Introduction
Adenosine diphosphate (ADP) ribosylation, which
includes mono-ADP-ribosylation, poly-ADP-ribosylation, ADP-ribose
cyclization and formation of O-acetyl-ADP-ribose, is involved in a
wide range of human physiological and pathological processes and
serves important roles in cell signal transduction, transcriptional
regulation, genetic stability maintenance, cell proliferation and
differentiation, adhesion and migration (1). Mono-ADP-ribosyltransferases (ART),
the enzymes of mono-ADP-ribosylation, consist of seven members
(ART1-7). ART1 catalyzes the mono-ADP-ribosylation of nicotinamide
adenine dinucleotide to arginine residues in proteins, thereby
releasing nicotinamide, which may alter the structure and chemical
property of acceptor proteins resulting in a change in their
activity and function (2).
Research on ART1 is mainly concentrated on the inflammatory
response and on non-neoplastic cells (3,4). In
the epithelial cells of the respiratory tract and the
bronchoalveolar lavage fluid of people with asthma, ART1 may
catalyze the mono-ADP-ribosylation of human neutrophil peptide-1
(3), resulting in an inflammatory
response. Yau et al (5)
demonstrated that meta-iodobenzylguanidine (MIBG), a selective
inhibitor of arginine-specific mono-ADP-ribosylation (6), is able to suppress the proliferation
and differentiation of vascular smooth muscle cells. The
researchers hypothesized that mono-ADP-ribosylation is involved in
a Rho-dependent signaling pathway.
However, although ART1 is associated with the
proliferation and migration of colon cancer cells in vivo,
its molecular mechanism has yet to be fully elucidated. In the
present study, mouse colon carcinoma CT26 cells were infected with
a lentivirus to change the expression of ART1 in CT26 cells. To
observe the effect of ART1 on the development of colon carcinoma
in vivo, CT26 cells with ART1 silencing or overexpression
were injected into BALB/c mice to construct a subcutaneously
transplanted tumor model or a spleen transplant tumor model. Growth
of the tumor and liver metastases were observed. In addition, the
expression of focal adhesion kinase (FAK), Ras homolog gene family
member A (RhoA) and their downstream factors, c-myc, c-fos and
cyclooxygenase-2 (COX-2) proteins, were measured. The potential
role of ART1 in the proliferation and invasion of CT26 cells and
its possible mechanism in vivo were explored.
Materials and methods
Cell lines and animals
The mouse colon adenocarcinoma CT26 cell line was
obtained from Professor Yu-Quan Wei (Sichuan University, Chengdu,
Sichuan, China), Tang et al and Kuang et al (7,8),
having successfully constructed ART1-short hairpin RNA (shRNA),
ART1-overexpression and vector-control CT26 cells. BALB/c mice (6–8
weeks old, 18–22 g) were obtained from the animal experimental
center of Chongqing Medical University (Chongqing, China) and
placed in the specific pathogen-free feeding room (20–26°C, 12 h:12
h light/dark cycle) of the animal experimental center at Chongqing
Medical University.
Subcutaneously transplanted tumor
model of CT26 cells in BALB/c mice
Each experimental group consisted of 12 mice. Each
mouse was anesthetized by the intraperitoneal injection of 2%
chloral hydrate (0.3 g/kg). CT26 cell suspension (1×107/mlx50 µl)
was subcutaneously injected into the lateral skin of the right
armpit of each mouse. After 14 days, six mice were randomly
selected from each group for sacrifice, and the weight and volume
of the subcutaneous tumor was recorded. The survival time of the
rest of the mice in each group was recorded. Tumor volume was
calculated according to the formula: Volume=the maximum diameter ×
the most trails2 × ½ (9).
Spleen transplant tumor model of CT26
cells in BALB/c mice to observe liver metastases
A total of 48 BALB/c mice were randomly divided into
four groups. Following the method described by Liu et al
(10), each mouse was anesthetized
with 2% chloral hydrate (0.015 ml/g) injected into the abdominal
cavity. Subsequently, the abdominal wall was incised along with the
left subcostal margin layer by layer. The spleen was identified in
the abdominal cavity, and then CT26 cell suspension (1×107/ml × 50
µl) was injected under the capsule of the spleen. Finally, the
abdominal wall was sutured. The entire procedure was performed
under sterile conditions to ensure the survival rate of the mice.
While being reared the mice were provided with standard chow and
tap water ad libitum. After 14 days, six mice were randomly
selected from each group for sacrifice and the remaining mice of
each group continued to be fed until their natural death to enable
the recording of the survival time and the plotting of a
Kaplan-Meier survival curve.
