Introduction
Pancreatic cancer is a fatal malignancy, which has
been predicted to become the second most common cause of
cancer-associated mortality within the next two decades (1). The incidence of pancreatic cancer has
gradually increased during recent years, predominantly due to the
increased rates of obesity, smoking, alcohol abuse, pre-existing
chronic pancreatitis and prior abdominal radiotherapy (2). At present, surgery and chemotherapy
during the early stages of the disease provide the best prognosis
for long-term survival; however, the 5-year survival rate is still
<5% and the median survival rate is rarely >20 months
(3,4). Due to the persistent poor prognosis
associated with pancreatic cancer, it is clear that more effective
agents are required, and novel therapeutic strategies should be
developed for the prevention and treatment of pancreatic
cancer.
Oxidative stress occurs as a result of an imbalance
between excessive reactive oxygen species (ROS) production and
antioxidant depletion. ROS exert a critical role in several
cellular processes associated with cancer development, metastasis,
progression and survival (5). The
majority of chemotherapeutic and radiotherapeutic agents may be
selectively toxic to tumor cells by increasing oxidative stress
beyond their limit, which is thought to be contribute to sustained
activation of cell-cycle inhibitors, cell death induction, and
macromolecular damage-induced senescence. At present, augmentation
of oxidative stress represents the best opportunity for the
development of novel therapeutic strategies for cancer treatment
(6–8).
Natural products from traditional Chinese medicine
(TCM) exhibit marked potential as anticancer drugs, including
paclitaxel (6), camptothecin
(7) and vincristine (8). The Dendrobium species
(Orchidaceae) has been widely used in TCM, since members of this
species exert a broad spectrum of beneficial health effects,
including antipyretic, eye health-promoting, immunomodulatory and
anti-aging activities. This species has been used in China, India,
and other countries in subtropical and Southeast Asia, for
>2,000 years (9–11). For decades, bibenzyls, which are
the main bioactive components derived from Dendrobium
species, have been subjected to extensive investigation as likely
candidates for cancer treatment (12–19).
For example, erianin exhibits anti-angiogenic activity via inducing
endothelial cytoskeletal disorganization and activating the c-Jun
N-terminal kinase (JNK)/stress-associated protein kinases (SAPK)
signaling pathway (12,13). Dendrofalconerol A exerts
antimetastatic effects via the suppression of
epithelial-to-mesenchymal transition and integrin proteins in lung
cancer (14). In addition,
gigantol inhibits migration of non-small cell lung cancer cells via
a decrease in caveolin-1 protein expression, and the activation of
Akt and cell division cycle 42 (15). In our preliminary study,
moscatilin, which was isolated from the Dendrobium
aurantiacum var. denneanum, was revealed to exert
proapoptotic effects in pancreatic cancer cells. Moscatilin has
been reported to exert proapoptotic effects in esophageal cancer,
antimetastatic effects in lung and breast cancer, and
anti-angiogenic activities against malignant tumors (16–19).
The present study aimed to investigate whether the cytotoxicity of
moscatilin on Panc-1 cells was associated with intracellular ROS
production, and to determine the underlying mechanisms.
Materials and methods
Chemicals and reagents
Moscatilin (Fig.
1A) was isolated from the Dendrobium aurantiacum var.
denneanum. Its structure was determined using 1H-nuclear
magnetic resonance (NMR) and 13C-NMR spectral analyses, and its
purity was >98%, as determined by high-performance liquid
chromatography analysis as described (20). Moscatilin was dissolved in absolute
dimethyl sulfoxide (DMSO), and was further diluted with culture
medium on the experimental day.
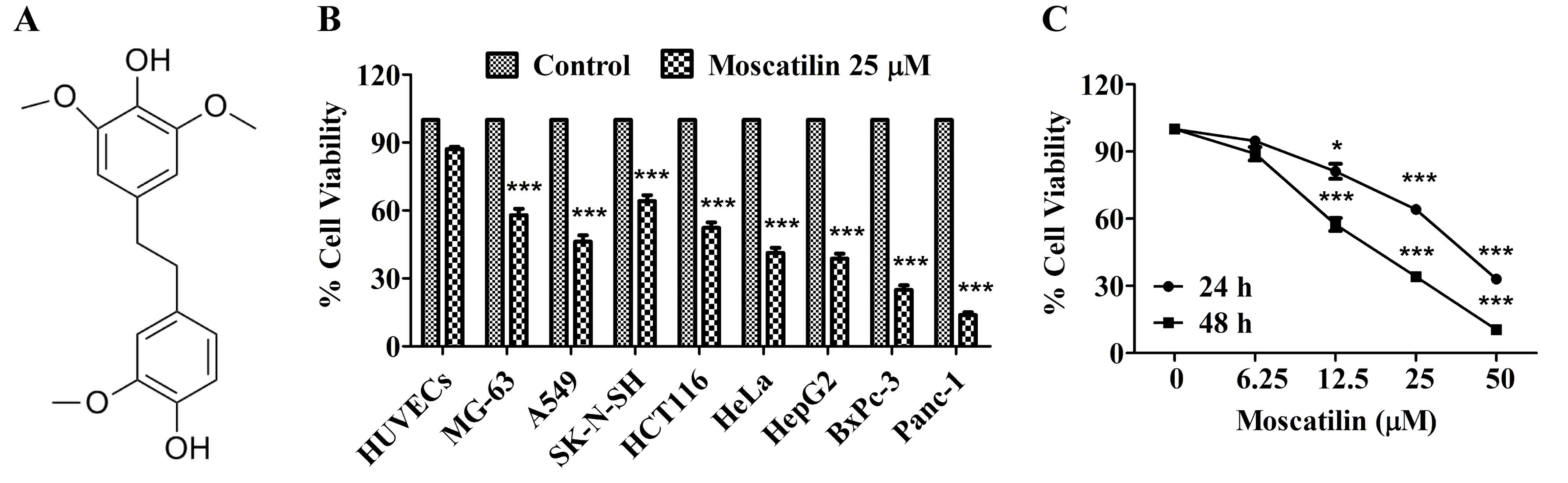 | Figure 1.Moscatilin inhibited cell viability
in vitro. (A) Chemical structure of moscatilin. (B) Cell
viability was measured following treatment with moscatilin (25 µM)
for 48 h in various cell lines, including HUVECs, MG-63, A549,
SK-N-SH, HCT116, HeLa, HepG2, BxPc-3 and Panc-1. Cell viability was
determined using MTT assay. (C) Cell viability was measured
following treatment with moscatilin (0–50 µM) for 24 and 48 h. Cell
viability was determined using MTT assay. Data are presented as the
mean ± standard error of the mean (n=3). *P<0.05, ***P<0.001,
compared with the control group. HUVECs, human umbilical vein
endothelial cells. |
Antibodies against phosphorylated (p)-JNK/SAPK (cat.
no. 9251), JNK/SAPK (cat. no. 9252), B-cell lymphoma 2 (Bcl2) (cat.
no. 2872), Bcl2-associated X protein (Bax) (cat. no. 2772), Bcl2
homologous antagonist killer (Bak; cat. no. 12105), caspase 3 (cat.
no. 9662), cleaved-caspase 3 (cat. no. 9661), poly (ADP-ribose)
polymerase (PARP) (cat. no. 9542) and GAPDH (cat. no. 2118) were
all purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Peroxidase-conjugated goat anti-rabbit immunoglobulin G (H+L;
cat. no. 111-065-003) was purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA). Cell culture reagents
were obtained from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA), 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium
bromide (MTT) and other reagents, unless otherwise indicated, were
purchased from Sigma-Aldrich (Merck Millipore, Darmstadt,
Germany).
