Introduction
Hypertension, a disorder associated with structural
and functional vascular alterations, has been recognized as a major
risk to cardiovascular health (1).
As hypertension is correlated with coronary artery disease, left
ventricular hypertrophy, congestive heart failure, obesity,
hyperlipidemia, diabetes and cerebrovascular complications, it
represents the biggest single contributor to the global burden of
disease and to global mortality (1–4).
Recent studies have demonstrated that some gene variants may be
involved in hypertension (2,3).
Dysfunction of the renin-angiotensin-aldosterone system (RAAS), and
over-activation of angiotensin II (Ang II), have been revealed as
most important factors in the onset and progression of hypertension
(5), cardiac remodeling (6), renal injury (7) and aortic aneurysm (8–11).
Consequently, inhibiting the action of Ang II using
angiotensin-converting enzyme inhibitors (ACEI) and Ang II type 1
receptor inhibitors have been widely accepted as a cornerstone in
the treatment of hypertension (12,13).
Although the biological functions and molecular mechanisms of Ang
II in cardiovascular system have not yet been not fully elucidated,
the injury of vascular smooth muscle cells (VSMCs) induced by
hypertension, as well as the accompanying high blood Ang II
concentration, are considered to be primary causes for
hypertension-associated vascular injury, including remodeling
(14), thrombi transformation
(15), neointimal hyperplasia
(16) and calcification (17).
Transient receptor potential melastatin 8 (TRPM8) is
a thermally-regulated Ca2+-permeable channel that is
activated by cold sensations (18). As a universal cold receptor in the
thermoregulation system, TRPM8 regulates body temperature (18,19).
Mice and rats experience a transient reduction in core body
temperature following administration of TRPM8 antagonists (20). By contrast, activation of TRPM8
channels by selective agonists, such as menthol and icilin,
demonstrates the opposite physiological hyperthermic effect by
acting on peripheral neurons rather than the central nervous system
(21,22). In addition to this thermoregulatory
effect, TRPM8 is involved in carcinogenesis (23), pain (24), inflammation (25), obesity (26), testosterone sensing (27) and eye-blinking regulation (28). Although previous studies have
demonstrated that TRPM8 functions as a regulator of vascular tone
that may be a promising therapeutic target for hypertension
(29,30), the role of TRPM8 in vascular
biology is not completely understood.
In the current study, it was hypothesized that TRPM8
serves an important role in VSMC biology under hypertensive
conditions. Therefore, the expression of TRPM8 was investigated in
the aorta media from rats subject to a two-kidney one-clip
operation (2K1C), which is a widely-used renovascular hypertension
model. In addition, the effect of benazepril, a commonly used ACEI,
on the protein expression levels of TRPM8 was studied. Furthermore,
the effects of exogenous Ang II treatment on TRPM8 expression in
cultured VSMCs were explored. Furthermore, the potential influence
of TRPM8 activation by a pharmacological agonist on oxidative
stress, ras homolog gene family, member A (RhoA)-rho-associated
protein kinase 2 (Rock2) signaling activation and janus kinase 2
(JAK2) signaling activation induced by Ang II treatment in VSMCs
were investigated.
Materials and methods
Animals
A total of 24 male Sprague-Dawley rats (age, 8
weeks; weight, 220–260 g) were obtained from Sino-British SIPPR/BK
Lab Animal Co., Ltd. (Shanghai, China) and housed under conditions
involving a 12 h/12 h light/dark cycle and a temperature of 23–25°C
with free access to food and water. All animals used in this work
received humane care in compliance with the institutional animal
care guidelines and the Guide for Care and Use of Laboratory
Animals published by the National Institutes of Health (Bethesda,
MA, USA). The animal experiments were approved by the Animal Care
and Use Committee of Tongji University (Shanghai, China).
2K1C hypertension model
The 2K1C hypertension model was performed as
described previously (31). Rats
were anesthetized with a combination of ketamine (40 mg/kg i.p.;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)/xylazine (13
mg/kg i.p.; Sigma-Aldrich; Merck Millipore), the right renal artery
was isolated through a flank incision, and a silver clip (0.2 mm
internal gap) was placed on the renal artery. Sham-operated rats
underwent the same surgical procedure except for the placement of
the renal artery clip, and served as controls. The rats were
returned to the cage after waking from the operation, and were
maintained for 4 weeks. Blood pressure and heart rate (HR)
measurements were recorded at 4 weeks post-2K1C surgery in
conscious rats as described in a previous study by the authors
(31). For ACEI benazepril
treatment, benazepril (1.5 mg/kg/day; Beijing Novartis Pharma Co.,
Ltd., Beijing, China) was administrated via the chow for an
additional 4 weeks in 2K1C rats.
Primary VSMC culture
Rat VSMCs were isolated using a standard enzymatic
digestion technique as described previously (32,33).
Primary VSMCs were cultured in Dulbecco's modified Eagle's medium
(DMEM; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in 95% O2 and 5%
CO2. Experiments were performed using cells between
passages 3 and 8 (34).
Cell study
The VSMCs (1×106) were seeded in wells of a 6-wells
plate. Cells were grown to 60% confluence, and switched to
serum-starved medium (0.1% FBS) for 12 h prior to further
treatments to achieve synchronization. At the second day, the cells
were switched to normal medium (DMEM + 10% FBS) and treated with
Ang II (10–7 M; Sigma-Aldrich; Merck Millipore) or menthol (100 mM;
Sigma-Aldrich; Merck Millipore) for 5 days at 37°C. Cells were then
washed with PBS three times. Subsequently, cells were lysed with
RIPA solution (Beyotime Institute of Biotechnology, Haimen, China)
for determination of mRNA and protein levels. In some experiments,
cells were induced by incubation of H2O2 (150
mM) for 12 h and then lysed for determination of the effect of ROS
on TRPM8 expression.
Oxidative stress
The levels of reactive oxygen species (ROS) were
measured using a flow cytometer (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA) with a 2′,7′-dichlorofluorescein-diacetate
fluorescent assay (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions and as described
previously (35). The level of
H2O2 was evaluated using the Hydrogen
Peroxide assay kit (Abcam, Cambridge, MA, USA) according to the
manufacturer's instructions and using the methods described
previously (36). In the presence
of horseradish peroxidase, the OxiRed Probe reacted with
H2O2 to produce product with color (λmax=570
nm) and red fluorescence (excitation/emission=535/587 nm).