The volume of the spleen tumors was calculated
according to the formula volume=the maximum diameter × the most
trails2 × ½ (9).
Nodules of liver metastases were graded as follows: Grade 0, no
visible metastatic nodule in liver; grade 1, 1–5 metastatic nodules
in liver; grade 2, 6–10 metastatic nodules in liver; grade 3,
>10 metastatic nodules, or fused nodules difficult to count
exactly (10).
Expression levels of ART1, RhoA,
c-myc, c-fos and COX-2 detected with western blotting in the
subcutaneously transplanted tumor
The subcutaneous tumors were cut into small pieces,
weighed, homogenized, and then lysed with radio-immunoprecipitation
assay (RIPA) lysis buffer (100 µl of RIPA lysis buffer/10 mg
tissue; Beyotime Institute of Biotechnology, Shanghai, China) for
30 min on ice. The lysate was transferred into a 1.5 ml centrifuge
tube and centrifuged at 4°C 12,000 rpm (8,418 g) for 5 min. A
bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of
Biotechnology) was used to measure the concentration of protein.
Protein (80 µg/lane) was electrophoresed on 10% polyacrylamide gels
(SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF)
membranes. The membranes were blocked with 5% non-fat dried milk
dissolved in Tris-buffered saline with Tween-20 (TBST) at room
temperature for 2 h, and incubated respectively with primary
antibodies of ART1 (cat. no. AP2311a; Abgent, Inc., San Diego, CA,
USA), RhoA (cat. no. BS6470), and c-fos (cat. no. BS6433; Bioworld
Technology, Inc., St. Louis, MO, USA), c-myc (cat. no. C10262;
Anbo, Inc., San Francisco, CA, USA), COX-2 (cat. no. 12375-1-AP;
Proteintech Group, Inc., Chicago, IL, USA) and β-actin (cat. no.
BA2305; Boster Systems, Wuhan, China) overnight at 4°C. The most
effective working concentration of these primary antibodies was
1:500. The membranes were washed three times with TBST, and then
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG secondary antibody at a dilution of 1:1,000 (ZSGB-BIO, Beijing,
China) for 1.5 h at room temperature. The membranes were washed
three times with TBST, and then dipped into BeyoECL Plus (Beyotime
Institute of Biotechnology, Shanghai, China) for exposure and
imaging (Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin
was used as a loading control for the western blotting
experiments.
Western blot analysis of expression
levels of ART1, RhoA and FAK in transplanted spleen tumors
Total protein was extracted from transplanted spleen
tumors. The tissue was washed with phosphate-buffered saline (PBS)
and then homogenized prior to being lysed with RIPA lysis buffer
(100 µl/10 mg) for 30 min on ice. The homogenate was transferred to
a pre-cooled centrifuge tube, and then centrifuged at 4°C, 12,000
rpm (8,418 g) for 10 min. The rest of the procedure was as detailed
in the previous paragraph with the exception that the primary
antibodies, ART1 (Abgent, Inc.), RhoA and FAK (cat. no. BS6899;
Bioworld Technology, Inc.) at a dilution of 1:500, and β-actin
(Boster Systems) at a dilution of 1:1,000 were used to incubate the
PVDF membranes.
Statistical analysis
Data were presented as the mean ± standard
deviation. Analysis of variance statistical evaluation was used and
analyses were performed using SPSS software, version 17.0 (SPSS,
Inc., Chicago, IL, USA). The Kruskal-Wallis and Nemenyi methods
were used to analyze the level of metastatic nodules in the liver.
The differences in tumor-bearing mice survival time were analyzed
using the log-rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of ART1 on the growth of
subcutaneous transplanted CT26 tumors in BALB/c mice
Compared with subcutaneous transplanted
vector-control and untransfected CT26 tumors, the volume and weight
of subcutaneous transplanted tumors were decreased in the
ART1-shRNA group (P<0.05) and increased in the
ART1-overexpression group (P<0.05; Fig. 1A-C).
Effects of ART1 on the growth of
spleen transplanted CT26 tumor in BALB/c mice
The volume and weight of spleen-transplanted
ART1-shRNA CT26 tumors were significantly decreased compared with
the spleen-transplanted vector-control and untransfected CT26
tumors (P<0.05). However, the volume and weight of
spleen-transplanted ART1-overexpression CT26 tumors were increased
(P<0.05). No significant differences were identified between the
spleen-transplanted vector-control and untransfected CT26 tumors
(P>0.05; Fig. 2A-C).
