Introduction
Ulcerative colitis (UC) is progressively emerging as
a global threat (1), and an
intractable disease by the World Health Organization (2). Colonic mucosal healing is currently
considered the gold standard for treatment (3). 5-aminosalicylic acid (5-ASA), a
conventional anti-inflammatory drug, has been a first-line
therapeutic agent in the treatment of mild to moderate active UC
for several decades, which allows for remission to be maintained
(4). However, when 5-ASA is orally
administered, a large quantity of the drug is absorbed by the upper
gastrointestinal (GI) tract and enters into the systemic
circulation, with only a fraction of the active compound reaching
the inflamed target colon areas. As a result, targeting 5-ASA to
the site of inflammation has remained a challenge and an unmet
requirement in the treatment of UC. In the current study,
5-ASA-loaded silicon dioxide nanoparticles (5-ASA-SiO2
NPs) were designed and prepared, and their therapeutic efficacy in
a dextran sodium sulfate (DSS)-induced mice model of UC was
investigated.
Materials and methods
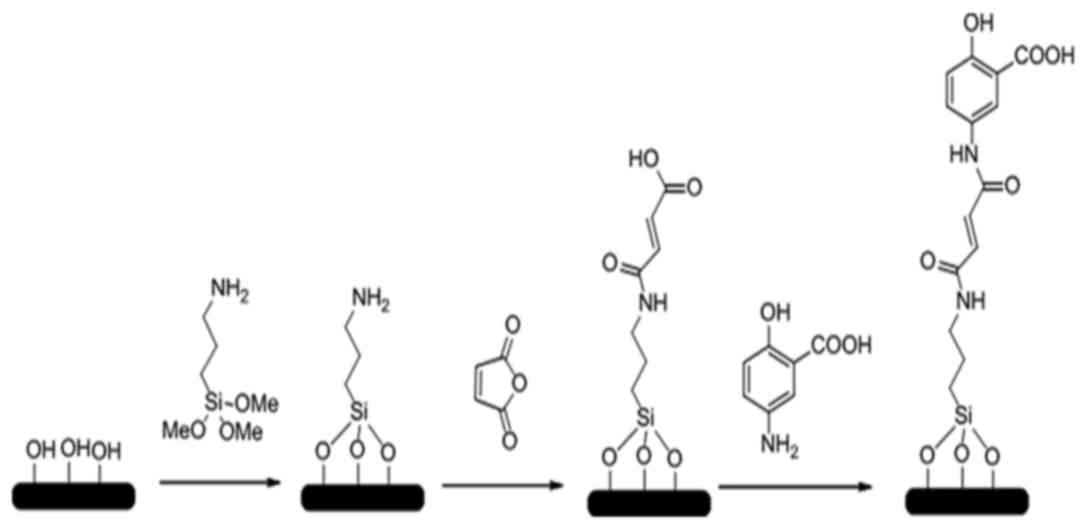
Preparation and characterization of
SiO2 NPs and 5-ASA-SiO2 NPs
SiO2 NPs and 5-ASA-SiO2 NPs
were prepared and synthesized by the Dalian Institute of Chemical
Physics, Chinese Academy of Sciences (Dalian, China). The brief
procedures were illustrated in Fig.
1. The micro-emulsion method was used to prepare the
SiO2 NPs according to the literature (5). This method involved use of an
appropriate amount of cyclohexane (31 ml), butanol (8 ml),
polyoxyethylene nonlyphenol ether (15 ml), ammonia water (0.5 ml),
and distilled H2O (6 ml) were mixed thoroughly for 5
min. Then, 20 mmol tetraethyl orthosilicate was added to the
mixture and reacted for 12 h. A total of 20 ml alcohol was then
added into the mixture to stop emulsification. The NPs were
collected by centrifuging the mixture at 8,000 × g for 5 min
at room temperature, prior to being re-suspended in ethanol. The
NPs suspension was added into a pressure bottle and mixed for 5 min
in an 80°C oil bath; and this step was repeated four times. The
SiO2 NPs were obtained by drying the suspension. After
reaction with APTES and dehydrated toluene, the amination of
SiO2 NPs were obtained; then the amination of
SiO2 NPs (0.2 g) were anhydride modified by reacting
with succinic anhydride (1 g), acetonitrile (10 ml) in a pressure
bottle for oil bath at 80°C with magnetic stirring. After reaction
for 24 h, the anhydride modified SiO2 NPs were collected
with centrifuging (8,000 × g, 5 min), washing with acetonitrile and
drying. SiO2 NPs were dispersed in deionized water to
prepare 1 mg/ml suspension. Following ultrasonic dispersion for 30
min, the suspension was taken out, and a small amount of dispersion
suspension was drained by copper mesh. The morphology of the
nanoparticles was observed by transmission electron microscopy
(TEM). The anhydride-SiO2 NPs (0.2 g), 5-ASA (0.2 g;
TCI, Tokyo, Japan) and acetonitrile (20 ml) were mixed in a
pressure bottle for another 24 h oil bath at 90°C; and the drying
precipitate was washed with dimethyl formamide (30 ml); then the
target agent of 5-ASA-SiO2 NPs were obtained after
drying.