Tissue sampling
At the end of 4-week treatment, rats were deeply
anesthetized by chloral hydrate (0.4 g/kg; Shanghai Meilian
Biological Technology R&D Co., Ltd., Shanghai, China) and then
decapitated. The aortae were carefully harvested and washed by
distilled water three times. Subsequently, the tissues were frozen
and stored at −80°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as described previously
(37,38). Total RNA was isolated from frozen
tissues and the VSMCs cultured in 6-well dishes using RNAiso Plus
reagent (Takara Bio, Inc., Otsu, Japan) and reverse transcribed
with M-MLV (Promega Corporation, Madison, WI, USA) into cDNA using
the methods described previously (39). The following primers were used for
qPCR analysis: TRPM8, forward, 5′-GCTACGGACCAGCATTTCAT-3′, and
reverse, 5′-GCTTGTCAATGGGCTTCTT-3′; β-actin, forward,
5′-CCCATCTATGAGGGTTACGC-3′, and reverse,
5′-TTTAATGTCACGCACGATTTC-3′. β-actin expression was used as an
internal control. Quantification of mRNA was performed using the
ABI Prism 7500 (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using a PrimeScript™ RT-PCR kit (Takara Bio, Inc.). The specificity
of the RT-qPCR assay was assessed by melting point analysis and gel
electrophoresis. The relative quantities of TRPM8 were calculated
using the 2-ΔΔCq method with β-actin as an internal
control (40,41).
Western blotting
Western blotting analysis was performed as described
previously (42,43). Tissues and the VSMCs cultured in
the 6-well dishes were homogenized in radioimmunoprecipitation
lysis buffer containing 5% protease inhibitor cocktail (Pierce;
Thermo Fisher Scientific, Inc.). Protein quantity was determined
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) (44). Protein
samples (30–50 µg) were boiled for 15 min and run on 12% SDS-PAGE
gels, electroblotted onto nitrocellulose membranes, and
immunoblotted at 4°C overnight with anti-TRPM8 (#ab3243; dilution,
1:2,000; Abcam), anti-NADPH oxidase (NOX) 1 (#ab55831; dilution,
1:2,000; Abcam), anti-NOX 4 (#ab60940; dilution, 1:2,000; Abcam),
anti-phosphorylated (p)-JAK2 (#ab32101; dilution, 1:1,000; Abcam),
anti-total (t)-JAK2 (#ab39636; dilution, 1:1,000; Abcam), anti-RhoA
(#sc-32; dilution, 1:600; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-Rock2 (#sc-1851; dilution, 1:800; Santa Cruz
Biotechnology, Inc.) and anti-tubulin (#sc-365791; dilution,
1:5,000; Santa Cruz Biotechnology, Inc.) followed by corresponding
horseradish peroxidase-conjugated goat anti-mouse IgG (#BA1050;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) and and
goat anti-rabbit IgG (#BA1054; Wuhan Boster Biological Technology,
Ltd.) secondary antibodies (45).
The signals were measured using the Amersham ECL Western Blotting
assay kit (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA)
(46). Quantification of blots was
conducted using the Quantity One software system, version 4.6.2
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each experiment
was repeated at least three times.
Statistical analysis
Data are represented as mean ± standard error of the
mean. Differences were evaluated by one-way analysis of variance
followed by Tukey post hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Downregulation of TRPM8 in the aorta
media from hypertensive rats
The systolic blood pressure (SBP), diastolic blood
pressure (DBP) and HR of 2K1C rats were significantly higher when
compared with that of Sham-operated rats (P=0.0017, P=0.0037 and
P=0.0055, respectively; Fig. 1A).
Benazepril treatment was associated with a significant reduction in
the 2K1C-induced increase in SBP, DBP and HR in 2K1C rats
(P=0.0078, P=0.0024 and P=0.0015, respectively; Fig. 1A). In the aortae of 2K1C
hypertensive rats, the mRNA level of TRPM8 in de-nuded aortae
(without endothelium and perivascular fat pad) was decreased by
~60% compared with that in Sham-operated rats (P=0.0076; Fig. 1B). However, benazepril treatment
partially inhibited the downregulation of TRPM8 (Fig. 1B). Similar alterations were
observed in TRPM8 protein expression levels (2K1C vs.
Sham-operated, P=0.015; Fig. 1C).
These results suggest that TRPM8 is downregulated in the aortae of
2K1C hypertensive rats, which may be partially reversed by ACEI
treatment. This suggests that TRPM8 downregulation in hypertension
may be associated with the Ang II.