Effect of ART1 on liver metastasis of
colon carcinoma in BALB/c mice
The number of liver metastatic tumor nodules in the
spleen-transplanted CT26 tumor model were counted in each group.
The number of liver metastatic tumor nodules in the ART1-shRNA
group was lower than in the vector-control and untransfected groups
(P<0.05). The number of liver metastatic tumor nodules in the
ART1-overexpression group was higher compared with the
vector-control and untransfected groups (P<0.05). No significant
differences were identified in the number or the appearance of
liver metastatic tumor nodules in the vector-control and
untransfected groups (P>0.05; Fig.
3 and Table I).
 | Table I.The quantity and grading of metastases
in liver (n=6, mean ± standard deviation). |
Table I.
The quantity and grading of metastases
in liver (n=6, mean ± standard deviation).
|
|
| Grade |
|---|
|
|
|
|
|---|
| Group | Quantity of
metastases in the liver | 0 | 1 | 2 | 3 |
|---|
| ART1-shRNA |
0.33±0.82a | 5 | 1 | 0 | 0 |
| Vector | 2 2.33±12.11 | 0 | 0 | 1 | 5 |
| Untransfected |
23.83±10.340 | 0 | 0 | 1 | 5 |
|
ART1-overexpression |
39.50±8.38b | 0 | 0 | 0 | 6 |
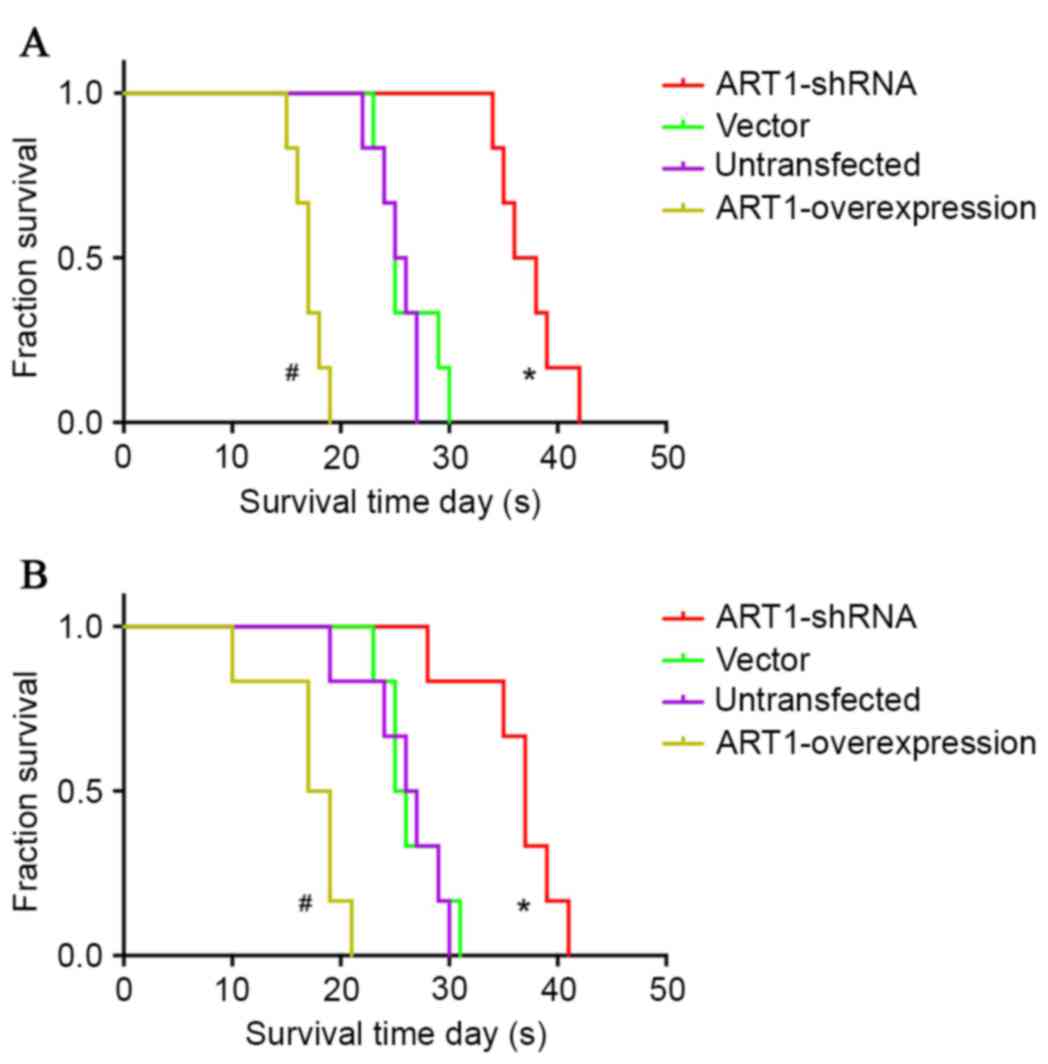
Influence of ART1 on the survival time
of BALB/c mice with subcutaneous transplanted CT26 tumor or spleen
transplanted CT26 tumor
The average survival time of BALB/c mice with
subcutaneously transplanted CT26 tumors was extended in the
ART1-shRNA group (P<0.05), and was shortened in the
ART1-overexpression group (P<0.05). However, no significant
differences were identified between the vector-control and
untransfected groups (P>0.05; Fig.