Assessment of drug loading efficiency
and 5-ASA-SiO2 NPs toxicity in Caco-2 cells
5-ASA-SiO2 NPs were dissolved in 1 M NaOH
solution (pH=9.0) and mixed at 15 × g in a 50°C oil bath for
12 h. The suspension was filtered with a 0.22 µm filter membrane
(Thomas Scientific, Swedesboro, NJ, USA) and detected using high
performance liquid chromatography (HPLC; Agilent Technologies,
Inc., Santa Clara, USA). The loading efficiency of
5-ASA-SiO2 NPs was calculated using the following
equation: (Total 5-ASA volume/total 5-ASA-SiO2 NPs
volume) × 100%. The detection was performed in triplicate.
Concentration of the logarithmic growth of Caco-2
cells (Cell Resource Center/PUMC, Beijing, China). in Minimum
Essential Medium (MEM) was adjusted to 2×106/ml. Cell
suspension (100 µl) was added to one well of the 96-well plate and
cultured in CO2 incubator for 48 h. The medium in the
plate was discarded and the cells washed with PBS twice. Then, 100
µl 5-ASA, 100 µl SiO2 NPs and 100 µl
5-ASA-SiO2 NPs solution were added into different wells
and incubated for 12 h. For different treatments, the working
concentrations of the solutions were adjusted by MEM to 15.6 µg/ml,
31.2 µg/ml, 62.5 µg/ml, 125 µg/ml, 250 µg/ml, 500 µg/ml and 1
mg/ml. Following the incubation, the medium was replaced by 90 µl
new medium with 10 µl Cell Counting Kit-8 (CCK-8) solution (Beijing
Zoman Biotehnlogy Co., Ltd., Beijing, China) and incubated for
another 2 h before recording the optical density (OD). The negative
control group (wells with Caco-2 cells and CCK-8 solution) and the
blank control group (wells with CCK-8 solution only) were also set
up as described above. Each treatment was represented by three
replicates.
The OD values in different wells were recorded using
a microplate reader at 450 nm. The survival rates (%) of different
treatments were calculated as (OD value in treatment group-OD value
in blank control group)/(OD value in negative control group-OD
value in blank control group) × 100%.
Colitis model
A total of 60 male healthy BALB/c mice (8–9 weeks of
age, body weight 20–25 g) were provided by the Animal Center,
Dalian Medical University (Dalian, China). Colitis in mice was
induced by 5% (w/v) DSS supplemented in the drinking water for
seven days (6,7). Mice were maintained in a room with
constant temperature (22±1°C) and a dark-light cycle (12/12 h), and
housed in cages (<5 mice per cage). They were fed with standard
laboratory food and water for one week before the experiments. All
animal experiments were conducted in the accordance with the
Institutional Animal Ethics Committee and Animal Care Guidelines of
Dalian Medical University (Dalian, China) governing the use of
experimental animals.
Mice were randomly divided into six groups (10 per
group): Group A, control group; group B, model group (UC model
mice); group C, normal dose 5-ASA group (200 mg/kg.day−1
for 7 days); group D, high dose 5-ASA group (400
mg/kg.day−1 for 7 days); group E, SiO2 NPs
group (100 mg/kg.day−1 for 7 days); group F,
5-ASA-SiO2 NPs group (100 mg/kg day−1 for 7
days).
Evaluation of colitis severity by
disease activity index (DAI), colon mucus histological assessment
and measurements of myeloperoxidase activity and cytokines
The DAI scores of different treatments were recorded
every day, according to previous studies (Table I) (8,9). On
the eighth day of the treatment, animals were anesthetized using 1%
pentobarbital sodium (40 mg/kg; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) through intraperitoneal injection and
sacrificed by cervical dislocation. Eyeball blood samples were then
collected and colon samples were obtained. Hematoxylin and eosin
staining was conducted on colon samples. Histology of the colon was
evaluated using a microscope. Levels of interleukin-6 (IL-6), tumor
necrosis factor-α (TNF-α) in blood samples were detected utilizing
IL-6 (cat. no. EK0411) and TNF-α (cat. no. EK0527) ELISA kits
(Wuhan Boster Biological Technology., Ltd, Wuhan, China); and
myeloperoxidase (MPO) activity in colon mucosa samples were
measured with an MPO ELISA kit (cat. no. DRE 30329; Xinfan Biomart
Co., Ltd., Shanghai, China) following the manufacturer's
instructions. Total RNA in colon tissue samples was extracted by
miRCURY™ RNA Isolation kit (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol, and reverse transcribed
into cDNA using a PrimeScript™ RT reagent kit (Takara Bio, Inc.)
according to the manufacturer's protocol. The expression of IL-6
and TNF-α was determined using GAPDH as a loading control. The
reaction mixture totaled 25 µl and included, 12.5 µl of 2X
SYBR® Premix Ex Taq (Takara Bio, Inc.), 0.5 µl of each
primer (Table II), 0.5 µl of 50X
ROX Reference Dye II (Takara Bio, Inc.), 2 µl DNA template and 9 µl
distilled H2O. One quantitative reverse transcriptase
PCR (RT-qPCR) cycle consisted of 95°C for 30 sec, 95°C 5 sec, 55°C
for 30 sec and 72°C for 30 sec. This was repeated for 40 cycles.
