7,8).
The restoration of the affected area is the main aim
of quality wound rehabilitation. Surgical wounds account for the
vast majority of skin injuries, with ~80% of these wounds requiring
the use of some form of closure product, such as sutures, staples
or tape, while numerous wound management strategies use hemostasis
products, fabric bandages and surgical dressings (9,10).
Wound healing is a normal biological process in the
human body that is achieved via four rigidly coordinated phases:
Hemostasis, inflammation, proliferation and remodeling (4,6).
Wound healing, therefore, involves a number of processes, including
inflammation, cell proliferation and contraction of the collagen
lattice (11–13). In addition, the healing process may
be hampered by the presence of oxygen free radicals or microbial
infection (5,6). Over the last 15 years, the in
vitro tests developed to investigate wound healing have
exploited all of these processes as targets for enhancing its
management. Since the different phases of the wound healing process
overlap, an ideal plant-based remedy should affect at least two
phases before it can be considered to have scientific support for
its use (12–14). Despite significant advances in the
pharmaceutical industry, the pursuit of effective and low cost
therapies for wound healing remains a challenge for modern medicine
due to the potentially chronic nature of injuries and the side
effects associated with current therapies (15–19).
In the search for novel therapeutic options, plants and their
metabolites represent an important potential source of
biomolecules.
Research on wound healing agents is one of the
emerging areas in modern biomedical sciences (33–37).
For medicinal plants to properly contribute to affordable
healthcare, they must be scientifically assessed; phytochemical
screenings are often considered as the first step towards the
discovery of useful drugs (38,39).
Successive solvent extraction techniques, as well as
chromatographic separations and spectroscopic methods, have been
used to determine the chemical constituents of plants and study
their bioactivity as it applies to traditional medicine (32,37).
A number of secondary metabolites and active compounds isolated
from plants have been demonstrated as active facilitators of wound
healing in animal models. Important examples include: Tannins from
Terminalia arjuna (40);
oleanolic acid from Anredera diffusa (41); polysaccharides from Opuntia
ficus-indica (42);
gentiopicroside, sweroside and swertiamarin from Gentiana
lutea (43); shikonin
derivatives (deoxyshikonin, acetyl shikonin, 3-hydroxy-isovaleryl
shikonin and 5,8-odimethyl acetyl shikonin) from Onosma
argentatum (44);
asiaticoside, asiatic acid and madecassic acid from Centella
asiatica (45–47); quercetin, isorhamnetin and
kaempferol from Hippophae rhamnoides (48); curcumin from Curcuma longa
(49); oleoresin from Copaifera
langsdorffii (50);
proanthocyanidins and resveratrol from grapes (51,52),
acylated iridoid glycosides from Scrophularia nodosa
(53); phenolic acids
(protocatechuic, p-hydroxybenzoic, p-coumaric, ferulic and vanillic
acids) from C. odorata (54,55);
glycoprotein fraction of Aloe vera (56); (+)-epi-alpha-bisabolol from
Peperomia galioides (57);
fukinolic and cimicifugic acids from Cimicifuga spp.
(58); and xyloglucan from
Tamarindus indicus (59).
A major challenge to the pharmacological validation
of wound healing plants, is that the exact mechanism of the process
of wound healing is not clearly understood; wound healing is known
to be a complicated process involving a number of stages, including
inflammation, epithelization, antioxidant defense and biochemical
changes (hydroxyproline), granulation, neovascularization and wound
contraction (60), however, the
precise mechanistic details remain unclear. The majority of
research studies into medicinal plants are, therefore, restricted
to screening plants to simply evaluate their wound healing effects
and investigate the mechanistic details. This review aims to
evaluate the current knowledge and information available regarding
the underutilized and neglected medicinal plant C. odorata,
including its mechanism of action in vitro and in
vivo, and to examine the ethnopharmacological claims of the
usefulness of this plant. This may contribute to encouraging the
global acceptance of wound healing agents of plant origin and the
recognition of their important natural role in wound healing. To
support this, a study reported that ~31% of these medicinal plants
have been used to treat wounds, 29% have been used to treat cuts,
10% to treat burns, and 22% to treat cuts and wounds (27,61).
Wound healing is an intricate process by which the
skin or other organs and tissues self-repair following injury
(103). In normal skin, the
epidermis and dermis form a protective barrier against the external
environment. The moment the injury occurs and this protective
barrier breaks, the process of wound healing is immediately
activated, and can continue for months or years (3). Wound healing involves continuous
cell-cell and cell-matrix interactions (104), and requires the collaborative
efforts of numerous different tissues and cell types, including
platelet aggregation and blood clotting, fibrin formation,
inflammation, angiogenesis and reepithelialization (105,106). Healing is considered complete
once the disrupted surfaces are firmly knitted together by collagen
(107). Optimal wound healing
involves minimizing tissue damage and providing adequate tissue
perfusion and oxygenation, proper nutrition and a moist wound
healing environment to restore function to the injury site
(108).