4A).
The average survival time of BALB/c mice with
spleen-transplanted ART1-shRNA CT26 tumors was longer compared with
vector-control and untransfected groups (P<0.05). The average
survival time of BALB/c mice with spleen-transplanted
ART1-overexpression CT26 tumors was shorter than in the control
groups (P<0.05). No significant differences were identified
between the vector-control and untransfected groups (P>0.05;
Fig. 4B).
Effect of ART1 on the expression
levels of RhoA, c-myc, c-fos and COX-2 in subcutaneously
transplanted CT26 tumor tissue of BALB/c mice
The expression levels of ART1, RhoA, c-myc, c-fos
and COX-2 in subcutaneously transplanted ART1-overexpression CT26
tumors were all higher than those in the control groups
(P<0.05). However, the expression levels of ART1, RhoA, c-myc,
c-fos and COX-2 in subcutaneously transplanted ART1-shRNA CT26
tumors were lower than in those in the control groups (P<0.05).
No significant differences were identified between the
vector-control and untransfected groups (P>0.05; Fig. 5A and B).
 | Figure 5.Effect of ART1 on the expression
levels of RhoA, c-myc, c-fos and COX-2 in subcutaneously
transplanted CT26 tumor tissue of BALB/c mice. (A) A representative
western blot showing the expression levels of ART1, RhoA, c-myc,
c-fos, COX-2 in subcutaneously transplanted CT26 tumor tissue in
BALB/c mice. (B) Quantitative analysis revealed that the expression
levels of ART1, RhoA, c-myc, c-fos and COX-2 in subcutaneously
transplanted ART1-overexpression CT26 tumors were increased.
However, the expression levels of ART1, RhoA, c-myc, c-fos and
COX-2 in subcutaneously transplanted ART1-shRNA CT26 tumors were
decreased. *P<0.05, ART1-shRNA group vs. vector and
untransfected groups; #P<0.05, ART1-overexpression
group vs. vector and untransfected groups. ART1,
ADP-ribosyltransferase 1; RhoA, Ras homolog gene family member A;
COX-2, cyclooxygenase-2; ART1-shRNA, ART1-short hairpin RNA. |
Effect of ART1 on the expression
levels of RhoA and FAK in spleen-transplanted CT26 tumor tissue of
BALB/c mice
Compared with the vector-control and untransfected
groups, the expression levels of ART1, RhoA and FAK in spleen
transplanted ART1-shRNA CT26 tumors were decreased, and the
expression levels of these proteins in spleen-transplanted
ART1-overexpression CT26 tumors were increased (P<0.05). No
significant differences were identified between the expression
levels of these proteins in the vector-control and untransfected
groups (P>0.05; Fig. 6A and
B).