The program was conducted in Agilent 3500-qPCR System (Agilent
Technologies, Inc.).
 | Table I.Disease activity index scoring
criteria. |
Table I.
Disease activity index scoring
criteria.
| Score | Weight loss
(%) | Shape of feces | Hematochezia |
|---|
| 0 | 0 | Normal | Negative |
| 1 | 1–5 |
|
|
| 2 |
6–10 | Slight loose | Occult blood |
| 3 | 11–15 |
|
|
| 4 | >15 | Loose | Hematochezia |
 | Table II.Primer information. |
Table II.
Primer information.
| Gene | Sequence
(5′-3′) | Tm
(°C) | Production length
(bp) |
|---|
| IL-6 | Forward:
CCACTTCACAAGTCGGAGGCTTA | 78 | 169 |
|
| Reverse:
CCAGTTTGGTAGCATCCATCATTTC |
|
|
| TNF-α | Forward:
TATGGCCCAGACCCTCACA | 80.6 | 199 |
|
| Reverse:
GGAGTAGACAAGGTACAACCCATC |
|
|
| GAPDH | Forward:
AAATGGTGAAGGTCGGTGTGAAC | 75.6 | 90 |
|
| Reverse:
CAACAATCTCCACTTTGCCACTG |
|
|
Statistical analysis
All the data are expressed as mean ± standard
deviation. Analysis of variance and Fisher's least significant
difference tests were performed and P<0.05 was considered to
indicate a statistically significant difference. All the
statistical analysis was conducted using SPSS, Inc. (version, 16.0;
Chicago, IL, USA).
Results
Morphology and drug loading efficiency
of the SiO2 NPs
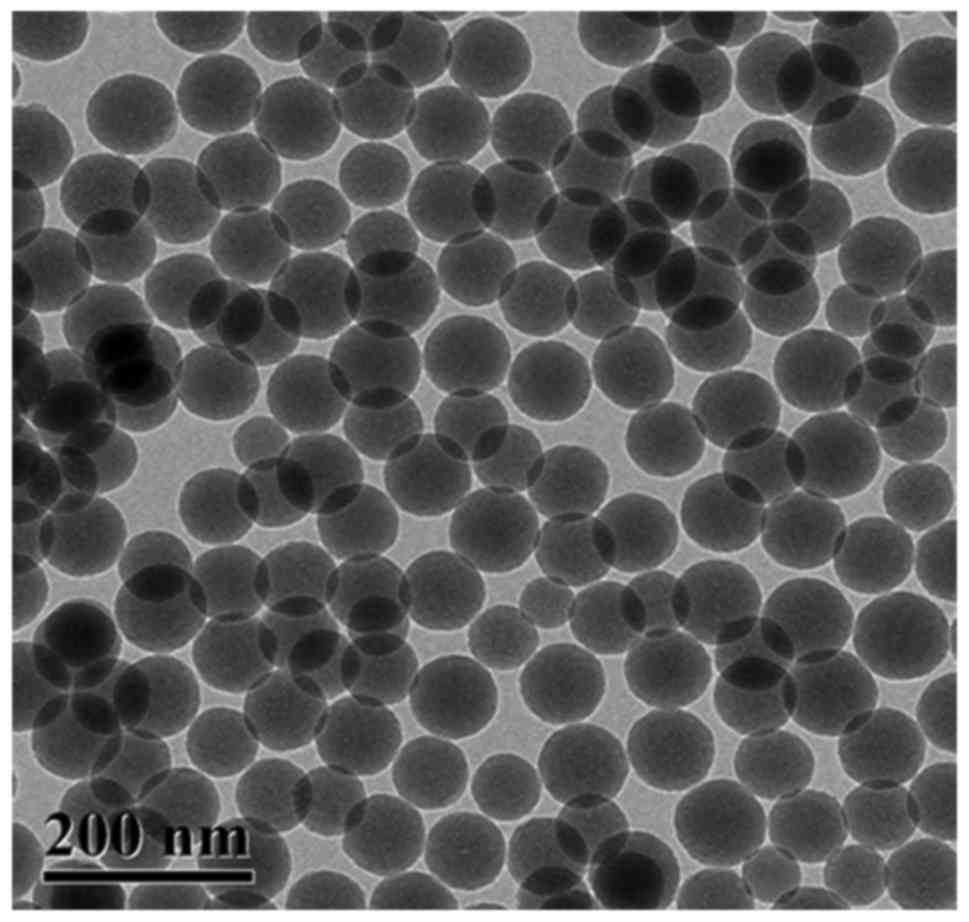
Morphological characteristics of SiO2 NPs
were presented in Fig. 2.