Cell lines and cell culture
conditions
The MG-63 human osteosarcoma cancer cell line, the
A549 human lung cancer cell line, the SK-N-SH human neuroblastoma
cancer cell line, the HCT116 human colon cancer cell line, the HeLa
human cervical cancer cell line, the HepG2 human hepatic cancer
cell line, and the Panc-1 and BxPc-3 human pancreatic cancer cell
lines were purchased from the Chinese Academy of Science Committee
Type Culture Collection Cell Bank (Shanghai, China). Panc-1 cells
were cultured in Dulbecco's modified Eagle' medium (DMEM)
supplemented with 10% (v/v) fetal bovine serum (FBS) and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. MG-63, SK-N-SH, HCT116, HepG2 and
HeLa cells were cultured in minimum essential medium (MEM)
supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin at
37°C in a humidified atmosphere containing 5% CO2.
BxPc-3 cells were cultured in RPMI-1640 containing 10% (v/v) FBS
and 1% penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. A549 cells were cultured in F12K
medium supplemented with 10% (v/v) FBS and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. Human umbilical vein endothelial cell
(HUVEC) was supplied by Dr. Wentao Zhou, from Institute of
Nutrition Science, Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China) in 2014. HUVECs were
cultured as previously described (21). Cell viability assay. Cells were
seeded at a density of 5×103 cells/well into 96-well plates in DMEM
supplemented with 10% FBS. Following attachment, cells were treated
with moscatilin (0–50 µM) for 24 and 48 h. Following treatment, MTT
solution was added and incubated for 4 h at 37°C. The medium was
then removed, 100 µl DMSO was added and absorbance was quantified
at 570 nm. Cell viability was normalized as a percentage of
control. For the blocking study, cells were pretreated with 5 mM
N-acetylcysteine (NAC; Sigma-Aldrich; Merck Millipore), 50 µM
Z-VAD-FMK (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or 20
µM SP600125 (Cell Signaling Technology, Inc.) for 30 min, and then
treated with moscatilin (25 µM) for 24 h at 37°C.
Colony formation assay
Cells were resuspended in 1.5 ml growth media
containing 0.3% low-melting temperature agarose, and were plated in
6-well plates over a base layer of 1.5 ml growth media containing
0.6% low-melting temperature agarose. The cells were allowed to
form colonies for 15 days at 37°C in a humidified atmosphere
containing 5% CO2. The colonies were fixed with 4%
paraformaldehyde for 30 min at room temperature and were then
stained with 0.04% crystal violet for 30 min at room temperature.
Colonies with >50 cells were counted as one positive colony
under a microscope (IX81; Olympus Corporation, Tokyo, Japan). The
inhibition of colony formation was exhibited as a percentage of
vehicle control.
Hoechst 33342 staining assay
Cells (1×105 cells/well) were seeded into 6-well
plates in DMEM supplemented with 10% FBS. After attachment, cells
were treated with various concentrations of moscatilin (0–25 µM)
for 24 h. After treatment, the cells were incubated with Hoechst
33342 (10 µg/ml) for 5 min at room temperature in the dark. After
incubation, stained cells were observed under a fluorescent
microscope.
DNA fragmentation assay
Cells (3×105 cells/well) were seeded into 60 mm
plates in DMEM supplemented with 10% FBS. After attachment, cells
were treated with moscatilin (0–25 µM) for 24 h. After treatment,
cells were lysed and the fragmented DNA in the lysate was extracted
using a DNA extraction kit (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocols. Briefly,
the fragmented DNA in the lysate was extracted with
phenol/chloroform/isopropyl alcohol (25:24:1, v/v), and then
precipitated for 10 min in liquid nitrogen with chilled 100%
ethanol and 3 M sodium acetate. The DNA pellet produced by
centrifuging at 12,000 × g for 15 min at 4°C, the pellet was then
washed with 70% ethanol and resuspended in Tris-HCl (pH 8.0) with
100 µg/ml RNase A for 1 h at 37°C. The DNA fragments were separated
by 1.5% agarose gel electrophoresis, stained with ethidium bromide
and images were captured under ultraviolet light.
Measurement of ROS formation
After treatment with moscatilin (0–25 µM), the cells
were stained with DCFH-DA for 30 min at 37°C in the dark.
Subsequently, stained cells were observed under a fluorescent
microscope and absorbance was measured at 488 nm (excitation
wavelength) and 525 nm (emission wavelength).
Western blotting
After treatment with moscatilin (0–25 µM), the cells
were lysed in lysis buffer containing 50 mM Tris (pH 7.5), 1 mM
EDTA, 150 mM NaCl, 20 mM NaF, 0.5% NP-40, 10% glycerol, 1 mM
phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 10 µg/ml
leupeptin and 10 µg/ml pepstatin A. Protein concentration was
determined using Bradford protein assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Protein samples (30 µg) were separated using 10 or 12% premade
SDS-PAGE gels, and were then transferred to a nitrocellulose filter
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membrane was blocked with 10% bovine serum albumin (Santa Cruz
Biotechnology, Inc.) in 1X Tris-buffered saline-0.05% Tween-20
(TBST) for 2 h at room temperature. Following blocking, the
membrane was incubated with primary antibody (1:1,000) at 4°C
overnight. The membrane was washed three times with TBST and then
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody (1:5,000) for 1 h at room temperature. The protein bands
were visualized by RapidStep™ ECL reagent (Merck Millipore) and
semi-quantified with ImageJ version 1.6.0 (imagej.nih.gov/ij/).
Xenograft mouse model
Specific pathogen-free nude male mice (age, 8 weeks;
weight, 18–20 g) were provided by Shanghai Experimental Animal
Center of Chinese Academy of Sciences (Shanghai, China). Mice were
housed under specific pathogen-free conditions according to the
guidelines of the association for Assessment and Accreditation of
Laboratory Animal Care (Shanghai, China). They were housed under
standard conditions (25°C, 12-h light/dark cycle) with access to
food and water ad libitum. All studies were conducted in a
manner aiming to minimize animal suffering and to reduce the number
of animals used. Nude mice (n=6/group) were subcutaneously injected
with Panc-1 cells (1×106 cells/mouse) into the left front leg.