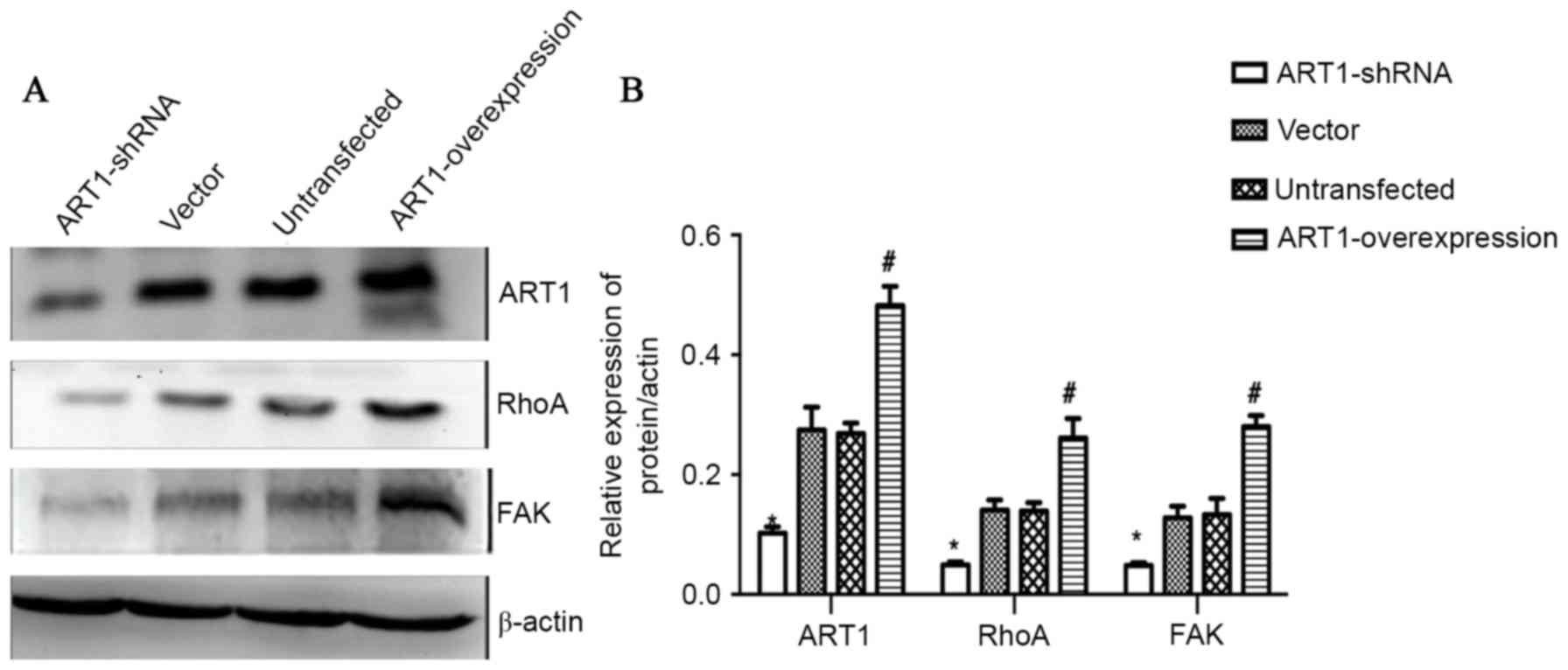 | Figure 6.Effect of ART1 on the expression
levels of RhoA and FAK in spleen-transplanted CT26 tumor tissue of
BALB/c mice. (A) A representative western blot showing the
expression levels of ART1, RhoA and FAK in spleen-transplanted CT26
tumor tissue of BALB/c mice. (B) Quantitative analysis revealed
that the expression levels of ART1, RhoA and FAK in
spleen-transplanted ART1-overexpression CT26 tumor were increased.
However, the expression levels of ART1, RhoA, c-myc, c-fos and
COX-2 in spleen-transplanted ART1-shRNA CT26 tumor were decreased.
*P<0.05, ART1-shRNA group vs. vector and untransfected groups;
#P<0.05, ART1-overexpression group vs. vector and
untransfected groups. ART1, ADP-ribosyltransferase 1; RhoA, Ras
homolog gene family member A; FAK, focal adhesion kinase; COX-2,
cyclooxygenase-2; ART1-shRNA, ART1-short hairpin RNA. |
Discussion
Yau et al (6) hypothesized that mono-ADP-ribosylation
enzymes may be associated with the progression of gastric cancer. A
previous study (11) has shown
that ART1 expression was increased in colorectal cancer and has a
positive correlation with the expression of vascular endothelial
growth factor (VEGF), which suggests that it may have an
association with tumor angiogenesis. It has also been observed that
the silencing of ART1 in CT26 cells may inhibit the proliferation
of cells by restraining cell cycle at the G0/G1 phase, suppressing
matrix adhesion and migration in vitro (12–14).