SiO2 NPs were uniformly distributed and the surface of
the NPs was smooth with an average diameter of 90 nm. Based on the
detection of HPLC, the average loading efficiency of
5-ASA-SiO2 NPs was 13.79±2.50 (wt%).
Cytotoxicity of the SiO2
NPs in Caco-2 cells
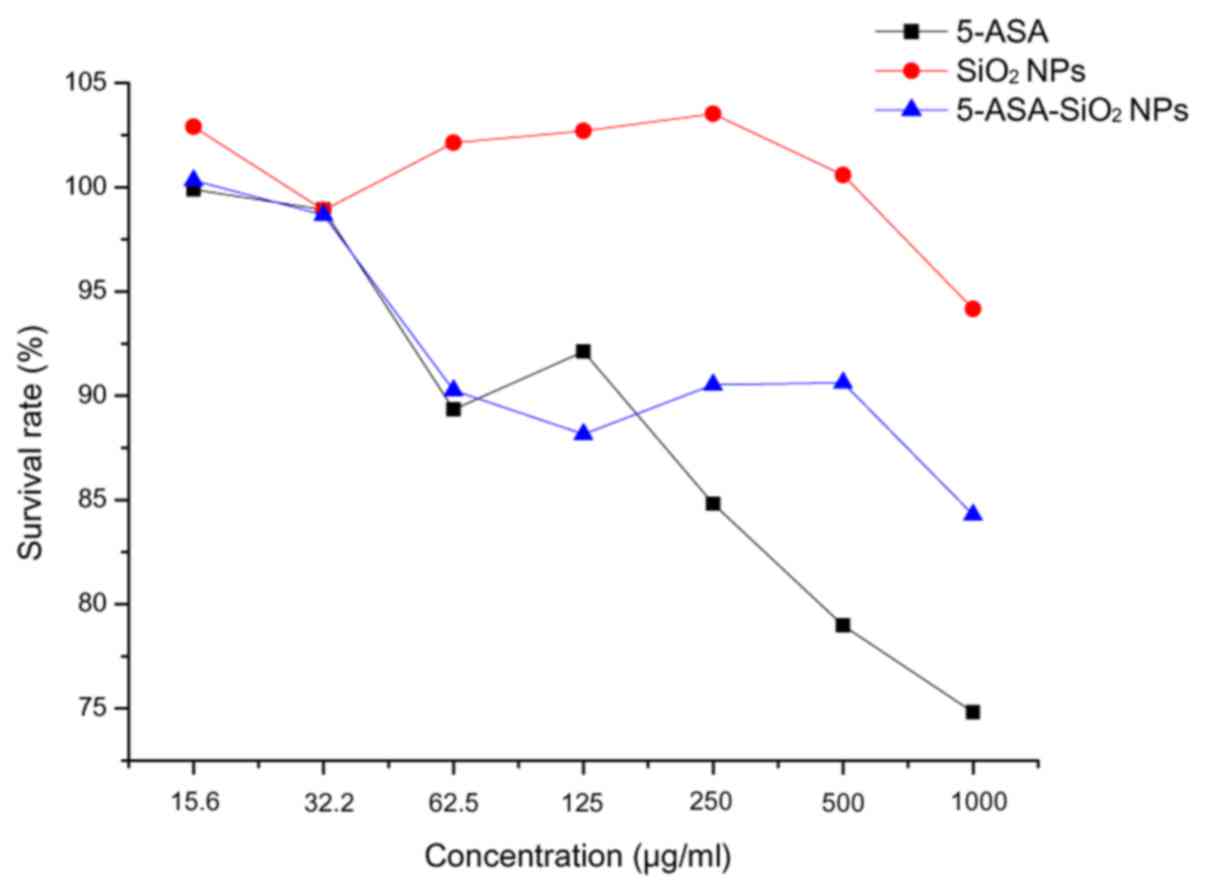
Survival rates of the Caco-2 cells decreased with
the increase in the concentration of the treatment agents (Fig. 3). There had no significant
difference in survival rate following treatment with
SiO2, 5-ASA and 5-ASA-SiO2 NPs in the 15.6,
31.25 and 62.5 µg/ml groups, respectively. But when the
concentration was above 250 µg/ml, a significant difference could
be observed between the three treatments, SiO2 NPs had
the strongest cytotoxicity and 5-ASA had the weakest cytotoxicity.
However, even for SiO2 NPs treatment of 1,000 µg/ml, the
survival rate was still more than 70%.