After tumors had been established (~30 mm3), the mice were
administered intraperitoneal injections of vehicle control (0.5%
DMSO and 0.5% Tween-80 in normal saline) or moscatilin (25 mg/kg in
vehicle control) every day. The body weight and tumor sizes of all
mice were recorded every 4 days. Tumor sizes were determined by
Vernier caliper measurements and were calculated as follows:
[(length × width2)/2]. After 21 days of treatment, mice were
sacrificed with pentobarbital sodium (150 mg/kg; delivered by
intraperitoneal injection), and their tumors were removed, weighed
and photographed.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Normal distributed data with equal variance were analyzed
using one- or two-way analysis of variance followed by Fisher's LSD
multiple comparisons test or Student's unpaired t-test for single
comparisons using SPSS 18.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Moscatilin inhibits viability of
pancreatic cancer cells
It is well known that cancer cell viability is an
important feature of cancer growth and development. The present
study initially investigated the cytotoxicity of moscatilin in a
panel of cell lines using the MTT assay. Following treatment with
moscatilin (25 µM) for 48 h, increased cytotoxicity was detected in
all tested cancer cell lines. These results indicate that
cytotoxicity was observed in all of the following tested cancer
cell lines: MG-63 (osteosarcoma), A549 (lung), SK-N-SH
(neuroblastoma), HCT116 (colon), HeLa (cervical), HepG2 (hepatic),
BxPc-3 and PanC-1 (pancreatic), following treatment with moscatilin
(25 µM) for 48 h (Fig. 1B). Among
the cell lines, the pancreatic cell lines were most sensitive to
moscatilin (Fig. 1B). Conversely,
moscatilin had little effect on the cell viability of HUVECs
(Fig. 1B). Subsequently, the
inhibitory effects of various concentrations of moscatilin were
detected on Panc-1 cell viability. As shown in Fig. 1C, 24 or 48 h treatment with
moscatilin (0–50 µM) markedly inhibited the viability of Panc-1
cells in a concentration-dependent manner.
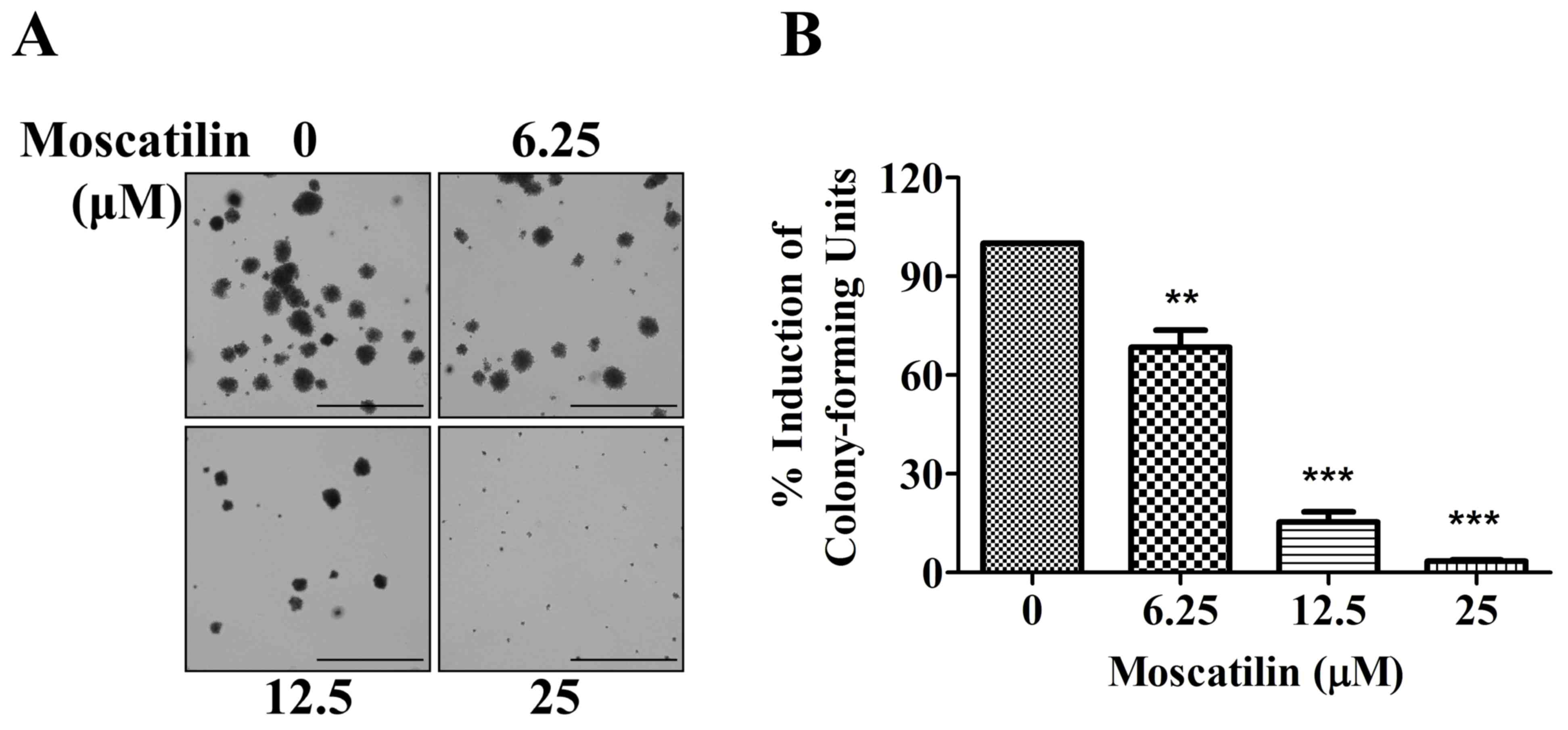
Moscatilin suppresses clonogenicity of
pancreatic cancer cells
To determine the potential effects of moscatilin on
long-term proliferation, a colony formation assay was conducted.
Panc-1 cells were exposed to moscatilin (0–25 µM) for 15 days. In
Panc-1 cells, colony formation was significantly decreased
following treatment with moscatilin. An initial decrease in
clonogenicity was observed following treatment with 6.25 µM
moscatilin, and a maximal response was detected following treatment
with 25 µM moscatilin (Fig. 2A and
B). These results suggest that treatment with moscatilin
suppressed clonogenicity in a dose-dependent manner compared with
the control group.