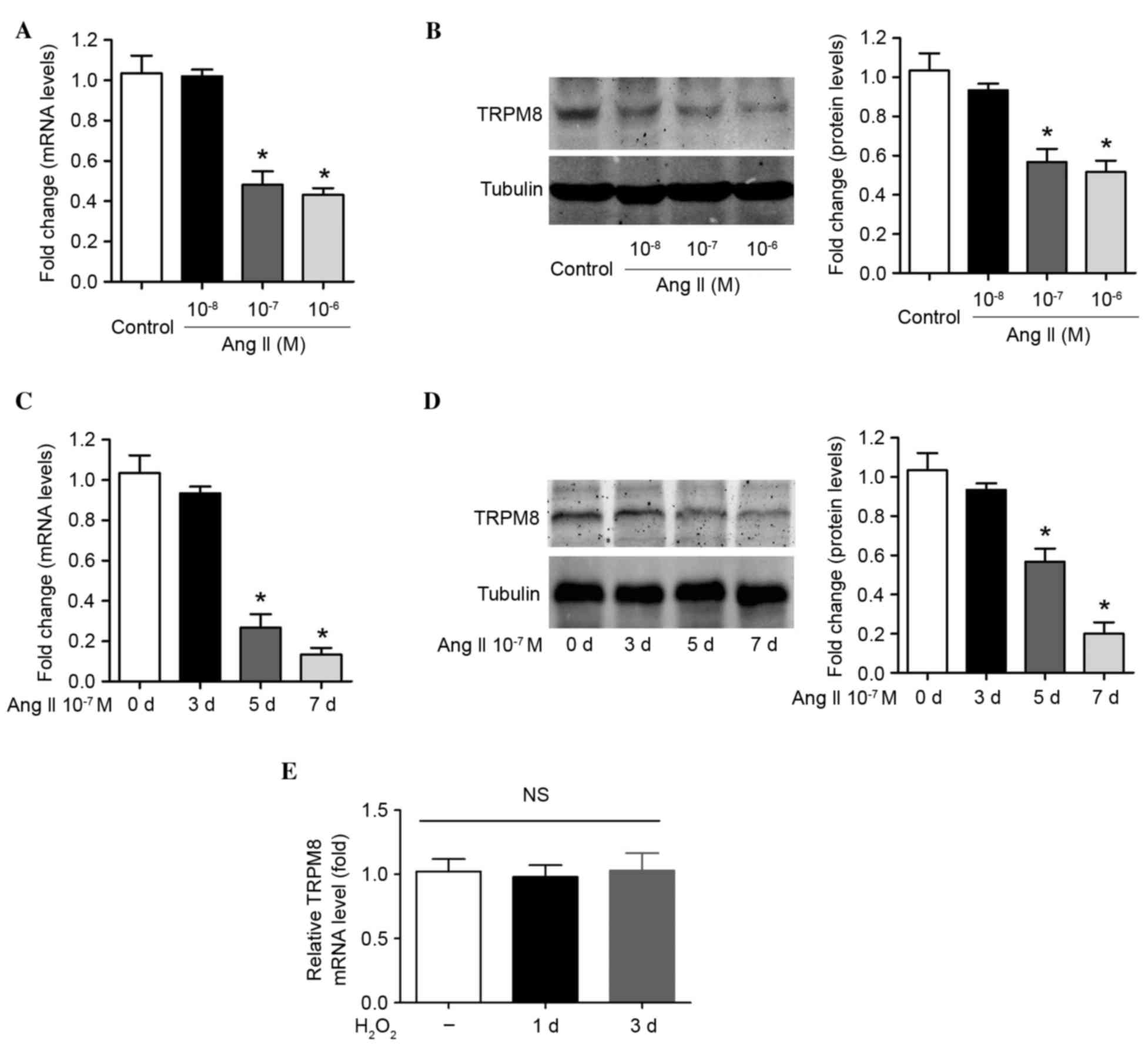
Ang II reduces TRPM8 expression in a
dose- and time-dependent manner in VSMCs
To determine whether TRPM8 downregulation in
hypertension is associated with Ang II, VSMCs were treated with
recombinant Ang II. As expected, incubation of cells with 10–7 M
and 10–6 M Ang II for 1 week induced a significant downregulation
in TRPM8 mRNA (P=0.014 and P=0.008, respectively; Fig. 2A) and protein expression (P=0.021
and P=0.015, respectively; Fig.
2B). However, treatment of cells with a low concentration of
Ang II (10–8 M) for 1 week was not associated with a significant
reduction in TRPM8 mRNA and protein expression (Figs. 2A and B). The influence of
incubation time on the Ang II-induced reduction in TRPM8 expression
was then evaluated. Ang II (10–7 M) treatment did not modulate
TRPM8 mRNA and protein expression at 3 days following treatment
(Fig. 2C and D). However, the mRNA
and protein levels of TRPM8 at 7 days post-treatment were lower
than those at 5 days post-treatment, which reached statistical
significance when compared with day 0 (TRPM8 mRNA at day 5 vs. day
0, P=0.019; TRPM8 mRNA at day 7 vs. day 0, P=0.003; TRPM8 protein
at day 5 vs. day 0, P=0.014; TRPM8 protein at day 7 vs. day 0,
P=0.006; Fig. 2C and D). The data
presented in the current study suggest that Ang II reduces TRPM8
expression in a dose- and time-dependent manner in VSMCs.
H2O2 was applied to VSMCs
directly to increase ROS production, in order to determine whether
ROS alters TRPM8 expression. TRPM8 mRNA expression was not altered
by H2O2 treatment (30 mM) for 1 day or 3 days
in VSMCs (Fig. 2E). This suggests
that the altered TRPM8 expression was not due to Ang II-induced ROS
production.
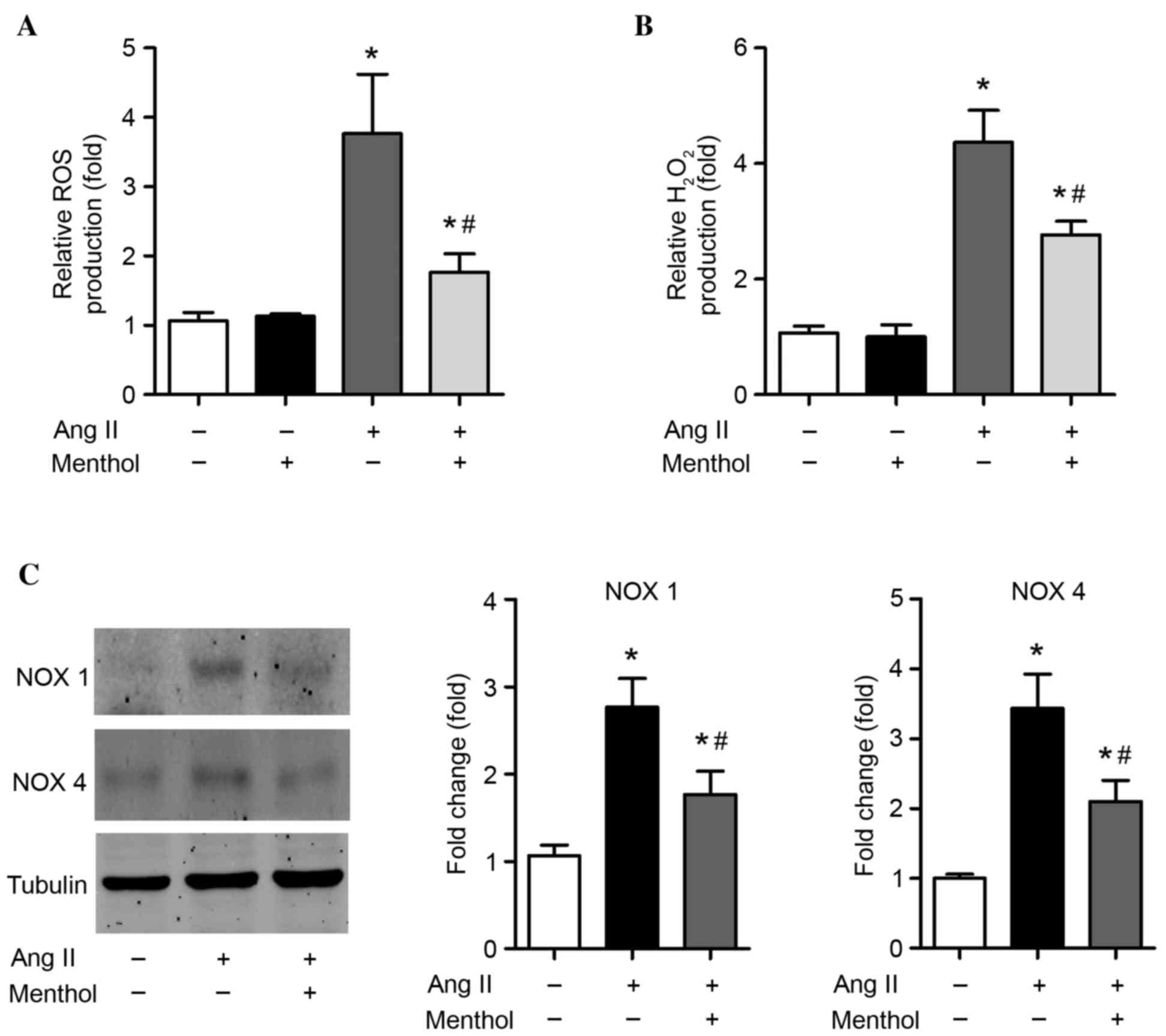
TRPM8 activation attenuates Ang