Evaluation of 5-ASA-SiO2
NPs in DDS-induced UC in mice
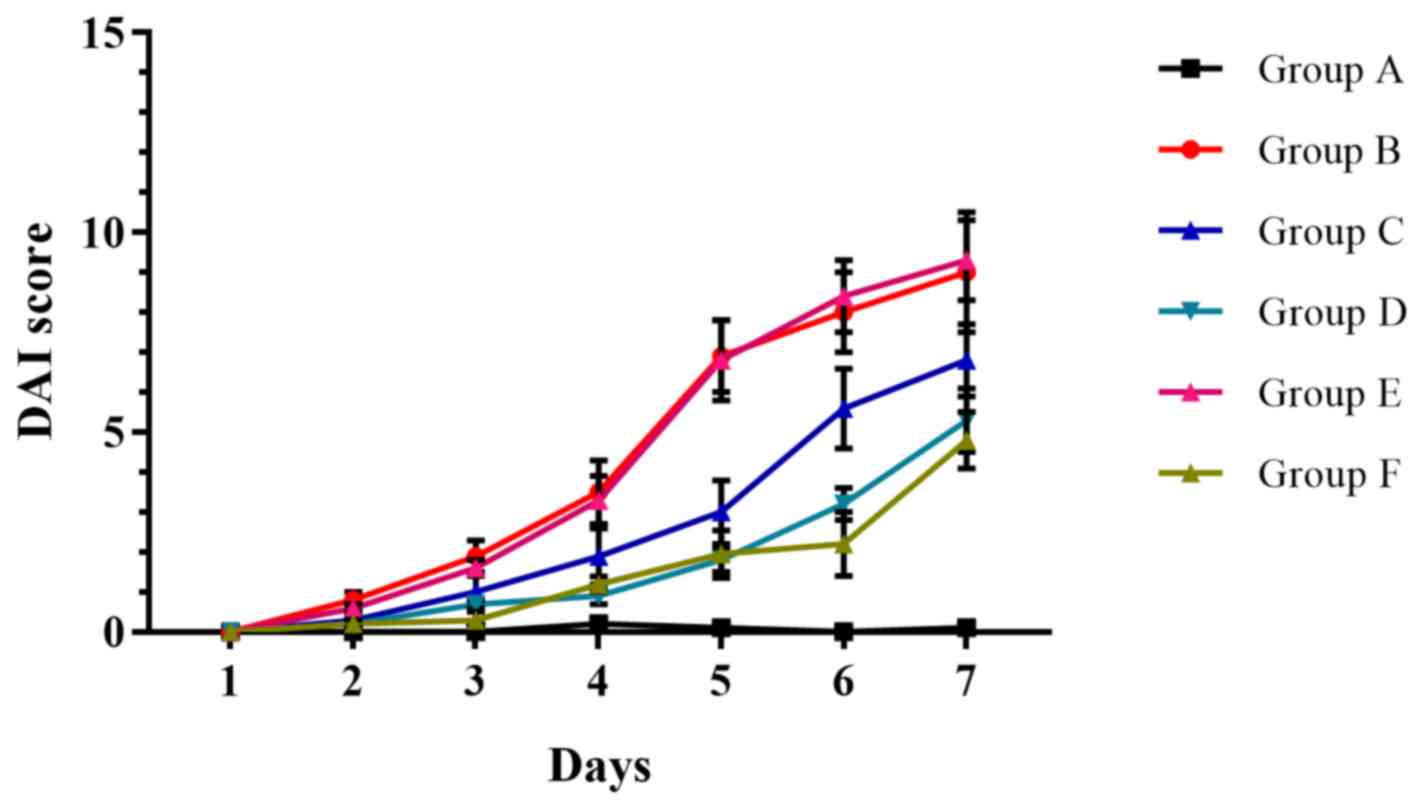
The result of DAI was showed in Fig. 4. There was no significant
difference in DAI scores between groups B and E throughout the
entire treatment period (Fig. 4).
On the 7th day, all 5-ASA treatment groups (groups C, D and F)
demonstrated reduced clinical activity compared with group B; and
all differences were statistically significant when compared with
the control group (group A) on the 7th day (Fig. 4).
H&E staining demonstrated that the colon mucosa
was injured as congestion, edema and a number of shallow ulcers;
and the structure of the glands were destroyed and disordered in
the model group (Fig. 5). Similar
pathological changes were observed in the colons of animals treated
with SiO2 NPs. The degree of leukocyte infiltration and
the mucosal edema as well as destroyed glands were reduced in the
case of the normal dose 5-ASA group (group C). Conversely, the
colon samples of animals treated with high dose 5-ASA group (group
D) and 5-ASA-SiO2 NPs group (group E) exhibited less
severe mucosal injury compared with the model and SiO2
NPs groups.