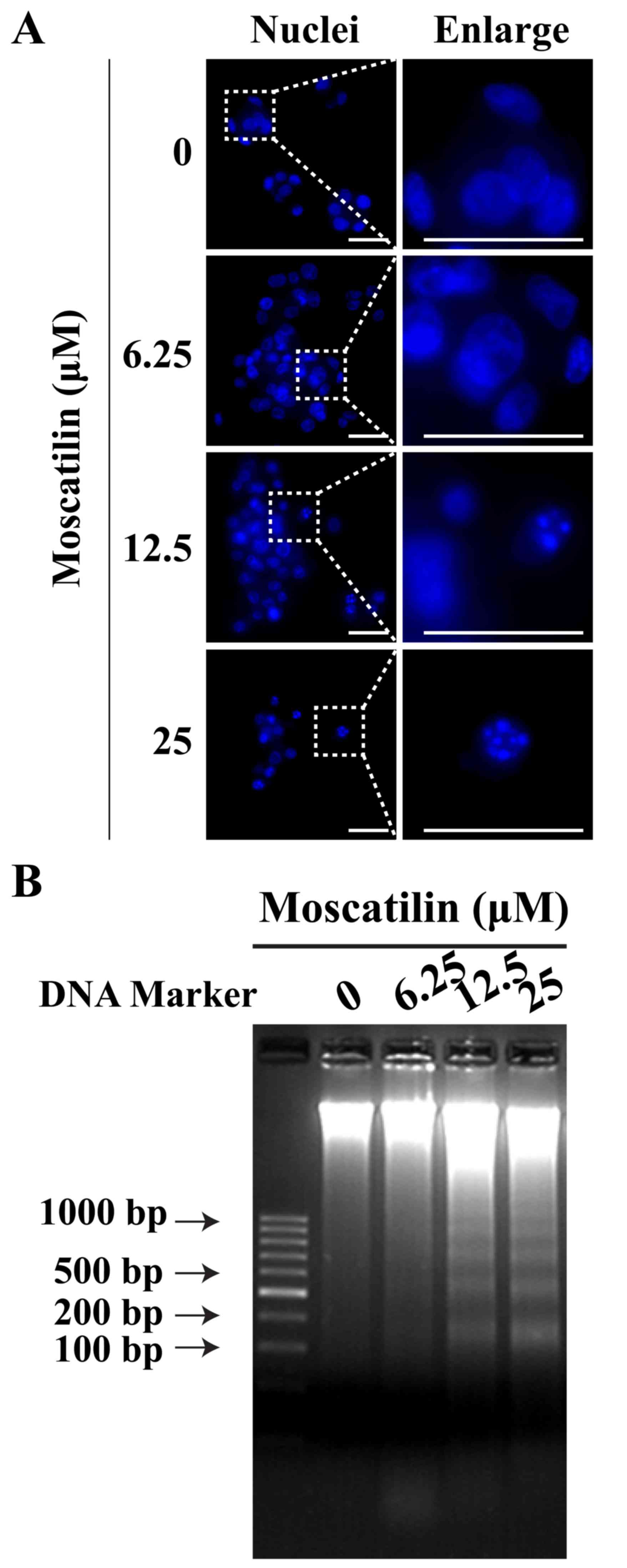
Moscatilin induces apoptosis of
pancreatic cancer cells
To determine whether the reduction in cell viability
by moscatilin was due to the induction of apoptosis, Hoechst
staining and a DNA fragmentation assay were conducted. In Panc-1
cells, treatment with moscatilin for 24 h led to nuclear
fragmentation and chromatin condensation in a
concentration-dependent manner, as determined by Hoechst staining
(Fig. 3A). Similarly, the present
study observed an induction in apoptotic DNA fragmentation
following treatment with moscatilin (0–25 µM) for 24 h, as
evidenced by the DNA fragmentation assay (Fig. 3B).
Moscatilin induces activation of the
mitochondrial apoptotic pathway
To investigate whether moscatilin induced apoptosis
via triggering the mitochondrial apoptotic pathway, the expression
levels of Bcl2 family proteins, including Bcl2, Bax and Bcl2
homologous antagonist killer (Bak), were detected in the presence
of moscatilin. The results indicated that treatment with moscatilin
induced a decrease in Bcl2 expression, and an increase in the
expression levels of Bak and Bax, which led to an increase in the
Bax/Bcl2 ratio (Fig. 4A and B). In
addition, treatment with moscatilin led to the cleavage of caspase
3 and PARP in Panc-1 cells (Fig.
4C). Similarly, treatment with the Pan-caspase inhibitor
Z-VAD-FMK prevented reductions in cell viability in response to
moscatilin (Fig. 4D). These
results indicate that moscatilin inhibits viability and induces
apoptosis via the mitochondrial apoptosis pathway.
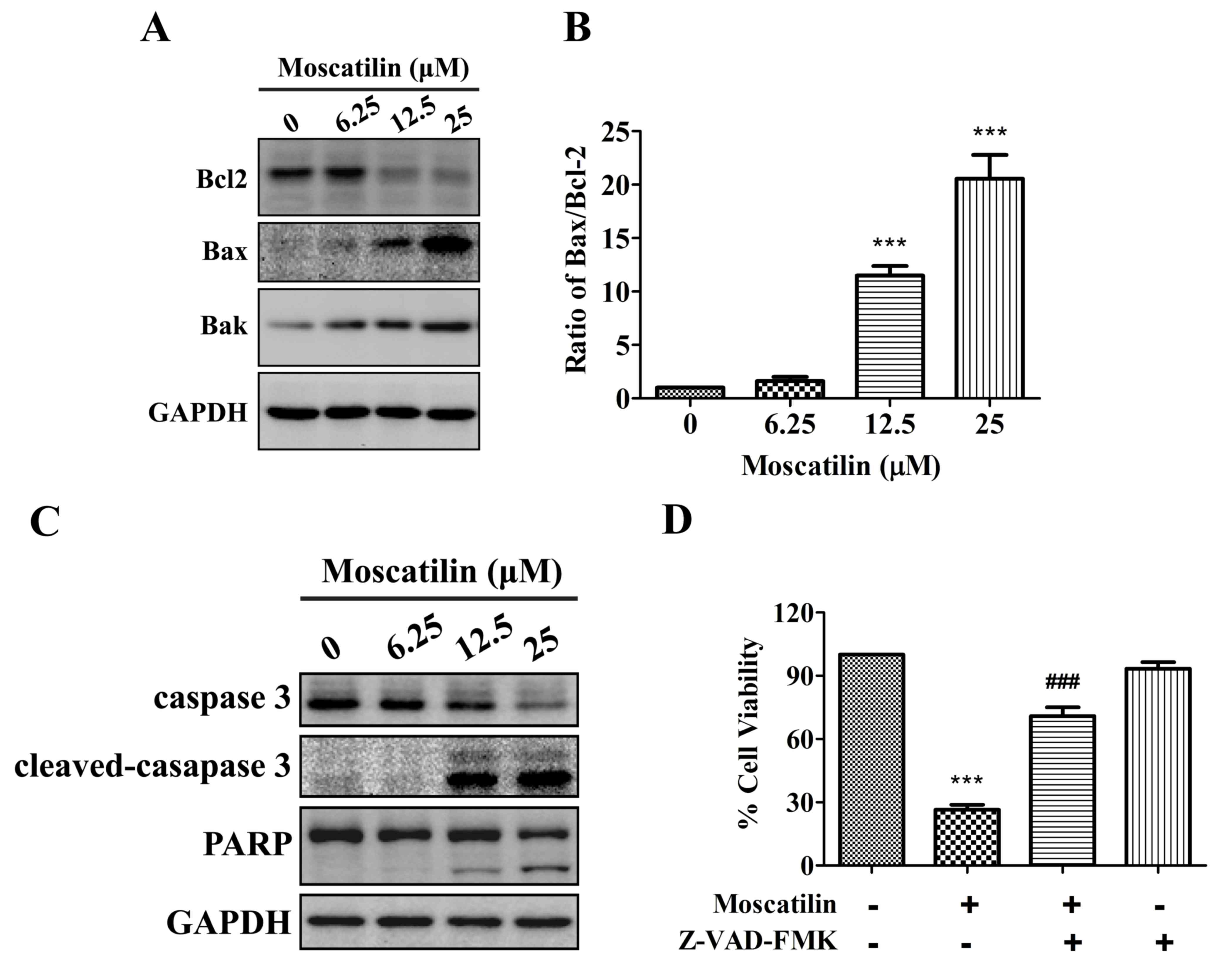 | Figure 4.Moscatilin induced the mitochondrial
apoptotic pathway. (A and B) Following treatment with moscatilin
(0–25 µM) for 24 h, the protein expression levels of Bcl2, Bax, Bak
and GAPDH were determined by western blotting in Panc-1 cells. (A)
Representative immunoblot. (B) Ratio of Bax/Bcl2 expression. (C)
Following treatment with moscatilin (0–25 µM) for 24 h, the protein
expression levels of caspase 3, cleaved-caspase 3, PARP and GAPDH
were determined by western blotting in Panc-1 cells. (D) Panc-1
cells were treated with moscatilin (25 µM) for 24 h following
pretreatment with Z-VAD-FMK (50 µM) for 30 min. Cell viability was
measured by MTT assay. Data are presented as the mean ± standard
error of the mean (n=3). ***P<0.001, compared with the control
group; ###P<0.001 compared with the moscatilin group.