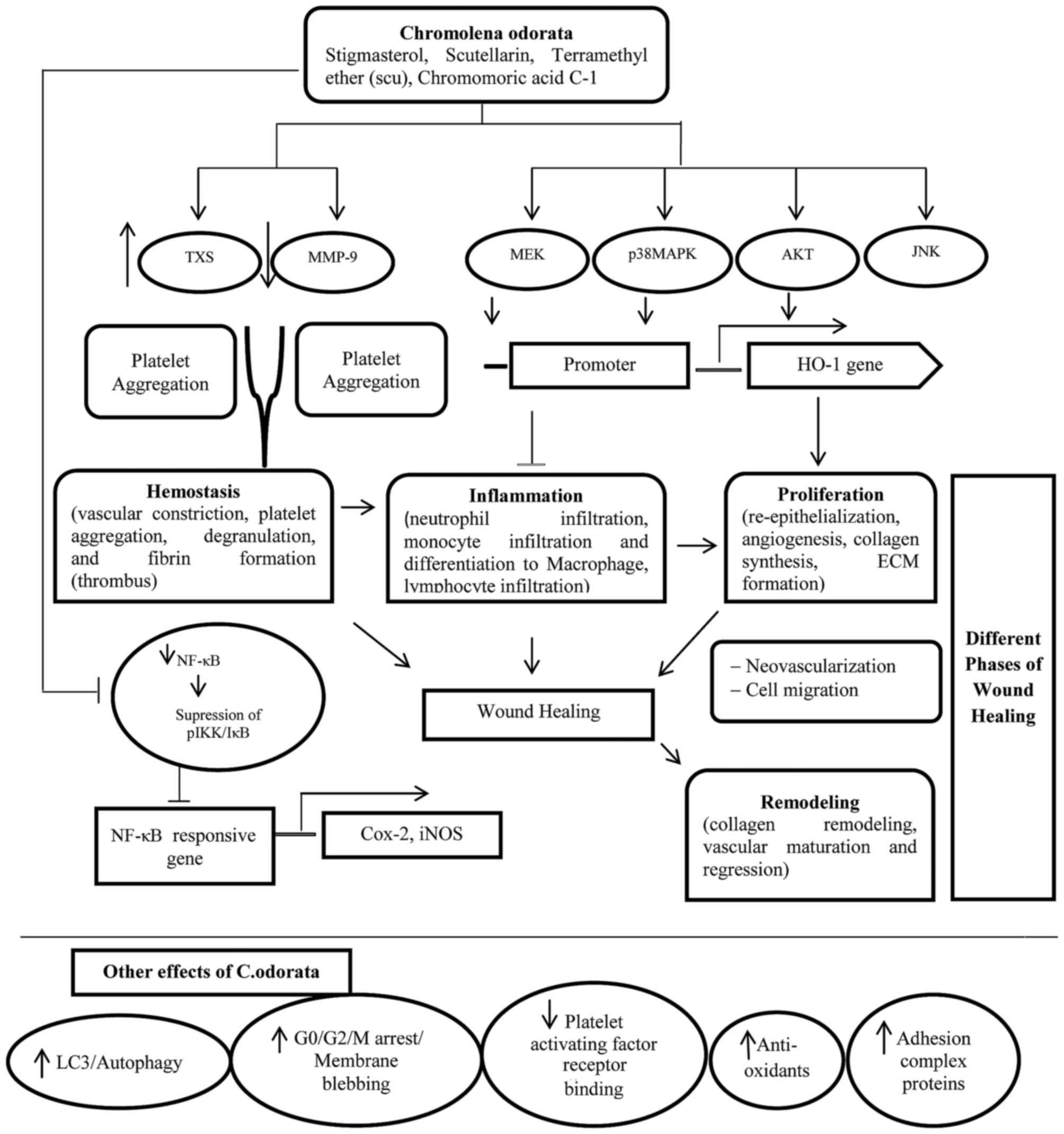
The process of wound healing involves four
overlapping phases: Hemostasis (cessation of bleeding),
inflammation, proliferation and remodeling (114–116). Thromboxane synthase is the
primary regulator of hemostasis; the enzyme that converts
prostaglandin H2 to thromboxane A2, which has
potent vasoconstriction and platelet aggregation activity (117). Plasminogen activator inhibitor
type 1 is also involved in hemostasis by inhibiting fibrinolysis,
which prevents failure of the hemostasis (118). Subsequently, during the
inflammatory phase, free radicals are released from neutrophils to
kill bacteria (119,120), and heme and heme proteins locally
accumulate at the wound site. Heme and heme proteins exert
prooxidative and proinflammatory effects via increasing the
expression level of adhesion molecules, increasing vascular
permeability and increasing leukocyte infiltration, which induce
wound healing. Heme oxygenase-1 (HO-1) is anti-inflammatory and is
an antioxidant involved in a variety of wound healing processes.
HO-1 produces biliverdin/bilirubin, iron, and carbon monoxide from
heme, which have potent antioxidant effects. HO-1 overexpression
accelerates wound healing by reducing inflammation, increasing
proliferation, and inhibiting endothelial cell apoptosis (121). Matrix metalloproteinases (MMPs)
are also important in wound healing via effects on remodeling of
the ECM (122). MMP-9 is key
effector among MMP proteins (123). Various reports have demonstrated
that C. odorata extract accelerates hemostasis (124–126) and wound healing (54,94,95).
In addition, Scu and stigmasterol also exhibit hemostatic (127) and anti-inflammatory activities
(101,102,128). The phytoprostane compound
chromomoric acid C-I has been identified in C. odorata as a
strong inducer of nuclear factor, erythroid 2 like 2 (Nrf2)
activity, which is a major regulator of various genes, including
NADPH:quinone reductase, glutathione S-transferase,
γ-glutamylcysteine synthetase, UDP-glucuronosyltransferases and
epoxide hydrolase, with defensive, anti-inflammatory and
detoxifying functions (100). The
mechanisms of the wound healing phases of C. odorata are
briefly demonstrated in Fig.
3.
Inflammation is a response to tissue injury caused
by infection, trauma, chemicals, heat, or unrecognized particles
(129). During the acute
response, inflammation causes an influx of neutrophils to the wound
area. Free radicals are produced from these cells as part of the
characteristic ‘respiratory burst’ activity (130). Free radicals are also generated
by wound-associated non-phagocytic cells by mechanisms involving
non-phagocytic NADPH oxidase (131). Therefore, the wound area has high
levels of oxygen- and nitrogen-centered reactive species, and their
derivative molecules. Oxidative stress is caused by these radicals
and causes lipid peroxidation, DNA damage and enzyme inactivation,
including free-radical scavenging proteins. Reports of the effects
of oxidants in the pathogenesis of various diseases suggests that
antioxidants may be useful as therapeutics in such conditions. In
patients, topical application of free-radical-scavenging molecules
has been reported to markedly improve wound healing and prevent
from oxidative damage to tissues (132).
Inflammation upregulates several proinflammatory
cytokines. Cyclooxygenase-2 and inducible nitric oxide synthase are
important proinflammatory enzymes in inflammation (133,134). They produce proinflammatory
mediators, prostaglandin E2 and nitric oxide, which enhance the
expression of proinflammatory cytokines including tumor necrosis
factor-α and interleukin-1β. C. odorata is capable of
anti-inflammatory activity in vitro and in vivo
(135–138). The Scu, isosakuranetin and
stigmasterol have also been reported to possess anti-inflammatory
activity (69,127,128,139–141).