ELISA results of IL-6 and TNF-α measurements taken
from eyeball blood samples and MPO from colon samples were
presented in Table III. The
IL-6, TNF-α and MPO levels in group B significantly increased
compared with group A. There was no significant difference between
groups B and E. The IL-6, TNF-α and MPO levels were significantly
reduced by administration of normal dose 5-ASA (group C) compared
with the control group; and the levels were lower in groups D and F
when compared with group C.
 | Table III.Protein levels of IL-6, TNF-α and MPO
after treatment of 5-ASA-SiO2 NPs. |
Table III.
Protein levels of IL-6, TNF-α and MPO
after treatment of 5-ASA-SiO2 NPs.
| Group | n | IL-6 (pg/ml) | TNF-α (pg/ml) | MPO (U/mg) |
|---|
| Group A | 10 | 53.2±9.0 | 42.2±7.0 | 2.0±0.8 |
| Group B | 9 |
315.8±31.1a |
284.5±27.1a |
34.5±2.2a |
| Group C | 10 |
226.2±17.5a,b |
153.3±17.9a,b |
25.7±2.0a,b |
| Group D | 10 |
126.5±8.9a–c |
84.0±9.3a–c |
10.3±1.3a–c |
| Group E | 9 |
317.8±18.9a |
282.0±22.7a |
34.1±1.7a |
| Group F | 10 |
135.1±10.9a,d,b,c |
87.5±7.0a,d,b,c |
10.9±1.4a,d,b,c |
Results of IL-6 and TNF-α mRNA expressions in
colonic samples of different groups have been presented in Table IV. IL-6 and TNF-α mRNA expressions
in group B were significantly higher than that of group A. In group
E that received SiO2 NPs, IL-6 and TNF-α mRNA
expressions were not significant to that of group B. IL-6 and TNF-α
in groups C-E were significantly reduced when compared with group
B. However, IL-6 and TNF-α were lower in groups D and F when
compared with group C.
 | Table IV.mRNA expression of IL-6,
TNF-α in colonic mucosa. |
Table IV.
mRNA expression of IL-6,
TNF-α in colonic mucosa.
| Group | n | IL-6 | TNF-α |
|---|
| Group A | 10 | 1.1±0.3 | 1.2±0.3 |
| Group B | 9 |
26.1±1.6a |
15.0±1.6a |
| Group C | 10 |
10.3±1.0a,b |
4.7±0.9a,b |
| Group D | 10 |
4.6±0.7a–c |
2.0±0.5a–c |
| Group E | 9 |
25.5±1.7a |
14.7±1.3a |
| Group F | 10 |
4.7±0.5a,d,b,c |
1.8±0.4a,d,b,c |
Discussion
The colon is the terminal section of the digestive
tract, and thus orally administered drugs targeting the colon must
pass through a detrimental environment including digestive enzymes,
pH and bacteria. Use of a novel oral colon-specific drug delivery
system has confirmed that it is difficult to release a drug in the
upper GI tract, and thus, the drug is released rapidly into the
colon following oral administration, allowing for the accumulation
of the drug in the inflamed target regions (10). Investigating this system would
hopefully result in a higher local drug concentration at the target
site and, consequently, reduced required doses, toxicity and
systemic side effects. Nanopharmacology has gained interest in the
clinic, and nanomedicines are promising therapeutics employed in
the treatment of diseases at the molecular level (11). NPs have received considerable
attention as a novel medical technology due to their non-toxic,
biocompatible, biodegradable and mucoadhesive properties. Among the
various pharmaceutical excipients, the SiO2 NPs are the
most common. Their particle size is adjustable, they can be easily
prepared and they are toxicologically safe, thus are favored by
numerous researchers (12). NPs
are an encouraging strategy to enhance the therapeutic benefit of
UC (13–16), however, NPs in UC therapy remain in
the initial stages.
The silica NPs demonstrated a mean size distribution
and NP diameter of 90 nm in the present study, which is appropriate
for uptake of NP in inflamed colonic mucosa (17). The particle size of the targeted
delivery system is a major parameter to control and affect the
particle transition into the mucosal tissue. As recorded in a
recent study, small sized NPs may be able to more rapidly transit
from the surface to deeper layer of intestinal wall, which reflects
a size-dependency (18). The NPs
in the current study had good spherical geometry, with transmission
electron microscopy images of SiO2 NPs demonstrating
that they have a solid and near consistent structure. The data
obtained suggested that mixing 5-ASA with SiO2 NPs had
no effect on the size and yield of 5-ASA-SiO2 NPs.
5-ASA-SiO2 NPs presented good loading efficiency through
detection using HPLC, with findings similar to those of Pertuit
et al (13), and with a
loading efficiency much higher than poly (ε-caprolactone) NPs
(15).