Bcl2, B-cell lymphoma 2; Bax, Bcl2-associated X protein; Bak, Bcl2
homologous antagonist killer; PARP, poly (ADP-ribose)
polymerase. |
ROS/JNK signaling pathway is involved
in cell apoptosis in response to moscatilin
ROS exert a pivotal role in various processes
associated with tumor progression. High ROS levels are required for
the initiation of apoptotic responses induced by some anticancer
agents (22,23). The present study demonstrated that
treatment with moscatilin led to an increase in ROS generation. The
ROS levels in Panc-1 cells were increased by moscatilin in a
dose-dependent manner, with 1.2-, 1.9- and 3.0-fold increases
detected following treatment with 6.25, 12.5 and 25 µΜ moscatilin,
respectively (Fig. 5A and B).
Similarly, treatment with the ROS scavenger NAC prevented the
reductions in cell viability in response to moscatilin treatment
(Fig. 5C).
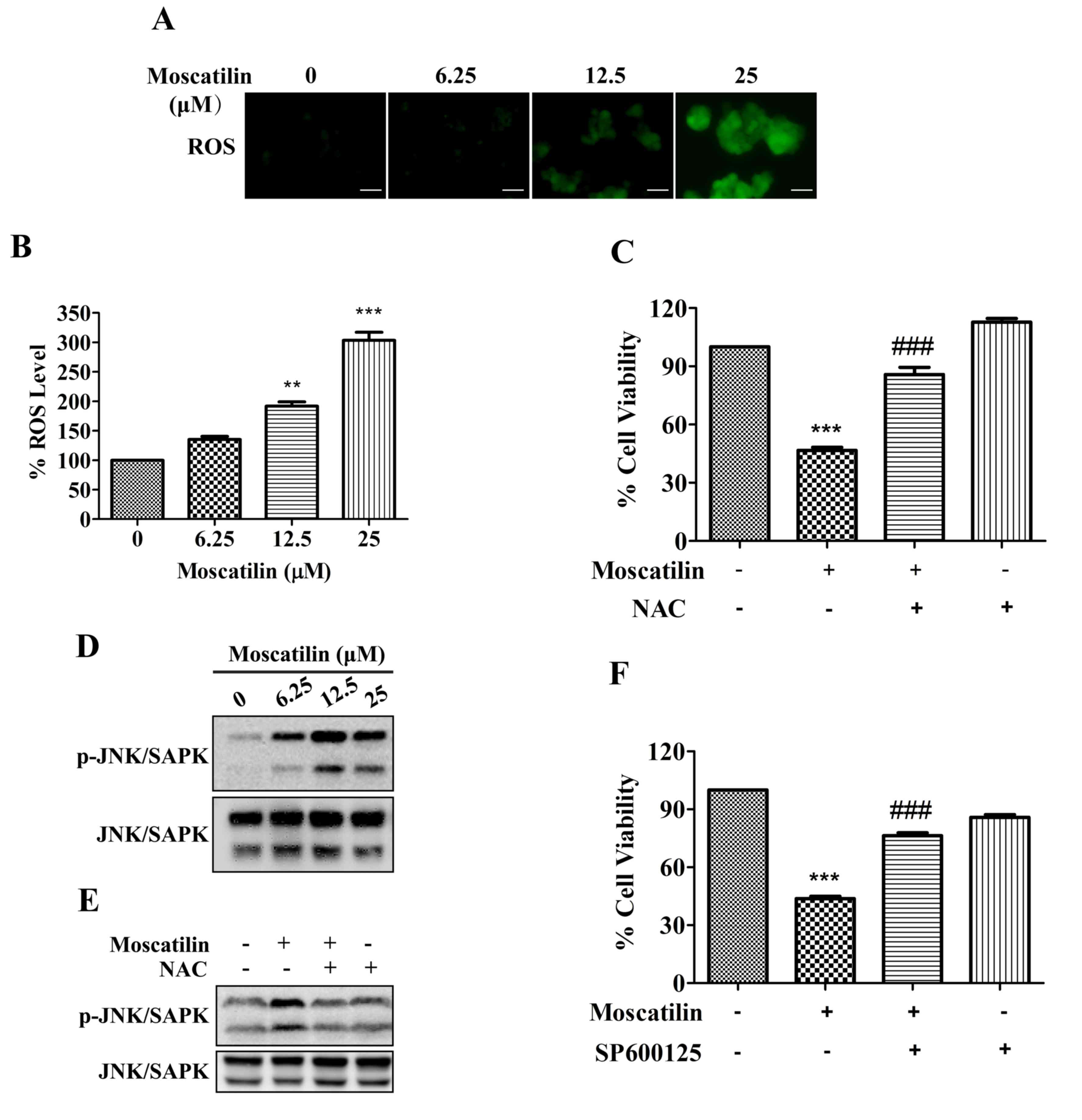 | Figure 5.ROS/JNK signaling pathway was
required for moscatilin-induced effects. (A and B) Following
treatment of Panc-1 cells with moscatilin (0–25 µM) for 24 h, ROS
were measured using the fluorescent dye
2′,7′-dichlorodihydrofluorescein diacetate. (A) Stained cells were
observed under an inverted fluorescent microscope and (B) the
fluorescence intensity units were measured at 488 nm (excitation
wavelength) and 525 nm (emission wavelength). Scale bar, 100 µm.