Reactive oxygen species (ROS) disrupt wound healing
due to their damaging effects on cells and tissues. ROS are able to
degrade absorbable synthetic biomaterials (150,151). Free-radical-scavenging enzymes
are cytoprotective and are essential for the reducing, deactivating
and removing of ROS, and for wound healing regulation. Inflammatory
disease pathology involves excessive ROS generation by
polymorphonuclear leukocytes (PMNLs). Natural compounds with
antioxidant activity may be useful in reducing or regulating the
oxidative damage caused by ROS produced from PMNLs. A previous
study reported that Chromolaena species inhibit ROS
generation via opsonized zymosan-stimulated PMNLs (83).
|
1
|
Bennet RG: Fundamentals of cutaneous
surgery. Mosby C.V.; St. Louis, MO: pp. 7781988
|
|
2
|
Singh M, Govindarajan R, Nath V, Rawat AK
and Mehrotra S: Antimicrobial, wound healing and antioxidant
activity of Plagiochasma appendiculatum Lehm. et Lind.
Journal of Ethnopharmacology. 107:67–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagori BP and Solanki R: Role of medicinal
plants in wound healing. Res J Med Plant. 5:392–405. 2011.
View Article : Google Scholar
|
|
4
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Edlich RF, Winters KL, Britt LD, Long WB
III, Gubler KD and Drake DB: Difficult wounds: An update. J Long
Term Eff Med Implants. 15:289–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wietecha MS and DiPietro LA: Therapeutic
approaches to the regulation of wound angiogenesis. Adv Wound Care
(New Rochelle). 2:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wound Management, Forecast to 2021:
Established and emerging products, technologies and markets in the
Americas, Europe, Asia/Pacific and Rest of World. http://woundcare-today.com/news/world-at-glance/projected-global-wound-prevalence-by-wound-typesMarch;2013.
|
|
8
|
WHO report. http://www.who.int/mediacentre/factsheets/fs365/en/2014.
|
|
9
|
Hostetler SG, Xiang H, Gupta S, Sen C and
Gordillo JM: Discharge patterns of injury-related hospitalizations
with an acute wound in the United States. Wounds. 18:340–351.
2006.
|
|
10
|
Belmont PJ, Schoenfeld AJ and Goodman G:
Epidemiology of combat wounds in operation iraqi freedom and
operation enduring freedom: Orthopaedic burden of disease. J Surg
Orthop Adv. 19:2–7. 2010.PubMed/NCBI
|
|
11
|
Bodeker G and Hughes MA: Wound healing,
traditional treatments and research policy. Etkin N, Prendergast H
and Houghton P: Plants for Food and Medicine. 345–349. 1998.
|
|
12
|
Bodeker GC, Ryan TJ and Ong CK:
Traditional Approaches to Wound Healing. Clin Dermatol. 17:93–98.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burford G, Bodeker G and Ryan TJ: Skin and
wound care: Traditional, complementary and alternative medicine in
public health dermatology. Bodeker G and Burford G: Traditional,
Complementary & Alternative Medicine: Policy and Public Health
Perspectives. Imperial College Press; London: 2007
|
|
14
|
Trung LT and Bodeker G: Traditional
medicine and the infrastructure for burn care in Vietnam. Trop
Doct. 27:39–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porras-Reyes BH, Lewis WH, Roman J,
Simchowitz L and Mustoe TA: Enhancement of wound healing by the
alkaloid taspine defining mechanism of action. Proc Soc Exp Biol
Med. 203:18–25. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suh DY and Hunt TK: Time line of wound
healing. Clin Podiatr Med Surg. 15:1–9. 1998.PubMed/NCBI
|
|
17
|
Udupa SL, Shetty S, Udupa AL and Somayaji
SN: Effect of Ocimum sanctum Linn. on normal and
dexamethasone suppressed wound healing. Ind J Exp Biol. 44:49–54.
2006.
|
|
18
|
Ghosh PK and Gaba A: Phyto-extracts in
wound healing. J Pharm Pharm Sci. 16:760–820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barreto RS, Albuquerque-Júnior RL, Araújo
AA, Almeida JR, Santos MR, Barreto AS, DeSantana JM, Siqueira-Lima
PS, Quintans JS and Quintans-Júnior LJ: A systematic review of the
wound-healing effects of monoterpenes and iridoid derivatives.
Molecules. 19:846–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soni H and Singhai AK: A recent update of
botanicals for wound healing activity. Int Res J Pharm. 3:1–7.
2013.
|
|
21
|
Fleischner AM: Plant extracts: To
accelerate healing and inflammation. Cosmet Toilet. 100:45–58.
1985.
|
|
22
|
Getie M, Mariam GT, Reitz R and Neubert
RH: Evaluation of the release profiles of flavonoids from topical
formulations of the crude extract of the leaves of Dodonea
viscosa (Sapindaceae). Pharmazie. 57:320–322. 2002.PubMed/NCBI
|
|
23
|
Tsuchiya H, Sato M, Miyazaki T, Fujiwara
S, Tanigaki S, Ohyama M, Tanaka T and Iinuma M: Comparative study
on the antibacterial activity of phytochemical flavaones against
methicillin-resistant Staphylococcus aureus. J
Ethnopharmacol. 50:27–34. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Divakar MC, Devi LS, Kumar S and Rao SB:
Studies on wound healing property of Polyscias scutellaria
leaf saponins. Ind J Nat Prod. 17:37–42. 2001.
|
|
25
|
Padmaja PN, Bairy KL and Kulkrani DR:
Pro-healing effect of betel nut and its polyphenols. Fitoterapia.
65:298–300. 2000.
|
|
26
|
Scortichini M and Rossi MP: Preliminary in
vitro evaluation of the antimicrobial activity of terpenes and
terpenoids towards Erwinia amylovora (Burrill). J Appl
Bacteriol. 71:109–112. 1991. View Article : Google Scholar
|
|
27
|
Biswas TK and Mukherjee B: Plants
medicines of Indian origin for wound healing activity: A review.