II-induced oxidative stress and upregulation of NOX enzymes in
VSMCs
The effect of TRPM8 downregulation in Ang II-treated
VSMCs was investigated. The induction of oxidative stress in VSMCs
by Ang II is an important factor that is detrimental to vascular
structure (1). Consistent with
previous studies (47),
significant enhancements of ROS (P=0.00044; Fig. 3A) and H2O2
(P=0.000016; Fig. 3B) levels were
detected in VSMCs treated with Ang II (10–7 M) for 5 days when
compared with untreated controls. Interestingly, activation of
TRPM8 by menthol, a selective TRPM8 agonist, significantly reduced
the production of ROS (P=0.0018; Fig.
3A) and H2O2 (P=0.0076; Fig. 3B) when compared with Ang II-only
treated cells, suggesting that activation of TRPM8 attenuates Ang
II-induced oxidative stress in VSMCs. As NOX enzymes are major
sources of ROS in the cardiovascular system (48), the expression of NOX1 and NOX4 was
determined. Treatment of VSMCs with Ang II (10–7 M) for 5 days
increased NOX1 and NOX4 protein expression by 2–3-fold in VSMCs
(Fig. 3C). However, TRPM8
activation by menthol significantly attenuated the Ang II-induced
upregulation of NOX1 and NOX4 (P=0.016 and P=0.012, respectively;
Fig. 3C).
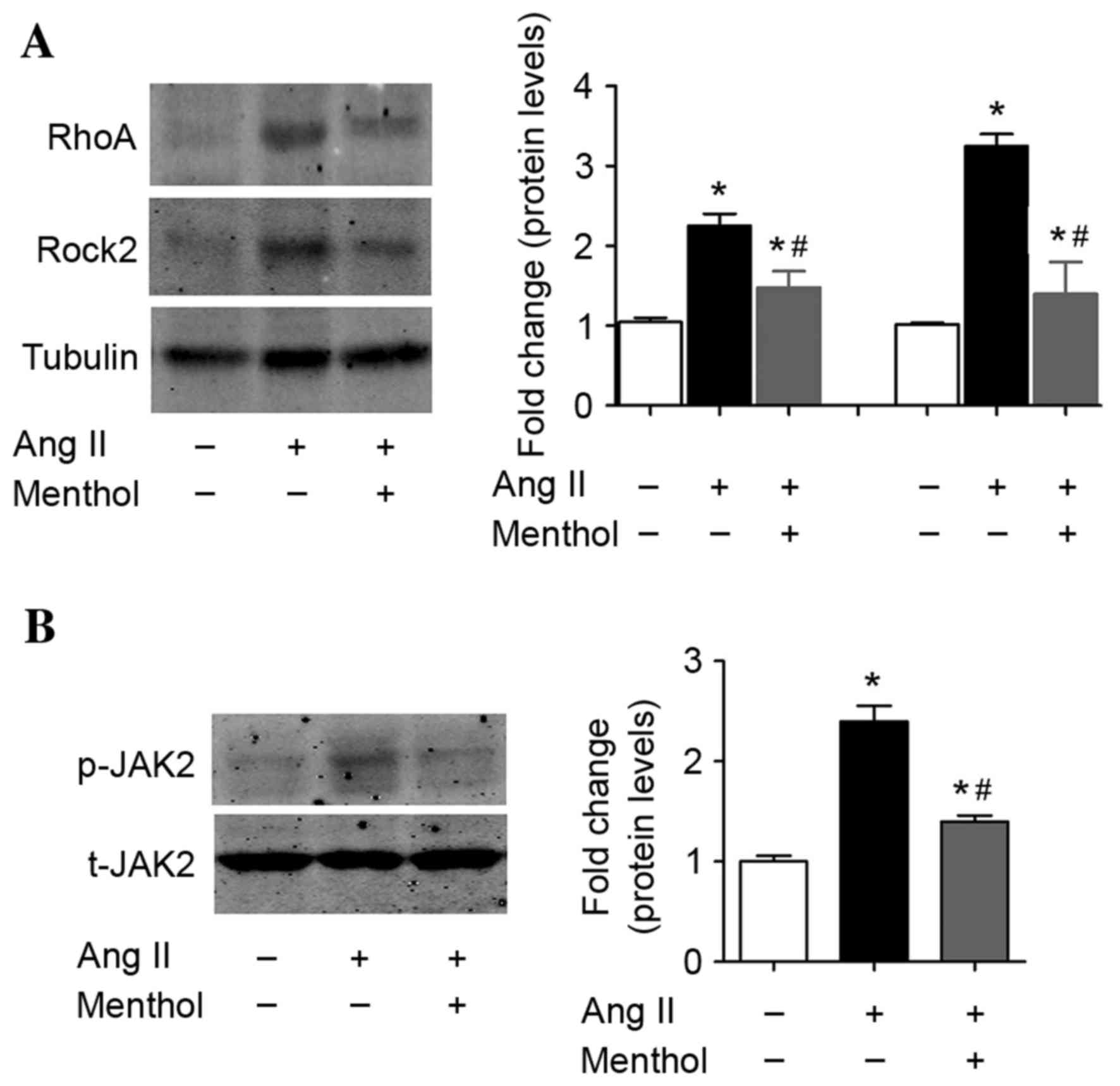
TRPM8 activation attenuates Ang
II-induced activation of RhoA-Rock2 and JAK2 signaling pathways in
VSMCs
Previous studies have demonstrated that the
RhoA-Rock2 and JAK2 signaling pathway in VSMCs is involved in the
pathogenesis of Ang II-dependent hypertension due to the increased
presence of ROS (49,50). Therefore, the present study
investigated whether TRPM8 activation may affect RhoA-Rock2 and
JAK2 activation by Ang II in VSMCs. Consistent with the results of
previous studies (49,50), Ang II treatment (10–7 M) for 5 days
activated RhoA-Rock2 (Fig. 4A) and
induced JAK2 phosphorylation (Fig.