However, whether the changes of ART1 in CT26 cells are able to
affect the proliferation and invasion in vivo has yet to be
fully elucidated. The present study demonstrated the reduction in
the volume and weight of subcutaneously transplanted ART1-shRNA
CT26 tumor tissue in BALB/c mice. However, there was an increase in
the volume and weight of subcutaneously transplanted
ART1-overexpression CT26 tumor tissue in BALB/c mice. The results
also demonstrated that a reduction in the volume and weight of
spleen-transplanted CT26 tumor tissue occurred in BALB/c mice with
the silencing of ART1 in CT26 cells, and an increase with the
overexpressing of ART1 in CT26 cells. The average survival time of
BALB/c mice with subcutaneously transplanted CT26 tumors or spleen
transplanted CT26 tumors was significantly shortened with the
overexpression of ART1, and was extended with the silencing of
ART1. Taken together, these data demonstrated that ART1 may affect
the growth and development of transplanted CT26 tumor in
vivo.
In skeletal muscle cells, ART1 catalyzes the
modification of mono-ADP-ribosylation on integrin α7β1, which may
promote the binding of integrin and laminin and lead to the
activation of FAK and of Rho, resulting in the formation of stress
fibers and the shrinkage of cells (15–18).
Integrin β1, an important signaling molecule on the cell membrane,
is able to associate with a variety of intracellular signaling
molecules, including FAK, Rho and integrin-linked kinase (ILK)
(19). It has been suggested that
phosphorylation of the Rho effector may also be inhibited by an
appropriate amount of MIBG (5).
The same study also hypothesized that arginine-specific
mono-ADP-ribosylation is involved in a Rho-dependent signaling
pathway. In the present study, expression levels of FAK and RhoA
decreased in the ART1-shRNA group, whereas they increased in the
ART1=overexpression group. Therefore, the change in the levels of
ART1 may exert an influence on the FAK and RhoA signaling pathways
in colon carcinoma.
FAK is known as a regulator of cell migration.
Schaller (20) demonstrated that
enhanced FAK signaling may promote cell motility, whereas inhibited
FAK signaling could suppress cell migration. Sieg et al
(18) demonstrated that integrin
β1-FAK is inactive in non-metastatic cancer cells, whereas it
exhibits strong activity in metastatic cancer cells. Silencing
integrin β1 could control the activity of FAK and further promote
cell migration. The small G-protein, RhoA, also may mediate the
RhoA/Rho-kinase (ROCK) and FAK signaling pathways, and have a
marked effect on tumor cell migration (18,21).
The present study demonstrated that expression levels of RhoA and
FAK in spleen-transplanted CT26 tumors were decreased significantly
due to ART1 gene silencing, and increased with ART1 overexpression
in CT26 cells.
RhoA, a member of the Rho GTPase family, has been
known to regulate the actin cytoskeleton in the formation of stress
fibers (22), cytoskeletal
dynamics, gene transcription, cell-cycle progression and cell
transformation (23). ROCK is an
important downstream effector of RhoA. It has been demonstrated
that the RhoA/ROCK pathway serves an important role in various
fundamental cellular functions, including proliferation (24). The c-myc proto-oncogene is an
important regulator of cell proliferation, growth and
differentiation (25). Kamaraju
and Roberts (26) indicated that
inhibition of Rho/ROCK activity is required for downregulation of
the expression levels of c-myc protein, and the subsequent
suppression of the growth of breast cancer cells. The
Rho-ROCK-c-myc cascade partly contributes to vascular endothelial
growth factor induction by lysophosphatidic acid in ovarian cancer
(27). C-myc silencing not only
efficiently downregulates the expression of c-myc, but also
inhibits the proliferation of HT-29 cells and suppresses the growth
of colon cancer cells in vivo (28). Rho is also involved in the
shear-stress induction of c-fos (29) and may stimulate the expression
levels of c-fos (30). The
inhibition of ROCK activity, and the subsequent disruption of actin
filaments, may induce a decrease in c-fos activity (29). C-fos-siRNA attenuated the invasive
ability of Lovo cells (31) and
the growth of human colon carcinoma cells in athymic mice (32). C-fos, the dysregulation of which
may lead to the development of cancer, is involved in important
cellular events, including cell proliferation, differentiation and
survival (33). RhoA may promote
the expression of COX-2 via a mechanism dependent on the
transcription factor, nuclear factor-κB (34). The inhibition of COX-2 may suppress
the growth of HCA-7 and Moser-S colon cancer cells (35). Increased COX-2 activity has a
positive effect on the progression of colorectal cancer (36). The present study demonstrated that
the expression levels of RhoA and the downstream factors, c-myc,
c-fos, and COX-2 proteins, were decreased significantly in
vivo due to ART1 gene silencing, and increased with ART1
overexpression in CT26 cells. Thus, it has been demonstrated that
the effect of ART1 on the proliferation of CT26 cells may be
associated with RhoA and its downstream signal-transduction
pathway.