Int J Low Extrem Wounds. 2:25–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Purna SK and Babu M: Collagen based
dressing - A review. Burns. 26:54–62. 2006.
|
|
29
|
Azaizeh H, Fulder S, Khalil K and Said O:
Ethnobotanical knowledge of local Arab practitioners in the Middle
Eastern Region. J Fitoterapia. 74:98–108. 2003. View Article : Google Scholar
|
|
30
|
Ragupathy S, Steven NG, Maruthakkutti M,
Velusamy B and Ul-Huda MM: Consensus of the ‘Malasars’ traditional
aboriginal knowledge of medicinal plants in the Velliangiri holy
hills, India. J Ethnobiol Ethnomed. 4:82008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Au DT, Wu J, Jiang Z, Chen H, Lu G and
Zhao Z: Ethnobotanical study of medicinal plants used by Hakka in
Guangdong, China. J Ethnopharmacol. 117:41–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar B, Vijayakumar M, Govindarajan R and
Pushpangadan P: Ethnopharmacological approaches to wound
healing-exploring medicinal plants of India. J Ethnopharmacol.
114:103–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Fátima A, Modolo LV, Sanches AC and
Porto RR: Wound healing agents: The role of natural and non-natural
products in drug development. Mini Rev Med Chem. 8:879–888. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fleck CA and Simman R: Modern collagen
wound dressings: Function and purpose. J Am Col Certif Wound Spec.
2:50–54. 2011.PubMed/NCBI
|
|
35
|
Korkina L, Kostyuk V, De Luca C and
Pastore S: Plant phenylpropanoids as emerging anti-inflammatory
agents. Mini Rev Med Chem. 11:823–835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morgan C and Nigam Y: Naturally derived
factors and their role in the promotion of angiogenesis for the
healing of chronic wounds. Angiogenesis. 16:493–502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Majumder P: Taxo-cognostic,
Phyto-physicochemical & Biological screening of a plant:
Taxo-cognostic, Phytochemical, Physicochemical parameters &
Biological screening of Zyziphus oenoplia (L.) Mill plant
root. LAP Lambert Academic Publishing; 2012
|
|
38
|
Akinmoladun AC, Obuotor EM and Farombi EO:
Evaluation of antioxidant and free radical scavenging capacities of
some Nigerian indigenous medicinal plants. J Med Food. 13:444–451.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kameshwaran S, Senthilkumar R, Thenmozhi S
and Dhanalakshmi M: Wound healing potential of ethanolic extract of
tecoma stans flowers in rats. Pharmacologia. 5:215–221. 2014.
View Article : Google Scholar
|
|
40
|
Chaudhari M and Mengi S: Evaluation of
phytoconstituents of Terminalia arjuna for wound healing
activity in rats. Phytother Res. 20:799–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moura-Letts G, Villegas LF, Marçalo A,
Vaisberg AJ and Hammond GB: In vivo wound-healing activity of
oleanolic acid derived from the acid hydrolysis of Anredera
diffusa. J Nat Prod. 69:978–979. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trombetta D, Puglia C, Perri D, Licata A,
Pergolizzi S, Lauriano ER, De Pasquale A, Saija A and Bonina FP:
Effect of polysaccharides from Opunta ficus-indica (L.)
cladodes on the healing of dermal wounds in the rat. Phytomedicine.
13:352–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Oztürk N, Korkmaz S, Oztürk Y and Başer
KH: Effects of gentiopicroside, sweroside and swertiamarine,
secoiridoids from Gentian (Gentiana lutea sp. symphyandra),
on cultured chicken embryonic fibroblasts. Planta Med. 72:289–294.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ozgen U, Ikbal M, Hacimuftuoglu A,
Houghton PJ, Gocer F, Dogan H and Coskun M: Fibroblast growth
stimulation by extracts and compounds of Onosma argentatum
roots. J Ethnopharmacol. 104:100–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maquart FX, Chastang F, Simeon A,
Birembaut P, Gillery P and Wegrowski Y: Triterpenes from
Centella asiatica stimulate extracellular matrix
accumulation in rat experimental wounds. Eur J Dermatol. 9:289–296.
1999.PubMed/NCBI
|
|
46
|
Shukla A, Rasik AM, Jain GK, Shankar R,
Kulshrestha DK and Dhawan BN: In vitro and in vivo wound healing
activity of asiaticoside isolated from Centella asiatica. J
Ethnopharmacol. 65:1–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hong SS, Kim JH, Li H and Shim CK:
Advanced formulation and pharmacological activity of hydrogel of
the titrated extract of C. asiatics. Arch Pharmacol Res.
28:502–508. 2005. View Article : Google Scholar
|
|
48
|
Fu SC, Hui CW, Li LC, Cheuk YC, Qin L, Gao
J and Chan KM: Total flavones of Hippophae rhamnoides
promotes early restoration of ultimate stress of healing patellar
tendon in a rat model. Med Eng Phys. 27:313–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jagetia GC and Rajanikant GK: Role of
curcumin, a naturally occurring phenolic compound of turmeric in
accelerating the repair of excision wound, in mice whole-body
exposed to various doses of gamma-radiation. J Surg Res.
120:127–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Paiva LA, de Alencar Cunha KM, Santos FA,
Gramosa NV, Silveira ER and Rao VS: Investigation on the wound
healing activity of oleo-resin from Copaifera langsdorffi in
rats. Phytother Res. 16:737–739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bråkenhielm E, Cao R and Cao Y:
Suppression of angiogenesis, tumor growth, and wound healing by
resveratrol, a natural compound in red wine and grapes. FASEB J.