(C) Panc-1 cells were treated with moscatilin (25 µM) for 24 h
following pretreatment with NAC (5 mM) for 30 min. Cell viability
was measured by MTT assay. (D) Following treatment with moscatilin
(0–25 µM) for 24 h, the protein expression levels of p-JNK/SAPK,
JNK/SAPK and GAPDH were determined by western blotting in Panc-1
cells. (E) Panc-1 cells were treated with moscatilin (25 µM) for 24
h following pretreatment with NAC (5 mM) for 30 min. The protein
expression levels of p-JNK/SAPK and JNK/SAPK were determined by
western blotting. (F) Panc-1 cells were treated with moscatilin (25
µM) for 24 h following pretreatment with SP600125 (20 µM) for 30
min. Cell viability was measured by MTT assay. Data are presented
as the mean ± standard error of the mean. (n=3). **P<0.01,
***P<0.001, compared with the control group;
###P<0.001 compared with the moscatilin group. ROS,
reactive oxygen species; NAC, N-acetylcysteine; p-, phosphorylated;
JNK, c-Jun N-terminal kinase; SAPK, stress-associated protein
kinases. |
The JNK/SAPK signaling pathway is downstream of the
ROS signaling pathway, which is implicated in controlling cell
proliferation, differentiation and apoptosis (24). As shown in Fig. 5D, phosphorylation of JNK/SAPK was
observed following treatment with 6.25 µΜ moscatilin, and was
maintained up to 25 µΜ. Conversely, moscatilin-induced activation
of JNK/SAPK was attenuated in Panc-1 cells following pretreatment
with the ROS scavenger NAC (Fig.
5E). Furthermore, the moscatilin-induced reduction in cell
viability was attenuated by the JNK/SAPK-specific inhibitor
SP600125 (Fig. 5F). These results
indicate that the ROS/JNK signaling pathway may serve a pivotal
role in apoptotic induction of Panc-1 cells by moscatilin.
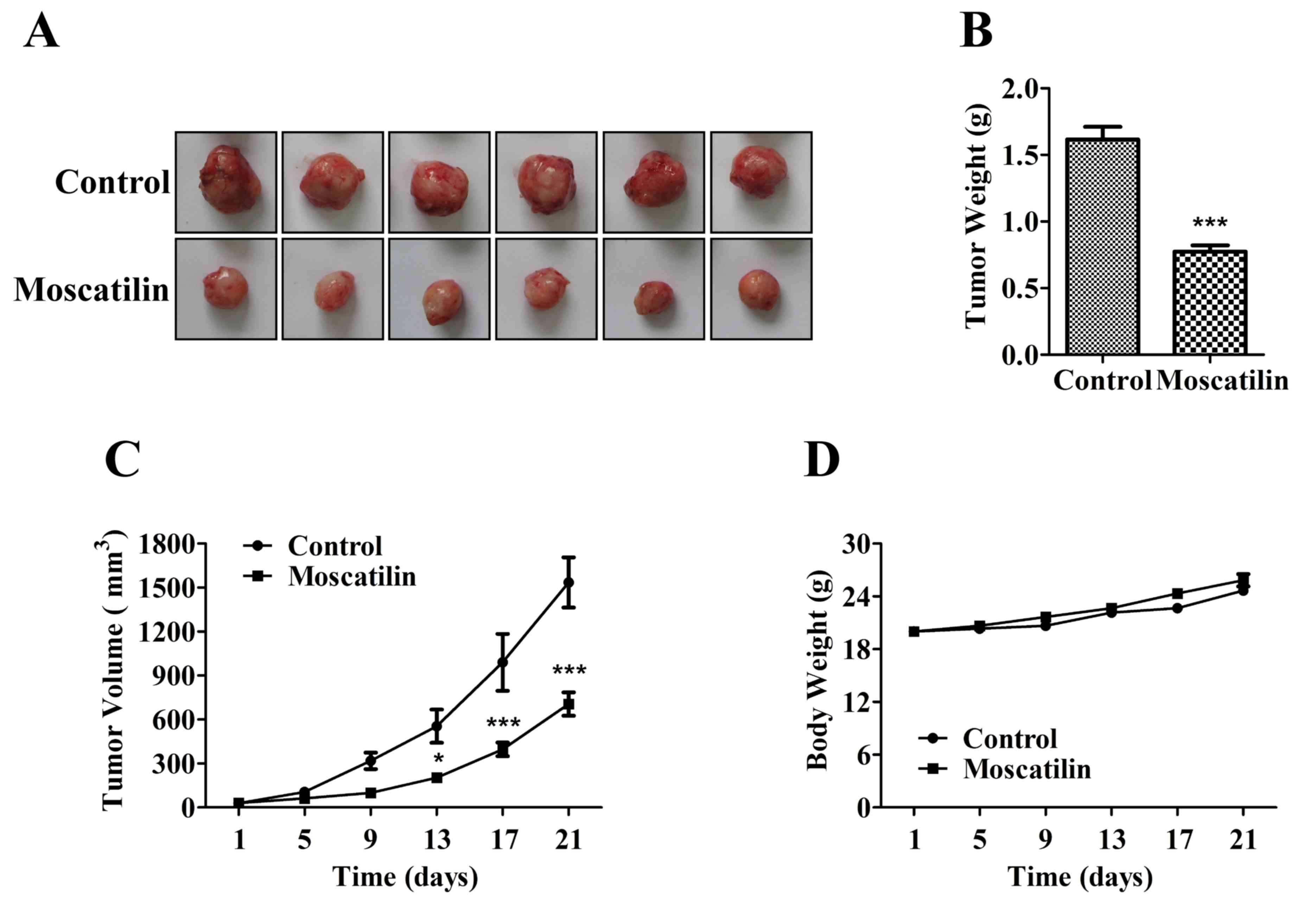
Moscatilin inhibits pancreatic cancer
growth in vivo
To determine the potential effects of moscatilin on
pancreatic cancer growth and development, equal numbers of Panc-1
pancreatic cancer cells were subcutaneously injected into the left
front leg of nude mice. Once the tumors reached ~30 mm3, the mice
were treated intraperitoneally with control (0.5% DMSO and 0.5%
Tween-80 in normal saline) or moscatilin (25 mg/kg) every day.
Following treatment with moscatilin for 21 days, tumor weight was
reduced by 52% compared with in the control group (Fig. 6A-C). There was no difference in
body weight between the control and moscatilin-treated groups
(Fig. 6D).