Thus, ART1 serves a facilitatory role in the
proliferation and migration of CT26 cells in vivo, and this
effect may be associated with the factors downstream of FAK and
RhoA, c-myc, c-fos, and COX-2. However, the underlying mechanisms
require further investigation.
Acknowledgements
The present study was supported by the Ministry of
Education Specialized Research Fund for the Doctoral Program of
Higher Education (grant no. 20105503110009), the Science and
Technology Program of Chongqing Municipal Education Commission
(grant no. KJ110322) and the National Nature Science Foundation of
China (grant no: 30870946).
References
|
1
|
Hassa PO, Haenni SS, Elser M and Hottiger
MO: Nuclear ADP-ribosylation reactions in mammalian cells: Where
are we today and where are we going? Microbiol Mol Biol Rev.
70:789–829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laing S, Unger M, Koch-Nolte F and Haag F:
ADP-ribosylation of arginine. Amino Acids. 41:257–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stevens LA, Levine RL, Gochuico BR and
Moss J: ADP-ribosylation of human defensin HNP-1 results in the
replacement of the modified arginine with the noncoded amino acid
ornithine. Proc Natl Acad Sci USA. 106:19796–19800. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corda D and Di Girolamo M: Functional
aspects of protein mono-ADP-ribosylation. EMBO J. 22:1953–1958.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yau L, Litchie B, Thomas S, Storie B,
Yurkova N and Zahradka P: Endogenous mono-ADP-ribosylation mediates
smooth muscle cell proliferation and migration via protein kinase
N-dependent induction of c-fos expression. Eur J Biochem.
270:101–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yau L, Molnar P, Moon MC, Buhay S, Werner
JP, Molnar K, Saward L, Rizzo DD and Zahradka P:
Meta-iodobenzylguanidine, an inhibitor of arginine-dependent
mono(ADP-ribosyl)ation, prevents neointimal hyperplasia. J
Pharmacol Exp Ther. 326:717–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang Y, Wang YL, Yang L, Xu JX, Xiong W,
Xiao M and Li M: Inhibition of arginine ADP-ribosyltransferase 1
reduces the expression of poly(ADP-ribose) polymerase-1 in colon
carcinoma. Int J Mol Med. 32:130–136. 2013.PubMed/NCBI
|
|
8
|
Kuang J, Wang YL, Xiao M, Tang Y, Chen WW,
Song GL, Yang X and Li M: Synergistic effect of arginine-specific
ADP-ribosyltransferase 1 and poly(ADP-ribose) polymerase-1 on
apoptosis induced by cisplatin in CT26 cells. Oncol Rep.
31:2335–2343. 2014.PubMed/NCBI
|
|
9
|
Huang W, Wu YL, Zhong J, Jiang FX, Tian XL
and Yu LF: Angiotensin II type 1 receptor antagonist suppress
angiogenesis and growth of gastric cancer xenografts. Dig Dis Sci.
53:1206–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu HY, Huang ZL, Yang GH, Lu WQ and Yu
NR: Inhibitory effect of modified citrus pectin on liver metastases
in a mouse colon cancer model. World J Gastroenterol. 14:7386–7391.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Wang YL, Sheng YT, Xiong W, Xu JX,
Tang Y and Li X: The correlation of ART1 expression with
angiogenesis in colorectal carcinoma and it relationship with VEGF
and integrin αVβ3 expressions. Basic Clin Med. 9:1064–1069.
2012.
|
|
12
|
Xiao M, Tang Y, Wang YL, Yang L, Li X,
Kuang J and Song GL: ART1 silencing enhances apoptosis of mouse
CT26 cells via the PI3K/Akt/NF-κB pathway. Cell Physiol Biochem.
32:1587–1599. 2013.PubMed/NCBI
|
|
13
|
Xiong W, Tang Y, Wang YL and Xu JX:
Effects of ART1 gene silencing on the ability of CT26 cellular
matrix adhesion and migration. Fudan Univer J Med Sci. 40:328–334.
2013.
|
|
14
|
Xu JX, Wang YL, Tang Y and Xiong W: Effect
of ART1 gene silencing by RNA interference on the proliferation of
mouse colon carcinoma cells and its possible mechanism. Tumor.