15:1798–1800. 2001.PubMed/NCBI
|
|
52
|
Khanna S, Venojarvi M, Roy S, Sharma N,
Trikha P, Bagchi D, Bagchi M and Sen CK: Dermal wound healing
properties of redox-active grape seed proanthocyanidins. Free Radic
Biol Med. 33:1089–1096. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Stevenson PC, Simmonds MS, Sampson J,
Houghton PJ and Grice P: Wound healing activity of acylated iridoid
glycosides from Scrophularia nodosa. Phytother Res. 16:33–35. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Phan TT, Wang L, See P, Grayer RJ, Chan SY
and Lee ST: Phenolic compounds of Chromolaena odorata
protect cultured skin cells from oxidative damage: Implication for
cutaneous wound healing. Biol Pharm Bull. 24:1373–1379. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Phan TT, Hughes MA and Cherry GW: Effects
of an aqueous extract from the leaves of Chromolaena odorata
(Eupolin) on the proliferation of human keratinocytes and on their
migration in an in vitro model of reepithelialization. Wound Repair
Regen. 9:305–313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Choi SW, Son BW, Son YS, Park YI, Lee S
and Chung MH: The wound-healing effect of a glycoprotein fraction
isolated from Aloe vera. Br J Dermatol. 145:535–545. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Villegas LF, Marçalo A, Martin J,
Fernández ID, Maldonado H, Vaisberg AJ and Hammond GB:
(+)-epi-Alpha-bisabolol [correction of bisbolol] is the
wound-healing principle of Peperomia galioides:
Investigation of the in vivo wound-healing activity of related
terpenoids. J Nat Prod. 64:1357–1359. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kusano A, Seyama Y, Nagai M, Shibano M and
Kusano G: Effects of fukinolic acid and cimicifugic acids from
Cimicifuga species on collagenolytic activity. Biol Pharm
Bull. 24:1198–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Burgalassi S, Raimondi L, Pirisino R,
Banchelli G, Boldrini E and Saettone MF: Effect of xyloglucan
(tamarind seed polysaccharide) on conjuctival cell adhesion to
laminin and corneal epithelium wound healing. Euro J Ophthalmol.
10:71–76. 2000.
|
|
60
|
Joshi A, Sengar N, Prasad SK, Goel RK,
Singh A and Hemalatha S: Wound-healing potential of the root
extract of Albizzia lebbeck. Planta Med. 79:737–743. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Singh MR, Saraf S, Vyas A, Jain V and
Singh D: Innovative approaches in wound healing: Trajectory and
advances. Artificial Cells Nanomed Biotechnol. 41:202–212. 2013.
View Article : Google Scholar
|
|
62
|
Heywood VH, Harborne JB and Turner BL:
Biology and Chemistry of the Compositae. 1. Academic Press; London:
1977
|
|
63
|
Binggeli P: Chromolaena odorata
(L.) King & Robinson (Asteraceae). http://members.lycos.co.uk/WoodyPlantEcologyWoody
Plant Ecology-Invasive Woody Plants. Pierre Binggeli; 1999
|
|
64
|
Bamikole MA, Ikhatua UJ and Osemwenkhae
AE: Converting bush to meat: A case of Chromolaena odorata
feeding to rabbits. Pakistan Journal of Nutrition. 3:258–261. 2004.
View Article : Google Scholar
|
|
65
|
Igboh MN, Ikewuchi JC and Ikewuchi CC:
Chemical profile of Chromolaena odorata L. (King and
Robinson) leaves. Pakistan Journal of Nutrition. 8:521–524. 2009.
View Article : Google Scholar
|
|
66
|
Akinmoladun AC, Ibukun EC and DanOloge IA:
Phytochemical constituents and antioxidant properties of extracts
from the leaves of Chromolaena odorata. Sci Res Essays.
2:191–194. 2007.
|
|
67
|
Akinmutimi AH and Akufo A: The effect of
graded levels of dietary inclusion of siam weed (Chromolina
odorata) leaf meal in grower rabbits diet in a tropical
environment. J Ani Vet Adv. 5:707–711. 2006.
|
|
68
|
King RM and Robinson H: Studies in the
Eupatoriae (Compositae). The Genus Chromolaena. Phytologia.
20:196–209. 1970. View Article : Google Scholar
|
|
69
|
Ling SK, Pisar MM and Man S:
Platelet-activating factor (PAF) receptor binding antagonist
activity of the methanol extracts and isolated flavonoids from
Chromolaena odorata (L.) King and Robinson. Biol Pharm Bull.
30:1150–1152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Owoyele VB, Adediji JO and Soladoye AO:
Anti-inflammatory activity of aqueous leaf extract of
Chromolaena odorata. Inflammopharmacol. 13:479–484. 2005.
View Article : Google Scholar
|
|
71
|
Akinmoladun AC, Obuotor EM and Farombi EO:
Evaluation of antioxidant and free radical scavenging capacities of
some Nigerian indigenous medicinal plants. J Med Food. 13:444–451.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ambika SR: Jayachandra: Influence of light
on seed germination in Eupatorium odoratum L. Ind Forester.
106:637–640. 1980.
|
|
73
|
Irobi ON: Activities of Chromolaena
odorata (Compositae) leaf extract against Pseudomonas
aeruginosa and Streptococcus faecalis. J Ethnopharmacol.
37:81–83. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bennett FD and Rao VP: Distribution of an
introduced weed Eupatorium odoratum L. in Asia and Africa
and possibilities of its biological control. PANS C. 14:277–281.
1968.
|
|
75
|
Rai SN: Eupatorium and weedicides. Ind
Forester. 102:449–454. 1976.
|
|
76
|
Warea O: Chromolaena (Siam) weed.
Pest Advisory Leaflet no. 43. Secretariat of the Pacific Community.
2004.
|
|
77
|
Bani G: Status and management of
Chromolaena odorata in congo. Proceedings of the fifth
international workshop on biological control and management of
Chromolaena odorata. 71–73. 2002.
|
|
78
|
Sajise PE, Palis RK, Norcio NV and Lales
JS: The Biology C. odorata L. King and Robinson. Flowering
behaviour, pattern of growth and nitrate metabolism. Phil Weed Sci
Bull. 1:17–24. 1974.
|
|
79
|
Muniappan R and Bamba J: Biological
control of Chromolaena odorata: Successes and Failures.
Proceedings of the X International Symposium on Biological Control
of Weeds. 81. 1999, Montana State University; Bozeman, Montana,
USA: Neal R: Spencer [ed.]. 2000; pp. 81–85
|
|
80
|
Rejitha G: Diuretic activity of
Eupatorium odoratum L inn. J Pharm Res. 2:844–846. 2009.
|
|
81
|
Vital PG and Windell LR: Antimicrobial
activity and cytotoxicity of Chromolaena odorata (L. f.)
King and Robinson and Uncaria perrottetii (A. Rich) Merr. Extracts.
J Med Plants Res. 3:511–518. 2009.
|
|
82
|
Hanh TT, Hang DT, Van Minh C and Dat NT:
Anti-inflammatory effects of fatty acids isolated from
Chromolaena odorata. Asian Pac J Trop Med. 4:760–763. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Taleb-Contini SH, Kanashiro A, Kabeya LM,
Polizello AC, Lucisano-Valim YM and Oliveira DC: Immunomodulatory
effects of methoxylated flavonoids from two Chromolaena
species: Structure-activity relationships. Phytother Res.