4B) in VSMCs. By contrast, TRPM8 activation by menthol
partially inhibited Ang II-induced activation of RhoA-Rock2
(Fig. 4A) and JAK2 (Fig. 4B) signaling pathways in VSMCs.
Discussion
The superfamily of TRP channels is composed of
proteins that were initially identified in the Drosophila
eye (29). The first mammalian
family of TRP channels identified was the transient receptor
potential cation channels (51).
In the following years, additional TRP families, such as TRPM
(melastatin), TRPML (mucolipidins), TRPV (vanilloid receptor), TRPP
(for polycystic kidney disease proteins) and TRPA (for ankyrin-rich
proteins) were described (51).
The TRPM family consists of 8 members that are subdivided into the
following 4 groups based on their sequence homology: TRPM1 and
TRPM3; TRPM4 and TRPM5; TRPM6 and TRPM7; and TRPM2 and TRPM8
(29). They regulate a number of
cellular functions in normal and pathophysiological conditions
(29). Among these members, TRPM7
has been most intensively studied for its role in the
cardiovascular system. Touyz et al (52) reported that TRPM7 expression was
lower in the VSMCs of spontaneously hypertensive rats in a genetic
hypertensive model. Bradykinin, a potent vasodilator that lowers
blood pressure, upregulated TRPM7 and its downstream target
annexin-1 in VSMCs through phospholipase C-dependent, protein
kinase C-dependent, c-Src-dependent and cAMP-independent pathways
(53,54). Ang II increased TRPM7 expression in
VSMCs and TRPM7 was a functionally important regulator of
Mg2+ homeostasis in VSMCs (55). In addition, magnesium negatively
regulates vascular calcification and osteogenic differentiation
through increased TRPM7 activity (56). Furthermore, upregulation of TRPM7
channels by Ang II contributes to the development of the
proliferative phenotype of ascending aortic VSMCs (57). However, the possible roles of
additional TRPM proteins in the biological functions of VSMCs have
been rarely studied.
Recently, it has been reported that TRPM8 has
regulatory function in the cardiovascular system, particularly in
blood vessels (57). TRPM8 is
expressed in pulmonary arterial and aortic smooth muscle (58). Notably, TRPM8 in smooth muscle was
demonstrated to not be involved in cold-induced contraction, as it
does in the nervous system. TRPM8 activation led to relaxation of
vessels (29). In addition, TRPM8
activation by chronic dietary menthol attenuated mesenteric
arterial constriction and lowered blood pressure in a genetic model
of rat hypertension via inhibition of RhoA-Rho kinase expression
and activity in an in vivo study (30). The RhoA-Rho kinase cascade serves
key roles in pressure overload-induced right ventricular
hypertrophy and dysfunction (59)
and neointima formation (60). In
addition, there is crucial Rho-kinase inhibition during cardiac
development in the pathogenesis of hypertension (61,62).
These results suggest that TRPM8 is involved in the regulation of
vascular tone. However, the precise role of TRPM8 in hypertension
and hypertension-associated pathophysiology is not completely
known. To the best of our knowledge, the results of the present
study are the first to demonstrate that TRPM8 is downregulated in
2K1C rat aortae. The results are consistent with a recent report by
Liu et al (63), which
demonstrated a significant decrease in TRPM8 mRNA and protein
expression in the pulmonary arteries of pulmonary hypertensive
rats. In the present study, when the hypertension in 2K1C rats was
partially reversed by ACEI treatment, the upregulation of TRPM8 was
also partially reversed, suggesting the TRPM8 downregulation may be
a direct consequence of hypertension or hypertension-associated
factors. In the following experiments, TRPM8 was observed to be
downregulated in Ang II-treated VSMCs, which supports the
hypothesis that the high blood Ang II concentration may be the
cause of TRPM8 downregulation during hypertension.
2K1C is a well-established hypertension animal
model. This model has been used for several decades (64–68),
and the authors of the present study have used this model in a
previous study (69). In this
model, not only is the blood level of Ang II increased, the local
tissue levels of Ang II are also enhanced. The enhanced Ang II
concentrations in the aorta, kidney and brain significantly
contribute to the pathogenesis of hypertension (65,68).
Benazepril, also known as Lotensin, is an ACEI that was approved by
the US Food and Drug Administration in 1991. There are numerous
pharmacodynamic data regarding benazepril (70–72).
According to an extensive review regarding the pharmacodynamic and
pharmacokinetic properties of benazepril (73), benazepril remarkably suppresses
human plasma and tissue Ang II levels via inhibiting ACE activity
by ~80–90% both in vitro and in vivo. Mochel et
al (74) recently provided a
comprehensive description of the effect of benazepril on the
dynamics of the RAAS in dogs. It was demonstrated that the plasma
Ang II concentration decreased markedly following a bolus
administration of benazepril. Consequently, existing data regarding
the inhibitory effects of benazepril on plasma and tissue Ang II
levels have been considered in the present study. The in
vitro experiments demonstrated that Ang II treatment reduced
TRPM8 expression in cultured VSMCs, while activation of TRPM8
reversed Ang II-induced oxidative stress and upregulated NOX
enzymes in VSMCs. These results suggest that Ang II may directly
regulate TRPM8.
Of particular note, the results of the present study
indicated that activation of TRPM8, using a pharmacological
agonist, partially reversed the Ang II-induced oxidative stress and
JAK2 signaling activation. Oxidative stress serves an important
role in the pathogenesis of hypertension (75,76).