Survival rates were investigated in order to choose
a safe range of concentrations to use for the transport
experiments. For all exposures, >70% survival percentage proved
to be safe. In vitro cytotoxicity investigations
demonstrated that there was only marginal difference between the
three agents when the concentration was lower than 62.5 µg/ml. A
significant difference could be detected when the concentration was
higher than 250 µg/ml, with the SiO2 NPs having the
strongest cytotoxicity. The lower toxicity of 5-ASA SiO2
NPs compared to SiO2 NPs may be explained by the
toxicity limiting the availability of bound compared to free drug.
The general toxicity of the new system could be considered as
minimal. However, we did not come to the conclusion that the
toxicity of 5-ASA SiO2 NPs or SiO2 NPs was
lower compared with 5-ASA, as previously indicated in the study of
Pertuit et al (13).
Together, the results of the present study demonstrate that
5-ASA-SiO2 NPs were successfully prepared, which may be
considered for use in animal experimentation.
The severity of disease can be assessed using
different parameters. The therapeutic effects of IBD can be
assessed with IBD disease activity, including clinical, endoscopic,
histological and radiological assessment tools (19). The clinical activity score allows
the assessment of the severity of the diseases in the living
animal. The treatment efficacy of 5-ASA-SiO2 NPs was
assessed in UC model mice. The DAI scores was significantly
decreased in the 5-ASA-SiO2 NPs group; the efficacy was
even comparable to 5-ASA dose of 400 mg/kg day−1.
Although the conditions of model mice did not return to normal
levels during the experiment period, the high efficacy of
5-ASA-SiO2 NPs on UC was still evident. Furthermore, in
histopathological examination, the injury to the colonic mucosa,
including edema, shallow ulcers and destroyed glands, relieved in
groups D and F. In the current study, 5-ASA SiO2 NPs
with a 5-ASA dose of 100 mg/kg indicated a comparable efficacy to a
5-ASA solution at a dose of 400 mg/kg. The 5-ASA-SiO2
NPs with its low drug dosage can achieve similar effect of high
dosage of 5-ASA, which is 32 times as much as that of
5-ASA-SiO2 NPs.
After the resection of the colon, different
inflammatory markers can be determined to evaluate the therapeutic
efficacy. A frequently used parameter is the activity of MPO. MPO
is an enzyme that is observed in mammalian granulocytes and the MPO
activity is used to determine the infiltration of tissue sections
with these cells. Inflammation can be assessed by measuring
cytokine levels, for example, TNF-α and IL-6 are suitable, as their
expression levels (as well as MPO expression) are closely
associated with the occurrence and development of UC. These
mediators have been demonstrated as being highly expressed in UC
samples (20) with a several-fold
increase of MPO levels also reported throughout the colonic mucosa
in patients with UC (21). Thus,
these three factors are excellent indicators for the severity of
inflammation. In the current study, the significant decrease of
IL-6, TNF-α and MPO in UC model mice after exposure to 5-ASA
SiO2 NPs was observed. The therapeutic potential of this
delivery system appears similar to the application of high dose
5-ASA. It was concluded that a much lower dose of 5-ASA
SiO2 NPs achieved similar results of high dose of 5-ASA
in treating UC. NPs delivered through oral administration were
determined to accumulate in the inflamed tissues and therefore
developed a more local effect through their accumulation at the
site of action (14,17,22).
Due to the tighter bond between the drug and carrier system, 5-ASA
SiO2 NPs present the advantage of targeting the drug to
the inflamed area. The efficient binding of the 5-ASA to the
SiO2 NPs is the key issue in the process. Additional
associated studies confirmed the therapeutic benefits of targeting
drug-loaded nanoparticles to the site of the disease and to
mitigate the clinical symptoms compared with conventional dosage
forms (23,24). In recent years, the incidence rate
of UC in China has increased markedly (25), and therefore, the application
5-ASA-SiO2 NPs would decrease the financial cost and
increase the safety of the treatment.
In conclusion, 5-ASA-SiO2 NPs is a
selective drug release strategy that targets the inflamed tissue
and highly increases therapeutic efficacy. The
5-ASA-SiO2 NPs at low dosage can achieve similar effects
to high dosages of 5-ASA. As this drug delivery system is
transposable to the majority of drugs in this therapeutic context,
it represents a promising alternative for future innovative
treatments of colon diseases.
Acknowledgements
The current study was supported by Science and
Technology Agency of Liaoning Province (grant no. 2012225018) and
the Science and Technology Bureau of Dalian (grant no.
2013E15SF154).
References
|
1
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimiol EI, Panaccione R, Ghosh S, Barkema
HW and Kaplan GG: Increasing incidence and prevalence of the
inflammatory bowel diseases with time, based on systematice review.
Gastroenterology. 142:46–54, e42; quiz e30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lakatos L and Lakatos LP: Is the incidence
and prevalence of inflammatory bowel diseases increasing in Eastern
Europe? Postgrad Med J. 82:332–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Pineton Chambrun G, Peyrin-Biroulet L,
Lémann M and Colombel JF: Clinical implications of mucosal healing
for the mangement of IBD. Nat Rev Gastroenterol Hepatol. 7:15–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Min Wang, Chen Chen, Jiping Ma and Jie Xu:
Preparation of superhydrophobic cauliflower-like silica nanosheres
with tunable water adhesion. J Mater Chem. 21:6962–6967. 2011.