20:573–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nasasombat S and Teckchuen N:
Antimicrobial, antioxidant and anticancer activities of Thai local
vegetables. J Med Plants Res. 3:443–449. 2009.
|
|
85
|
Thang PT, Patrick S, Teik LS and Yung CS:
Anti-oxidant effects of the extracts from the leaves of
Chromolaena odorata on human dermal fibroblasts and
epidermal keratinocytes against hydrogen peroxide and
hypoxanthine-xanthine oxidase induced damage. Burns. 27:319–327.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Torrenegra RD and Rodríguez OE: Chemical
and biological activity of leaf extracts of Chromolaena
leivensis. Nat Prod Commun. 6:947–950. 2011.PubMed/NCBI
|
|
87
|
Harun FB, Syed Sahil Jamalullail SM, Yin
KB, Othman Z, Tilwari A and Balaram P: Autophagic cell death is
induced by acetone and ethyl acetate extracts from Eupatorium
odoratum in vitro: Effects on MCF-7 and vero cell lines. Sci
World J. 439–479. 2012.
|
|
88
|
Kouamé PB, Jacques C, Bedi G, Silvestre V,
Loquet D, Barillé-Nion S, Robins RJ and Tea I: Phytochemicals
isolated from leaves of Chromolaena odorata: Impact on
viability and clonogenicity of cancer cell lines. Phytother Res.
27:835–840. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Subramoniam A, Asha VV, Nair SA,
Sasidharan SP, Sureshkumar PK, Rajendran KN, Karunagaran D and
Ramalingam K: Chlorophyll revisited: Anti-inflammatory activities
of chlorophyll a and inhibition of expression of TNF-α gene by the
same. Inflammation. 35:959–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Leonora PN and Elena SC:
Anti-immunosuppressive Effects of Chromolaena odorata (Lf.)
King & Robinson (Asteraceae) leaf extract in
cyclophosphamide-injected Balb/C mice. philippine. J Sci.
141:35–43. 2012.
|
|
91
|
Vaisakh MN and Pandey A: The invasive weed
with healing properties: A review on Chromolaena odorata.
Int J Pharmaceutical Sci Res. 3:80–83. 2013.
|
|
92
|
Suksamrarn A, Chotipong A, Suavansri T,
Boongird S, Timsuksai P, Vimuttipong S and Chuaynugul A:
Antimycobacterial activity and cytotoxicity of flavonoids from the
flowers of Chromolaena odorata. Arch Pharm Pres. 27:507–511.
2004. View Article : Google Scholar
|
|
93
|
Wang L, Waltenberger B, Pferschy-Wenzig
EM, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S,
Rollinger JM, Heiss EH, et al: Natural product agonists of
peroxisome proliferator-activated receptor gamma (PPARγ): A review.
Biochem Pharmacol. 92:73–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Phan TT, Hughes MA and Cherry GW: Enhanced
proliferation of fibroblasts and endothelial cells treated with an
extract of the leaves of Chromolaena odorata (Eupolin), an
herbal remedy for treating wounds. Plast Reconstr Surg.
101:756–765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Phan TT, Allen J, Hughes MA, Cherry G and
Wojnarowska F: Upregulation of adhesion complex proteins and
fibronectin by human keratinocytes treated with an aqueous extract
from the leaves of Chromolaena odorata (Eupolin). Eur J
Dermatol. 10:5222000.PubMed/NCBI
|
|
96
|
Raina R, Parwez S, Verma PK and Pankaj NK:
Medicinal plants and their role in wound healing. OnlineVet J.
3:212008.
|
|
97
|
Ayyanar M and Ignacimuthu S:
Ethnomedicinal plants used by the tribals of Tirunelveli hills to
treat poisonous bites and skin diseases. Ind J Trad Knowled.
4:229–236. 2005.
|
|
98
|
Akinmoladun AC, Ibukun EO and Don-Ologe
IA: Phytochemicals constituents and antioxidant properties of
extracts from the leaves of Chromolaena odorata. Sci Res
Essay. 2:191–194. 2007.
|
|
99
|
Zhang ML, Irwin D, Li XN, Sauriol F, Shi
XW, Wang YF, Huo CH, Li LG, Gu YC and Shi QW: PPARγ agonist from
Chromolaena odorata. J Nat Prod. 75:2076–2081. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Heiss EH, Tran TV, Zimmermann K, Schwaiger
S, Vouk C, Mayerhofer B, Malainer C, Atanasov AG, Stuppner H and
Dirsch VM: Identification of chromomoric acid C-I as an Nrf2
activator in Chromolaena odorata. J Nat Prod. 77:503–508.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pandith H, Zhang X, Thongpraditchote S,
Wongkrajang Y, Gritsanapan W and Baek SJ: Effect of Siam weed
extract and its bioactive component scutellarein tetramethyl ether
on anti-inflammatory activity through NF-κB pathway. J
Ethnopharmacol. 147:434–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pandith H, Zhang X, Liggett J, Min KW,
Gritsanapan W and Baek SJ: Hemostatic and wound healing properties
of chromolaena odorata Leaf Extract. ISRN Dermatol.
2013:1682692013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Okuno Y, Nakamura-Ishizu A, Kishi K, Suda
T and Kubota Y: Bone marrow-derived cells serves as proangiogenic
macrophages but no endothelial cells in wound healing. Blood.
117:5264–5272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Martin A: The use of antioxidants in
healing. Dermatol. Surg. 22:156–160. 1996.
|
|
105
|
Martin P: Wound healing-aiming for perfect
skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Martin P and Leibovich SJ: Inflammatory
cells during wound repair: The good, the bad and the ugly. Trends
Cell Biol. 15:599–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Buffoni F, Banchelli G, Cambi S, Ignesti
G, Pirisino R, Raimondi L and Vannelli G: Skin wound healing: Some
biochemical parameters in guinea-pig. J Pharm Pharmacol.