Ang II-mediated oxidative stress and DNA damage accelerates
cellular senescence in VSMCs (47). During this process, NOX1 and NOX4
are two major sources of ROS in Ang II-induced vascular hypertrophy
(48). In addition, RhoA-Rock2 and
JAK2 signaling pathways are involved in the pathogenesis of
hypertension or vascular remodeling due to the increased presence
of ROS (41,49,77).
In the present study, activation of TRPM8 significantly inhibited
the increase in NOX1 and NOX4 in Ang II-treated VSMCs, and
suppressed activation of the RhoA-Rock2 and JAK2 signaling
pathways. Recently, Sun et al (30) demonstrated that chronic dietary
menthol administration for 8 weeks prevented mesenteric arterial
constriction and lowered blood pressure in genetic hypertensive
rats with high Ang II levels. In addition, this study demonstrated
that TRPM8 effects were associated with inhibition of intracellular
calcium release from the sarcoplasmic reticulum and RhoA kinase
activity in arteries (30). The
results of the present study are consistent with this previous
study. In addition, the role of TRPM8 in additional vascular cells,
such as endothelial cells, may be of important interest. This
question requires further investigation in the future.
The RAAS modulates cardiovascular functions, as well
as a number of additional biological functions. As some
thermoregulatory proteins and RNAs have been demonstrated to be
involved in the development of brown and beige fat, which is
closely associated with metabolism (78–82),
the authors of the present study hypothesize that TRPM8 may be a
crucial regulator of adiposity and metabolism. Notably, the RAAS
serves an important role in metabolism via multiple molecular
mechanisms (83,84). Consequently, the association
between the RAAS and TRPM8 may be more complex than assumed in the
current study.
In conclusion, the results of the present study
demonstrated that TRPM8 may be involved in the pathophysiology of
hypertension, and support the notion that pharmacological
activation of TRPM8 may be a novel approach for treatment of
hypertension and other Ang II-induced vascular injuries, such as
aneurysm.
Acknowledgements
The present study was supported by grants from the
National Natural Foundation of China (grant nos. 81300081 and
81370558), the Shanghai Natural Science Foundation (grant no.
13ZR1459300) and the Fundamental Research Funds for the Central
Universities-Multi-Subjects Crossing of Tongji University (grant
no. 1501219097).
References
|
1
|
Poulter NR, Prabhakaran D and Caulfield M:
Hypertension. Lancet. 386:801–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He D, Fu M, Miao S, Hotta K, Chandak GR
and Xi B: FTO gene variant and risk of hypertension: A
meta-analysis of 57,464 hypertensive cases and 41,256 controls.
Metabolism. 63:633–639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu YL, Hu CY, Lu SS, Gong FF, Feng F, Qian
ZZ, Ding XX, Yang HY and Sun YH: Association between
methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C
polymorphisms and essential hypertension: A systematic review and
meta-analysis. Metabolism. 63:1503–1511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rocha NG, Templeton DL, Greiner JJ,
Stauffer BL and DeSouza CA: Metabolic syndrome and endothelin-1
mediated vasoconstrictor tone in overweight/obese adults.
Metabolism. 63:951–956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ait Aissa K, Lagrange J, Mohamadi A, Louis
H, Houppert B, Challande P, Wahl D, Lacolley P and Regnault V:
Vascular smooth muscle cells are responsible for a prothrombotic
phenotype of spontaneously hypertensive rat arteries. Arterioscler
Thromb Vasc Biol. 35:930–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang C, Li Y, Wang C, Wu Y, Cui W, Miwa
T, Sato S, Li H, Song WC and Du J: Complement 5a receptor mediates
angiotensin II-induced cardiac inflammation and remodeling.
Arterioscler Thromb Vasc Biol. 34:1240–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia Y, Jin X, Yan J, Entman ML and Wang Y:
CXCR6 plays a critical role in angiotensin II-induced renal injury
and fibrosis. Arterioscler Thromb Vasc Biol. 34:1422–1428. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Usui F, Shirasuna K, Kimura H, Tatsumi K,
Kawashima A, Karasawa T, Yoshimura K, Aoki H, Tsutsui H, Noda T, et
al: Inflammasome activation by mitochondrial oxidative stress in
macrophages leads to the development of angiotensin II-induced
aortic aneurysm. Arterioscler Thromb Vasc Biol. 35:127–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kigawa Y, Miyazaki T, Lei XF, Nakamachi T,
Oguchi T, Kim-Kaneyama JR, Taniyama M, Tsunawaki S, Shioda S and
Miyazaki A: NADPH oxidase deficiency exacerbates angiotensin
II-induced abdominal aortic aneurysms in mice. Arterioscler Thromb
Vasc Biol. 34:2413–2420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krishna SM, Seto SW, Jose RJ, Biros E,
Moran CS, Wang Y, Clancy P and Golledge J: A peptide antagonist of
thrombospondin-1 promotes abdominal aortic aneurysm progression in
the angiotensin II-infused apolipoprotein-E-deficient mouse.
Arterioscler Thromb Vasc Biol. 35:389–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davis FM, Rateri DL, Balakrishnan A,
Howatt DA, Strickland DK, Muratoglu SC, Haggerty CM, Fornwalt BK,
Cassis LA and Daugherty A: Smooth muscle cell deletion of
low-density lipoprotein receptor-related protein 1 augments
angiotensin II-induced superior mesenteric arterial and ascending
aortic aneurysms. Arterioscler Thromb Vasc Biol. 35:155–162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zreikat HH, Harpe SE, Slattum PW, Mays DP,
Essah PA and Cheang KI: Effect of Renin-Angiotensin system
inhibition on cardiovascular events in older hypertensive patients
with metabolic syndrome. Metabolism. 63:392–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristensen KE, Torp-Pedersen C, Gislason
GH, Egfjord M, Rasmussen HB and Hansen PR: Angiotensin-converting
enzyme inhibitors and angiotensin II receptor blockers in patients
with abdominal aortic aneurysms: Nation-wide cohort study.
Arterioscler Thromb Vasc Biol. 35:733–740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pruthi D, McCurley A, Aronovitz M, Galayda
C, Karumanchi SA and Jaffe IZ: Aldosterone promotes vascular
remodeling by direct effects on smooth muscle cell
mineralocorticoid receptors. Arterioscler Thromb Vasc Biol.