Discussion
Pancreatic cancer is the fourth leading cause of
cancer-associated mortality worldwide, and is one of the most
invasive and frequently diagnosed malignancies, due to its poor
prognosis (1,4). The high mortality rate associated
with pancreatic cancer indicates that the currently available
treatments, such as chemotherapy, are ineffective (3,4).
Therefore, there is an increasing need to identify novel
proapoptotic agents that can be used to treat tumor growth in the
advanced stages of pancreatic cancer.
Cell proliferation is considered to be an important
feature of cell growth and development. The present study
demonstrated that moscatilin markedly inhibited the viability of
various cancer cell lines, and exhibited higher sensitivity in
pancreatic cancer cell lines. Conversely, moscatilin failed to
affect the cell viability of normal HUVECs. These results indicated
the specific anti-tumor activity of moscatilin, and suggested it
possessed a favorable therapeutic index. Furthermore, moscatilin
was revealed to strongly suppress pancreatic tumor growth and
development, without any apparent toxicity, in vivo. In
previous studies, moscatilin has been demonstrated to possess
proapoptotic effects in esophageal cancer, antimetastatic effects
in lung and breast cancer, and anti-angiogenic activity against
malignant tumors (16–19). These previous data indicated that
moscatilin is worthy of being developed into a therapeutic agent
for the prevention and treatment of pancreatic cancer.
Apoptosis serves a pivotal role in the development
of cancer, including cancer initiation, progression and metastasis
(25,26). It has previously been reported that
apoptosis is characterized by certain hallmarks, such as
phosphatidyl serine exposure on the plasma membrane, activation of
caspase 3 and PARP, and DNA fragmentation (27). The results of the present study
indicated that activation of caspase 3 and PARP contribute to
moscatilin-induced apoptosis of Panc-1 cells. The key event in the
intrinsic apoptotic pathway is permeabilization of the
mitochondrial outer membrane, which occurs in response to various
stimuli, and is regulated by several cytoplasmic proteins,
including Bcl2 family members (28). Anti-apoptotic members, such as
Bcl2, prevent apoptosis, whereas proapoptotic members, such as Bax
and Bak, reside on the outer mitochondrial membrane or cytosol and
oligomerize under stress to facilitate the release of factors from
the mitochondria, which trigger apoptosis (29). Therefore, the Bax/Bcl2 ratio, as a
candidate prognostic biomarker for cancer, indicates the degree of
mitochondrial outer membrane permeabilization and hence the
entrance to the execution phase of the apoptotic pathway. The
present study indicated that an increase in Bak expression and an
increase in the Bax/Bcl2 (proapoptotic/anti-apoptotic) ratio
contribute to the involvement of the mitochondrial-mediated
intrinsic pathway in moscatilin-induced apoptosis.
ROS are recognized as intracellular secondary
messengers in various cell receptor signal transduction pathways.
Although higher than normal ROS levels are observed in cancer
cells, it has previously been suggested that cancer cells are more
vulnerable to intracellular ROS introduction (30). Therefore, cancer treatment by means
of enhancing intracellular ROS production may be considered an
effective approach. Natural compounds, such as nimbolide and
bufalin, are able to induce pancreatic cancer cell apoptosis by
increasing ROS levels (31,32).
The present study demonstrated that treatment with 25 µM moscatilin
led to an increase in ROS generation; however, moscatilin-inhibited
cell viability was abolished by NAC, thus suggesting that ROS is
responsible for the proapoptotic effects of moscatilin on
pancreatic cancer cells. Kowitdamrong et al (18) demonstrated that moscatilin (1 µM)
suppressed ROS generation and FeSO4-mediated ROS
generation, and the inhibition of ROS was critical for
moscatilin-mediated suppression of cell motility and invasion, but
not cell growth, in lung cancer. The different effects of
moscatilin on ROS levels may be due to the various concentrations
of moscatilin used, or may be attributed to different cancer cell
functions (cancer cell growth and cancer cell metastasis), or the
fact that Panc-1 pancreatic cancer cells and H23 lung cancer cells
are two different cancer cell lines.
Mitogen-activated protein kinases, including
extracellular signal-regulated kinase (ERK1/2), p38 and JNK/SAPK,
are primarily activated by exposure to ROS and serve important
roles in regulating cell proliferation, differentiation, mitosis,
survival and apoptosis (33,34).
Although the ERK1/2 pathway is considered a great contributor to
oncogenesis, a previous study demonstrated that it serves a lesser
role in mitogen-induced survival of pancreatic cancer (35). In addition, JNK/SAPK activation is
considered an important apoptosis-inducing factor that exerts
proapoptotic effects on apoptosis of cancer cells (36,37).
Previous studies have indicated that JNK/SAPK activation results in
an increase in the number of apoptotic cells in response to several
anticancer agents in pancreatic cancer (38,39).
The present study demonstrated that treatment with moscatilin
induced sustained activation of JNK/SAPK, and the phosphorylation
of JNK/SAPK was dependent on ROS generation, which was prevented by
treatment with the ROS scavenger NAC. Furthermore, treatment with a
JNK/SAPK inhibitor restored the viability of moscatilin-treated
Panc-1 cells. These observations indicate that elevation of ROS
generation and the subsequent activation of JNK/SAPK serves a
crucial role in the induction of programmed cell death in
pancreatic cancer in response to moscatilin.
In conclusion, the present study demonstrated that
moscatilin increases ROS generation and subsequently activates the
JNK/SAPK pathway, which modulates the Bax/Bcl2 ratio, thus leading
to the caspase-dependent mitochondrial apoptotic pathway. The
present study provided detailed mechanistic insights into the
proapoptotic effects of moscatilin in cells; these data strongly
support the application of moscatilin as a potential future
treatment against pancreatic cancer.
Acknowledgements
The present study was supported financially by the
Youth Foundation of Zhongshan Hospital Fudan University (grant no.
2014ZSQN39).
References
|
1
|
Rahib L, Smith BD, Aizenber R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paulson AS, Cao Tran HS, Tempero MA and
Lowy AM: Therapeutic advances in pancreatic cancer.
Gasroenterology. 144:1316–1326. 2013. View Article : Google Scholar
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexandre J, Hu Y, Lu W, Pelicano H and
Huang P: Novel action of paclitaxel against cancer cells: Bystander
effect mediated by reactive oxygen species. Cancer Res.
67:3512–3517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paduch R, Kandefer-Szerszeń M and Piersiak
T: The important of release of proinflammatory cytokines, ROS, and
NO in different stages of colon carcinoma growth and metastasis
after treatment with cytotoxic drugs. Oncol Res. 18:419–436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Groninger E, Meeuwsen-De Boer GJ, De Graaf
SS, Kamps WA and De Bont ES: Vincristine induced apoptosis in acute
lymphoblastic leukaemia cells: A mitochondrial controlled pathway
regulated by reactive oxygen species? Int J Oncol. 21:1339–1345.