32:949–954. 2012.
|
|
15
|
Sawhney RS, Liu W and Brattain MG: A novel
role of ERK5 in integrin-mediated cell adhesion and motility in
cancer cells via Fak signaling. J Cell Physiol. 219:152–161. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Z, Gruszczynska-Biegala J and
Zolkiewska A: ADP-ribosylation of integrin alpha7 modulates the
binding of integrin alpha7beta1 to laminin. Biochem J. 385:309–317.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shibue T and Weinberg RA: Integrin beta
1-focal adhesion kinase signaling directs the proliferation of
metastatic cancer cells disseminated in the lungs. Proc Natl Acad
Sci USA. 106:10290–10295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sieg DJ, Hauck CR and Schlaepfer DD:
Required role of focal adhesion kinase (FAK) for
integrin-stimulated cell migration. J Cell Sci. 112:2677–2691.
1999.PubMed/NCBI
|
|
19
|
Gilcrease MZ: Integrin signaling in
epithelial cells. Cancer Lett. 247:1–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schaller MD: Cellular functions of FAK
kinases: Insight into molecular mechanisms and novel functions. J
Cell Sci. 123:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dorfleutner A, Stehlik C, Zhang J, Gallick
GE and Flynn DC: AFAP-110 is required for actin stress fiber
formation and cell adhesion in MDA-MB-231 breast cancer cells. J
Cell Physiol. 213:740–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nobes CD and Hall A: Rho, rac, and cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia, and filopodia.
Cell. 81:53–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng
Y, Liu M, Chen J, Liu S, Qiu M, et al: RhoA regulates G1-S
progression of gastric cancer cells by modulation of multiple INK4
family tumor suppressors. Mol Cancer Res. 7:570–580. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
25
|
Levens DL: Reconstructing Myc. Gene Dev.
17:1071–1077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamaraju AK and Roberts AB: Role of
Rho/ROCK and p38 MAP kinase pathways in transforming growth
factor-beta-mediated Smad-dependent growth inhibition of human
breast carcinoma cells in vivo. J Biol Chem. 280:1024–1036. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song Y, Wu J, Oyesanya RA, Lee Z,
Mukherjee A and Fang X: Sp-1 and c-Myc mediate lysophosphatidic
acid-induced expression of vascular endothelial growth factor in
ovarian cancer cells via a hypoxia-inducible factor-1-independent
mechanism. Clin Cancer Res. 15:492–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Ge YL and Tian RH: The knockdown
of c-myc expression by RNAi inhibits cell proliferation in human
colon cancer HT-29 cells in vitro and in vivo. Cell Mol Biol Lett.
14:305–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shiu YT, Li S, Yuan S, Wang Y, Nguyen P
and Chien S: Shear stress-induced c-fos activation is mediated by
Rho in a calcium-dependent manner. Biochem Biophys Res Commun.
303:548–555. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueyama T, Sakoda T, Kawashima S, Hiraoka
E, Hirata KI, Akita H and Yokoyama M: Activated RhoA stimulates
c-fos gene expression in myocardial cells. Circ Res. 81:672–678.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia ZC, Wan YL, Tang JQ, Dai Y, Liu YC,
Wang X and Zhu J: Tissue factor/activated factor VIIa induces
matrix metalloproteinase-7 expression through activation of c-Fos
via ERK1/2 and p38 MAPK signaling pathways in human colon cancer
cell. Int J Colorectal Dis. 27:437–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pandey MK, Liu G, Cooper TK and Mulder KM:
Knockdown of c-Fos suppresses the growth of human colon carcinoma
cells in athymic mice. Int J Cancer. 130:213–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tulchinsky E: Fos family members:
Regulation, structure and role in oncogenic transformation. Histol
Histopathol. 15:921–928. 2000.PubMed/NCBI
|
|
34
|
Benitah SA, Valerón PF and Lacal JC: ROCK
and nuclear factor-kappaB-dependent activation of cyclooxygenase-2
by Rho GTPases: Effects on tumor growth and therapeutic
consequences. Mol Biol Cell. 14:3041–3054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fosslien E: Biochemistry of cyclooxygenase
(COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia.
Crit Rev Cl Lab Sci. 37:431–502. 2000. View Article : Google Scholar
|
|
36
|
Asting AG, Carén H, Andersson M, Lönnroth
C, Lagerstedt K and Lundholm K: COX-2 gene expression in colon
cancer tissue related to regulating factors and promoter
methylation status. BMC Cancer. 11:2382011. View Article : Google Scholar : PubMed/NCBI
|