34:355–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alias S, Redwan B, Panzenböck A, Winter
MP, Schubert U, Voswinckel R, Frey MK, Jakowitsch J, Alimohammadi
A, Hobohm L, et al: Defective angiogenesis delays thrombus
resolution: A potential pathogenetic mechanism underlying chronic
thromboembolic pulmonary hypertension. Arterioscler Thromb Vasc
Biol. 34:810–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manka D, Chatterjee TK, Stoll LL, Basford
JE, Konaniah ES, Srinivasan R, Bogdanov VY, Tang Y, Blomkalns AL,
Hui DY and Weintraub NL: Transplanted perivascular adipose tissue
accelerates injury-induced neointimal hyperplasia: Role of monocyte
chemoattractant protein-1. Arterioscler Thromb Vasc Biol.
34:1723–1730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Demer LL and Tintut Y: Inflammatory,
metabolic, and genetic mechanisms of vascular calcification.
Arterioscler Thromb Vasc Biol. 34:715–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peier AM, Moqrich A, Hergarden AC, Reeve
AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan
S and Patapoutian A: A TRP channel that senses cold stimuli and
menthol. Cell. 108:705–715. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McKemy DD, Neuhausser WM and Julius D:
Identification of a cold receptor reveals a general role for TRP
channels in thermosensation. Nature. 416:52–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almeida MC, Hew-Butler T, Soriano RN, Rao
S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, et al:
Pharmacological blockade of the cold receptor TRPM8 attenuates
autonomic and behavioral cold defenses and decreases deep body
temperature. J Neurosci. 32:2086–2099. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knowlton WM, Daniels RL, Palkar R, McCoy
DD and McKemy DD: Pharmacological blockade of TRPM8 ion channels
alters cold and cold pain responses in mice. PLoS One.
6:e258942011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morenilla-Palao C, Luis E, Fernández-Peña
C, Quintero E, Weaver JL, Bayliss DA and Viana F: Ion channel
profile of TRPM8 cold receptors reveals a role of TASK-3 potassium
channels in thermosensation. Cell Rep. 8:1571–1582. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borrelli F, Pagano E, Romano B, Panzera S,
Maiello F, Coppola D, De Petrocellis L, Buono L, Orlando P and Izzo
AA: Colon carcinogenesis is inhibited by the TRPM8 antagonist
cannabigerol, a Cannabis-derived non-psychotropic cannabinoid.
Carcinogenesis. 35:2787–2797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel R, Gonçalves L, Leveridge M, Mack
SR, Hendrick A, Brice NL and Dickenson AH: Anti-hyperalgesic
effects of a novel TRPM8 agonist in neuropathic rats: A comparison
with topical menthol. Pain. 155:2097–2107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ramachandran R, Hyun E, Zhao L, Lapointe
TK, Chapman K, Hirota CL, Ghosh S, McKemy DD, Vergnolle N, Beck PL,
et al: TRPM8 activation attenuates inflammatory responses in mouse
models of colitis. Proc Natl Acad Sci USA. 110:7476–7481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y,
Jin R, Ma L, Wang P, Zhu Z, et al: Activation of the cold-sensing
TRPM8 channel triggers UCP1-dependent thermogenesis and prevents
obesity. J Mol Cell Biol. 4:88–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asuthkar S, Elustondo PA, Demirkhanyan L,
Sun X, Baskaran P, Velpula KK, Thyagarajan B, Pavlov EV and
Zakharian E: The TRPM8 protein is a testosterone receptor: I.
Biochemical evidence for direct TRPM8-testosterone interactions. J
Biol Chem. 290:2659–2669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quallo T, Vastani N, Horridge E, Gentry C,
Parra A, Moss S, Viana F, Belmonte C, Andersson DA and Bevan S:
TRPM8 is a neuronal osmosensor that regulates eye blinking in mice.
Nat Commun. 6:71502015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson CD, Melanaphy D, Purse A,
Stokesberry SA, Dickson P and Zholos AV: Transient receptor
potential melastatin 8 channel involvement in the regulation of
vascular tone. Am J Physiol Heart Circ Physiol. 296:H1868–H1877.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun J, Yang T, Wang P, Ma S and Zhu Z, Pu
Y, Li L, Zhao Y, Xiong S, Liu D and Zhu Z: Activation of
cold-sensing transient receptor potential melastatin subtype 8
antagonizes vasoconstriction and hypertension through attenuating
RhoA/Rho kinase pathway. Hypertension. 63:1354–1363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen JK, Zhao T, Ni M, Li DJ, Tao X and
Shen FM: Downregulation of alpha7 nicotinic acetylcholine receptor
in two-kidney one-clip hypertensive rats. BMC Cardiovasc Disord.
12:382012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato Y, Yokoyama U, Yanai C, Ishige R,
Kurotaki D, Umemura M, Fujita T, Kubota T, Okumura S, Sata M, et
al: Epac1 deficiency attenuated vascular smooth muscle cell
migration and neointimal formation. Arterioscler Thromb Vasc Biol.
35:2617–2625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weise-Cross L, Taylor JM and Mack CP:
Inhibition of diaphanous formin signaling in vivo impairs
cardiovascular development and alters smooth muscle cell phenotype.
Arterioscler Thromb Vasc Biol. 35:2374–2383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernandez I, Martin-Garrido A, Zhou DW,
Clempus RE, Seidel-Rogol B, Valdivia A, Lassègue B, García AJ,
Griendling KK and San Martin A: Hic-5 mediates TGFβ-induced
adhesion in vascular smooth muscle cells by a Nox4-dependent
mechanism. Arterioscler Thromb Vasc Biol. 35:1198–1206. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song J, Li J, Hou F, Wang X and Liu B:
Mangiferin inhibits endoplasmic reticulum stress-associated
thioredoxin-interacting protein/NLRP3 inflammasome activation with
regulation of AMPK in endothelial cells. Metabolism. 64:428–437.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Omodei D, Pucino V, Labruna G, Procaccini
C, Galgani M, Perna F, Pirozzi D, De Caprio C, Marone G, Fontana L,
et al: Immune-metabolic profiling of anorexic patients reveals an
anti-oxidant and anti-inflammatory phenotype. Metabolism.
64:396–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang HJ, Jung TW, Hong HC, Seo JA, Kim
SG, Kim NH, Choi KM, Choi DS, Baik SH and Yoo HJ: LECT2 induces
atherosclerotic inflammatory reaction via CD209 receptor-mediated
JNK phosphorylation in human endothelial cells. Metabolism.