45:784–790. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Pierce GF and Mustoe TA: Pharmacologic
enhancement of wound healing. Annu Rev Med. 46:467–481. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ling SK, Mazura MP, Khoo MGH, Virmala S,
Ong BK, Mastura M, Azah Nor MA, Salbiah M, Siti Asha ABL and
Rasadah MA: Chemical constituents and therapeutic potential of the
leaf extracts from Chromolaena odorata. In: Highlights of
FRIM's IRPA Projects 2005. Identifying Potential Commercial
Collaboratons Project Evaluation Meeting. Nik Zanariah NM, Norhara
H, Nor Azman H and Chan HT: Forest Research Institute Malaysia;
Malaysia: pp. 108–114. 2005
|
|
110
|
Odunbaku OA, Ilusanya OA and Akasoro KS:
Antibacterial activity of ethanolic leaf extract of Ficus
exasperata on Escherichia coli and Staphylococcus
albus. Sci Res Essay. 3:562–564. 2008.
|
|
111
|
Owoyele BV, Oguntoye SO, Dare K, Ogunbiyi
BA, Aruboula EA and Soladoye AO: Analgesic, anti-inflammatory and
antipyretic activities from flavonoid fractions of Chromolaena
odorata. J Med Plants Res. 2:219–225. 2008.
|
|
112
|
Bamba D, Bessière JM, Marion C, Pélissier
Y and Fourasté I: Essential Oil of Eupatorium odoratum. Planta Med.
59:184–185. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ayyanar M and Ignacimuthu S: Herbal
medicines for wound healing among tribal people in Southern India:
Ethnobotanical and Scientific evidences. Int J Appl Res Nat Prod.
2:29–42. 2009.
|
|
114
|
Reddy JS, Rao PR and Reddy MS: Wound
healing effects of Heliotropium indicum, Plumbago zeylanicum and
Acalypha indica in rats. J Ethnopharmacol. 79:249–251. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gosain A and DiPietro LA: Aging and wound
healing. World J Surg. 28:321–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nguyen DT, Orgrill DP and Murphy GF: The
pathophysiologic basis for wound healing and cutaneous
regeneration. Biomaterials for Treating Skin Loss. Orgill D and
Blanco G: Woodhead Publishing; Cambrdige: pp. 22–57. 2009
|
|
117
|
Vezza R, Mezzasoma AM, Venditti G and
Gresele P: Prostaglandin endoperoxides and thromboxane A2 activate
the same receptor isoforms in human platelets. Thromb Haemost.
87:114–121. 2002.PubMed/NCBI
|
|
118
|
Aso Y: Plasminogen activator inhibitor
(PAI)-1 in vascular inflammation and thrombosis. Front Biosci.
12:2957–2966. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
119
|
Phan TT, Hughes MA, Cherry GW, Le TT and
Pham HM: An aqueous extract of the leaves of Chromolaena
odorata (formerly Eupatorium odoratum) (Eupolin)
inhibits hydrated collagen lattice contraction by normal human
dermal fibroblasts. J Altern Complement Med. 2:335–343. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Fialkow L, Wang Y and Downey GP: Reactive
oxygen and nitrogen species as signaling molecules regulating
neutrophil function. Free Radical Biol Med. 42:153–164. 2007.
View Article : Google Scholar
|
|
121
|
Wagener FA, van Beurden HE, von den Hoff
JW, Adema GJ and Figdor CG: The heme-heme oxygenase system: A
molecular switch in wound healing. Blood. 102:521–528. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Stamenkovic I: Extracellular matrix
remodelling: The role of matrix metalloproteinases. J Pathol.
200:448–464. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hirose Y, Chiba K, Karasugi T, Nakajima M,
Kawaguchi Y, Mikami Y, Furuichi T, Mio F, Miyake A, Miyamoto T, et
al: A functional polymorphism in THBS2 that affects alternative
splicing and MMP binding is associated with lumbar-disc herniation.
Am J Human Genetic. 82:1122–1129. 2002. View Article : Google Scholar
|
|
124
|
Akah PA: Mechanism of hemostatic activity
of Eupatorium odoratum. Int J Crude DrugRes. 28:253–256.
1990. View Article : Google Scholar
|
|
125
|
Wongkrajang Y, Muagklum S, Peungvicha P,
Jaiaraj P and Opartkiattikul N: Eupatorium odoratum linn: An
enhancer of hemostasis. Mahidol Univ J Pharmaceut Sci. 17:9–13.
1990.
|
|
126
|
Wongkrajang Y, Thongpraditchote S,
Nakornchai S, Chuakul W, Muangklum K and Jaiaraj P: Hemostatic
activities of Eupatorium odoratum Linn.: Calcium removal
extract. Mahidol Univ J Pharmaceut Sci. 21:143–148. 1994.
|
|
127
|
Triratana T, Suwannuraks R and
Naengchomnong W: Effect of Eupatorium odoratum on blood
coagulation. J Med Assoc Thailand. 74:283–287. 1991.
|
|
128
|
Gabay O, Sanchez C, Salvat C, Chevy F,
Breton M, Nourissat G, Wolf C, Jacques C and Berenbaum F:
Stigmasterol: A phytosterol with potential anti-osteoarthritic
properties. Osteoarthritis Cartilage. 18:106–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Parimala DB, Tamilchelvan N and
Ramasubramaniaraja R: Inflammation and medicinal plants-an
ethnomedical approach. J Phytol. 2:49–56. 2010.
|
|
130
|
Baboir BM: Oxygen-dependent microbial
killing by phagocytes (first of two parts). New Eng J Med.
29:629–668. 1978.
|
|
131
|
Griendling KK: NAD(P)H oxidase: Role in
cardiovascular biology and diseases. Circ Res. 86:494–501. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Thiem B and Goślińska O: Antimicrobial
activity of Rubus chamaemorus leaves. Fitoterapia. 75:93–95.
2003. View Article : Google Scholar
|
|
133
|
Kim JH, Kim DH, Baek SH, Lee HJ, Kim MR,
Kwon HJ and Lee CH: Rengyolone inhibits inducible nitric oxide
synthase expression and nitric oxide production by down-regulation
of NF-kappaB and p38 MAP kinase activity in LPS stimulated RAW
264.7 cells. Biochem Pharmacol. 71:1198–1205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Pan CH, Kim ES, Jung SH, Nho CW and Lee
JK: Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory
responses in murine macrophage RAW 264.7 cells. Arch Pharma Res.