View Article : Google Scholar
|
|
6
|
Hoffmann JC, Pawlowski NN, Kühl AA, Höhne
W and Zeitz M: Animal models of inflammatory bowel disease: An
overview. Pathobiology. 70:121–130. 2002.-2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hartmann G, Bidlingmaier C, Siegmund B,
Albrich S, Schulze J, Tschoep K, Eigler A, Lehr HA and Endres S:
Specific type IV phosphodiesterase inhibitor rolipram mitigates
experimental colitis in mice. J Pharmacol Exp Ther. 292:22–30.
2000.PubMed/NCBI
|
|
8
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
9
|
Murano M, Maemura K, Hirata I, Toshina K,
Nishikawa T, Hamamoto N, Sasaki S, Saitoh O and Katsu K:
Therapeutic effect of intracolonically administered nuclear factor
kappa B (p65) antisense oligonucleotide on mouse dextran sulphate
sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamprecht A: IBD: Selective nanoparticle
adhesion can enhance colitis therapy. Nat Rev Gastroenterol and
Hepatolo. 7:311–312. 2010. View Article : Google Scholar
|
|
11
|
Hock SC, Ying YM and Wah CL: A review of
the current scientific and regulatory status of nanomedicines and
the challenges ahead. PDA J Pharm Sci Technol. 65:177–195.
2011.PubMed/NCBI
|
|
12
|
Borbat PP, Costa-Filho AJ, Earle KA,
Moscicki JK and Freed JH: Electron spin resonance in studies of
membranes and proteins. Science. 291:266–269. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pertuit D, Moulari B, Betz T, Nadaradjane
A, Neumann D, Ismaïli L, Refouvelet B, Pellequer Y and Lamprecht A:
5-amio salicylic acid bound nanoparticles for the therapy of
inflammatory bowel disease. J Control Release. 123:211–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamprecht A, Yamamoto H, Takeuchi H and
Kawashima Y: Nanoparticles enhance therapeutic efficiency by
selectively increased local drug dose in experimental colitis in
rats. J Pharmacol Exp Ther. 315:196–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moulari B, Pertuit D, Pellequer Y and
Lamprecht A: The targeting of surface modified silica nanoparticles
to inflamed tissue in experimental colitis. Biomaterials.
29:4554–4560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kshirsagar SJ, Bhalekar MR, Patel JN,
Mohapatra SK and Shewale NS: Preparation and characterization of
nanocapsules for colon-targeted drug delivery system. Pharm Dev
Technol. 17:607–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lamprecht A, Schäfer U and Lehr CM:
Size-dependent bioadhesion of micro-and nanoparticulate carriers to
the inflamed colonic mucosa. Pharm Res. 18:788–793. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt C, Lautenschlaeger C, Collnot EM,
Schumann M, Bojarski C, Schulzke JD, Lehr CM and Stallmach A: Nano-
and microscaled particles for drug targeting to inflamed intestinal
mucosa: A first in vivo study in human patients. J Control Release.
165:139–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walsh AJ, Bryant RV and Travis SP: Current
best practice for disease activity assessment in IBD. Nat Rev
Gastroenterol Hepatol. 13:567–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M,
Lv P, An P, Liu SQ and Yu HG: Effects of Ginkgo biloba extract on
inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in
TNBS-induced colitis in rats. Mediators Inflamm. 2006:926422006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izzo RS, Witkon K, Chen AI, Hadjiyane C,
Weinstein MI and Pellecchia C: Interleukin-8 and neutrophil markers
in colonic mucosa from patients with ulcerative colitis. Am J
Gastroenterol. 87:1447–1452. 1992.PubMed/NCBI
|
|
22
|
Lamprecht A, Ubrich N, Yamamoto H, Schäfer
U, Takeuchi H, Maincent P, Kawashima Y and Lehr CM: Biodegradable
nanoparticles for the targeted drug delivery in treatment of
inflammatory bowel disease. J Pharmacol Exp Ther. 299:775–781.
2001.PubMed/NCBI
|
|
23
|
Niebel W, Walkenbach K, Béduneau A,
Pellequer Y and Lamprecht A: Nanoparticle-based clodronate delivery
mitigates murine experimental colitis. J Control Release.
160:659–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meissner Y, Pellequer Y and Lamprecht A:
Nanoparticles in inflammatory bowel disease: Particle targeting
versus pH-sensitive delivery. Int J Pharm. 316:138–143. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ng SC: Epidemiology of inflammatory bowel
disease: Focus on Asia. Best Pract Res Clin Gastroenterol.
28:363–372. 2014. View Article : Google Scholar : PubMed/NCBI
|