2002.PubMed/NCBI
|
|
9
|
Zhang X, Xu JK, Wang J, Wang NL, Kuriihara
H, Kitanaka S and Yao XS: Bioactive bibenzyl derivatives and
fluorenones from Dendrobium nobile. J Nat Prod. 70:24–28.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CC, Wu LG, Ko FN and Teng CM:
Antiplatelet aggregation principles of Dendrobium
loddigesii. J Nat Prod. 57:1271–1274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu JM, Chen JJ, Yu H, Zhao YX and Zhou J:
Two novel bibenzyls from Dendrobium trigonopus. J Asian Nat
Prod Res. 10:653–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong Y, Fan Y, Liu L, Wu D, Chang Z and
Wang Z: Erianin induces a JNK/SAPK-dependent metabolic inhibition
in human umbilical vein endothelial cells. In Vivo. 18:223–228.
2004.PubMed/NCBI
|
|
13
|
Gong YQ, Fan Y, Wu DZ, Yang H, Hu ZB and
Wang ZT: In vivo and in vitro evaluation of erianin, a novel
anti-angiogenic agent. Eur J Cancer. 40:1554–1565. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pengpaeng P, Sritularak B and
Chanvorachote P: Dendrofalconerol A suppresses migrating cancer
cells via EMT and integrin proteins. Anticancer Res. 35:201–205.
2015.PubMed/NCBI
|
|
15
|
Charoenrungruang S, Chanvorachote P,
Sritularak B and Pongrakhananon V: Gigantl, a bibenzyl from
Dendrobium draconis, inhibits the migratory behavior of
non-small cell lung cancer cells. J Nat Prod. 77:1359–1366. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CA, Chen CC, Shen CC, Chang HH and
Chen YJ: Moscatilin induces apoptosis and mitotic catastrophe in
human esophageal cancer cells. J Med Food. 16:869–877. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pai HC, Chang LH, Peng CY, Chang YL, Chen
CC, Shen CC, Teng CM and Pan SL: Moscatilin inhibits migration and
metastasis of human breast cancer MDA-MB-231 cells through
inhibition of Akt and Twist signaling pathway. J Mol Med (Berl).
91:347–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kowitdamrong A, Chanvorachote P,
Sritularak B and Pongrakhananon V: Moscatilin inhibits lung cancer
cell motility and invasion via suppression of endogenous reactive
oxygen species. Biomed Res Int. 2013:7658942013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai AC, Pan SL, Liao CH, Guh JH, Wang SW,
Sun HL, Liu YN, Chen CC, Shen CC, Chang YL and Teng CM: Moscatilin,
a bibenzyl derivative from the India orchid Dendrobium
loddigesii, suppresses tumor angiogenesis and growth in vitro
and in vivo. Cancer Lett. 292:163–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang L, Wang Y, Zhang G, Zhang F, Zhang Z,
Wang Z and Xu L: Stimulaneous quantitative and qualitative analysis
of bioactive phenols in Dendrobium aurantiacum var.
Denneanum by high-performance liquid chromatography coupled
with mass spectrometry and diode array detection. Biomed
Chromatogr. 21:687–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marin V, Kaplanski G, Grès S, Farnarier C
and Bongrand P: Endothelial cell culture: Protocol to obtain and
cultivate human umbilical endothelial cells. J Immunol Methods.
254:183–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jo GH, Kim GY, Kim WJ, Park KY and Choi
YH: Sulforaphane induces apoptosis in T24 human urinary bladder
cancer cells through a reactive oxygen species-mediated
mitochondrial pathway: The involvement of endoplasmic reticulum
stress and the Nrf2 signaling pathway. Int J Oncol. 45:1497–1506.
2014.PubMed/NCBI
|
|
23
|
Kello M, Drutovic D, Chripkova M, Pilatova
M, Budovska M, Kulikova L, Urdzik P and Mojzis J: ROS-dependent
antiproliferative effect of brassinin derivative homobrassinin in
human colorectal cancer Caco2 cells. Molecules. 19:10877–10897.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vriz S, Reiter S and Galliot B: Cell
death: A program to regenerate. Curr Top Dev Biol. 108:121–151.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li MX and Dewson G: Mitochondria and
apoptosis: Emerging concepts. F1000Prime Rep. 7:422015.PubMed/NCBI
|
|
26
|
Solary E, Dubrez L and Eymin B: The role
of apoptosis in the pathogenesis and treatment of diseases. Eur
Respir J. 9:1293–1305. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suliman A, Lam A, Datta R and Srivastava
RK: Intracellular mechanisms of TRAIL: Apoptosis through
mitochondrial-dependent and independent-pathways. Oncogene.
20:2122–2133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaux DL, Cory S and Adams JM: Bcl-2 gene
promotes haemopoietic cell survival and cooperates with c-myc to
immortalize pre-B cells. Nature. 335:440–442. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong LY, Chuang CC, Wu S and Zuo L:
Reactive oxygen species in redox cancer therapy. Cancer Lett.
367:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Surbramani R, Gonzalez E, Arumugam A,
Nandy S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S,
Narayan M, et al: Nimbolide inhibits pancreatic cancer growth and
metastasis through ROS-mediated apoptosis and inhibition of
epithelial-mesenchymal transition. Sci Rep. 6:198192016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian X, Dai S, Sun J, Jiang S, Sui C, Meng
F, Li Y, Fu L, Jiang T, Wang Y, et al: Bufalin induces
mitochondrial-dependent apoptosis in pancreatic and oral cancer
cells by downregulating hTERT expression via activation of the
JNK/p38 pathway. Evid Based Complement Alternat Med.
2015:5462102015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lei YY, Wang WJ, Mei JH and Wang CL:
Mitogen-activated protein kinase signal transduction in solid
tumors. Asian Pac J Cancer Prev. 15:8539–8548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pearson G, Robinson F, Gibson Beers T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perugini RA, McDade TP, Vittimeberga FJ Jr
and Callery MP: Pancreatic cancer cell proliferation is
phosphatidylinositol 3-kinase dependent. J Surg Res. 90:39–44.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tournier C: The 2 faces of JNK signaling
in cancer. Genes Cancer. 4:397–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takahashi R, Hirata Y, Sakitani K, Nakata
W, Kinoshita H, Hayakawa Y, Nakagawa H, Sakamoto K, Hikiba Y,
Ijichi H, et al: Theraputic effect of c-Jun N-terminal kinase
inhibition on pancreatic cancer. Cancer Sci. 104:337–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia G, Kong R, Ma ZB, Han B, Wang YW, Pan
SH, Li YH and Sun B: The activation of c-Jun
NH2-terminal kinase is required for
dihydroartemisinin-induced autophagy in pancreatic cancer cells. J
Exp Clin Cancer Res. 33:82014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Liang X and Yang X: Ursolic acid
inhibits growth and induces apoptosis in gemcitabine-resistant
human pancreatic cancer via the JNK and PI3K/Akt/NF-kB pathways.
Oncol Rep. 28:501–510. 2012.PubMed/NCBI
|