31:1447–1456. 2008. View Article : Google Scholar
|
|
135
|
Pisutthanan N, Liawruangrath S, Bremner J
and Liawruangrath B: Chemical constituents and biological
activities of Chromolaena odorata. J Sci Fac Chiang Mai
Univ. 32:139–148. 2005.
|
|
136
|
Lessio C, de Assunção Silva F, Glória MA,
Di Tommaso AB, Gori Mouro M, Di Marco GS, Schor N and Higa EM:
Cyclosporine A and NAC on the inducible nitric oxide synthase
expression and nitric oxide synthesis in rat renal artery cultured
cells. Kidney Int. 68:2508–2516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Park JW, Kwon OK, Jang HY, Jeong H, Oh SR,
Lee HK, Han SB and Ahn KS: A leaf methanolic extract of Wercklea
insignis attenuates the lipopolysaccharide-induced inflammatory
response by blocking the NF-κB signaling pathway in RAW 264.7
macrophages. Inflammation. 35:321–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sun J, Zhang X, Broderick M and Fein H:
Measurement of nitric oxide production in biological systems by
using griess reaction assay. Sensors. 3:276–284. 2003. View Article : Google Scholar
|
|
139
|
Lee IS, Jin W, Zhang X, Hung TM, Song KS,
Seong YH and Bae K: Cytotoxic and COX-2 inhibitory constituents
from the aerial parts of Aralia cordata. Arch Pharmacal Res.
29:548–555. 2006. View Article : Google Scholar
|
|
140
|
Dat NT, Lee K, Hong YS, Kim YH, Minh CV
and Lee JJ: A peroxisome proliferator-activated receptor-gamma
agonist and other constituents from Chromolaena odorata.
Planta Med. 75:803–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Karodi R, Jadhav M, Rub R and Bafna A:
Evaluation of the wound healing activity of a crude extract of
Rubia cordifolia L. (Indian madder) in mice. Int J Appl Res
Nat Prod. 2:12–18. 2009.
|
|
142
|
Csupor D, Blazsó G, Balogh A and Hohmann
J: The traditional Hungarian medicinal plant Centaurea
sadleriana Janka accelerates wound healing in rats. J
Ethnopharmacol. 127:193–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Trabucchi E, Baruffaldi Preis F, Baratti C
and Montorsi W: Topical treatment of experimental skin lesions in
rats: Macroscopic, microscopic and scanning electron-microscopic
evaluation of the healing process. Int J Tissue React. 8:533–544.
1986.PubMed/NCBI
|
|
144
|
Shukla A, Rasik AM and Dhawan BN:
Asiaticoside-induced elevation of antioxidant levels in healing
wounds. Phytother Res. 13:50–54. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cohen IK, Diegelmann RF and Lindblad WJ:
Wound healing. Biochem Clin Asp Philadelphia; Saunders: 1992
|
|
146
|
Szabo S, Kusstatscher S, Sakoulas G,
Sandor Z, Vincze A and Jadus M: Growth factors: New ‘endogeneous
drug’ for ulcer healing. Scand J Gastroenterol Suppl. 210:15–18.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Buntrock P, Jentzsch KD and Heder G:
Stimulation of wound healing, using brain extract with fibroblast
growth factor (FGF) activity. II. Histological and morphometric
examination of cells and capillaries. Exp Pathol. 21:62–67. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Habibipour S, Oswald TM, Zhang F, Joshi P,
Zhou XC, Dorsett-Martin W and Lineaweaver WC: Effect of sodium
diphenylhydantion on skin wound healing in rats. Plast Reconstr
Surg. 112:1620–1627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Aliyev E, Sakallioğlu U, Eren Z and
Açikgöz G: The effect of polylactide membranes on the levels of
reactive oxygen species in periodontal flaps during wound healing.
Biomaterials. 25:4633–4637. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Umachigi SP, Kumar GS, Jayaveera K,
Kishore KD, Ashok KC and Dhanapal R: Antimicrobial, wound healing
and antioxidant activities of Anthocephalus cadamba. Afr J
Tradit Complement Altern Med. 4:481–487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Shirwaikar A, Malini S and Kumari SC:
Protective effect of Pongamia pinnata flowers against cisplatin and
gentamicin-induced nephrotoxicity in rats. Ind J Exp Biol.
41:58–62. 2003.
|
|
152
|
Sumitra M, Manikandan P and Suguna L:
Efficacy of Butea monosperma on dermal wound healing in
rats. J Biochem Cell Biol. 37:566–573. 2005. View Article : Google Scholar
|
|
153
|
Saleem M and Aftab A: Tephrosia purpurea
ameliorates benzoyl peroxide induced cutaneous toxicity in mice:
Diminution of oxidative stress. Pharma Pharmacol Commun. 5:455–461.
1999. View Article : Google Scholar
|
|
154
|
Lewis DA and Hanson PJ: Antiulcer drugs of
plant origin. Prog Med Chem. 8:210–229. 1991.
|
|
155
|
Srinivasa Rao K, Chaudhury PK and Pradhan
A: Evaluation of anti-oxidant activities and total phenolic content
of Chromolaena odorata. Food Chem Toxicol. 48:729–732. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Onkaramurthy M, Veerapur VP, Thippeswamy
BS, Reddy TN, Rayappa H and Badami S: Anti-diabetic and
anti-cataract effects of Chromolaena odorata Linn., in
streptozotocin-induced diabetic rats. J Ethnopharmacol.
145:363–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ukwueze SE, Duru O and Shorinwa O:
Evaluation of the cutaneous wound healing activity of solvent
fractions of Chromolaena odorata linn. IAJPR. 3:3316–3323.
2013.
|
|
158
|
Boudjeko T, Megnekou R, Woguia AL, Kegne
FM, Ngomoyogoli JE, Tchapoum CD and Koum O: Antioxidant and
immunomodulatory properties of polysaccharides from Allanblackia
floribunda Oliv stem bark and Chromolaena odorata (L.)
King and H.E. Robins leaves. BMC Res Notes. 8:7592015. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pitakpawasutthi Y, Thitikornpong W,
Palanuvej C and Ruangrungsi N: Chlorogenic acid content, essential
oil compositions, and in vitro antioxidant activities of
Chromolaena odorata leaves. J Adv Pharm Technol Res.
7:37–42. 2016. View Article : Google Scholar : PubMed/NCBI
|