64:1175–1182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kurano M, Hara M, Satoh H and Tsukamoto K:
Hepatic NPC1L1 overexpression ameliorates glucose metabolism in
diabetic mice via suppression of gluconeogenesis. Metabolism.
64:588–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou XB, Feng YX, Sun Q, Lukowski R, Qiu
Y, Spiger K, Li Z, Ruth P, Korth M, Skolnik EY, et al: Nucleoside
diphosphate kinase B-activated intermediate conductance potassium
channels are critical for neointima formation in mouse carotid
arteries. Arterioscler Thromb Vasc Biol. 35:1852–1861. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee MJ, Kim EH, Lee SA, Kang YM, Jung CH,
Yoon HK, Seol SM, Lee YL, Lee WJ and Park JY:
Dehydroepiandrosterone prevents linoleic acid-induced endothelial
cell senescence by increasing autophagy. Metabolism. 64:1134–1145.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ray Hamidie RD, Yamada T, Ishizawa R,
Saito Y and Masuda K: Curcumin treatment enhances the effect of
exercise on mitochondrial biogenesis in skeletal muscle by
increasing cAMP levels. Metabolism. 64:1334–1347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kadota Y, Toyoda T, Hayashi-Kato M,
Kitaura Y and Shimomura Y: Octanoic acid promotes branched-chain
amino acid catabolisms via the inhibition of hepatic branched-chain
alpha-keto acid dehydrogenase kinase in rats. Metabolism.
64:1157–1164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fu L, Bruckbauer A, Li F, Cao Q, Cui X, Wu
R, Shi H, Zemel MB and Xue B: Leucine amplifies the effects of
metformin on insulin sensitivity and glycemic control in
diet-induced obese mice. Metabolism. 64:845–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Soe NN, Sowden M, Baskaran P, Smolock EM,
Kim Y, Nigro P and Berk BC: Cyclophilin A is required for
angiotensin II-induced p47phox translocation to caveolae in
vascular smooth muscle cells. Arterioscler Thromb Vasc Biol.
33:2147–2153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu YJ, Guo X, Li CJ, Li DQ, Zhang J, Yang
Y, Kong Y, Guo H, Liu DM and Chen LM: Dipeptidyl peptidase-4
inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in
association with its effects suppressing endoplasmic reticulum
stress in db/db mice. Metabolism. 64:226–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seshiah PN, Weber DS, Rocic P, Valppu L,
Taniyama Y and Griendling KK: Angiotensin II stimulation of NAD
(P)H oxidase activity: Upstream mediators. Circ Res. 91:406–413.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lasségue B, San M, artín A and Griendling
KK: Biochemistry, physiology, and pathophysiology of NADPH oxidases
in the cardiovascular system. Circ Res. 110:1364–1390. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kirabo A, Kearns PN, Jarajapu YP, Sasser
JM, Oh SP, Grant MB, Kasahara H, Cardounel AJ, Baylis C, Wagner KU
and Sayeski PP: Vascular smooth muscle Jak2 mediates angiotensin
II-induced hypertension via increased levels of reactive oxygen
species. Cardiovasc Res. 91:171–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Montezano AC, Callera GE, Yogi A, He Y,
Tostes RC, He G, Schiffrin EL and Touyz RM: Aldosterone and
angiotensin II synergistically stimulate migration in vascular
smooth muscle cells through c-Src-regulated redox-sensitive RhoA
pathways. Arterioscler Thromb Vasc Biol. 28:1511–1518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Clapham DE, Runnels LW and Strübing C: The
trp ion channel family. Nat Rev Neurosci. 2:387–396. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Touyz RM, He Y, Montezano AC, Yao G,
Chubanov V, Gudermann T and Callera GE: Differential regulation of
transient receptor potential melastatin 6 and 7 cation channels by
Ang II in vascular smooth muscle cells from spontaneously
hypertensive rats. Am J Physiol Regul Integr Comp Physiol.
290:R73–R78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yogi A, Callera GE, Tostes R and Touyz RM:
Bradykinin regulates calpain and proinflammatory signaling through
TRPM7-sensitive pathways in vascular smooth muscle cells. Am J
Physiol Regul Integr Comp Physiol. 296:R201–R207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Callera GE, He Y, Yogi A, Montezano AC,
Paravicini T, Yao G and Touyz RM: Regulation of the novel Mg2+
transporter transient receptor potential melastatin 7 (TRPM7)
cation channel by bradykinin in vascular smooth muscle cells. J
Hypertens. 27:155–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He Y, Yao G, Savoia C and Touyz RM:
Transient receptor potential melastatin 7 ion channels regulate
magnesium homeostasis in vascular smooth muscle cells: Role of
angiotensin II. Circ Res. 96:207–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Montezano AC, Zimmerman D, Yusuf H, Burger
D, Chignalia AZ, Wadhera V, van Leeuwen FN and Touyz RM: Vascular
smooth muscle cell differentiation to an osteogenic phenotype
involves TRPM7 modulation by magnesium. Hypertension. 56:453–462.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang Z, Wang M, Fan XH, Chen JH, Guan YY
and Tang YB: Upregulation of TRPM7 channels by angiotensin II
triggers phenotypic switching of vascular smooth muscle cells of
ascending aorta. Circ Res. 111:1137–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang XR, Lin MJ, McIntosh LS and Sham JS:
Functional expression of transient receptor potential melastatin-
and vanilloid-related channels in pulmonary arterial and aortic
smooth muscle. Am J Physiol Lung Cell Mol Physiol. 290:L1267–L1276.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ikeda S, Satoh K, Kikuchi N, Miyata S,
Suzuki K, Omura J, Shimizu T, Kobayashi K, Kobayashi K, Fukumoto Y,
et al: Crucial role of rho-kinase in pressure overload-induced
right ventricular hypertrophy and dysfunction in mice. Arterioscler
Thromb Vasc Biol. 34:1260–1271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gadang V, Konaniah E, Hui DY and Jaeschke
A: Mixed-lineage kinase 3 deficiency promotes neointima formation
through increased activation of the RhoA pathway in vascular smooth
muscle cells. Arterioscler Thromb Vasc Biol. 34:1429–1436. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ellawindy A, Satoh K, Sunamura S, Kikuchi
N, Suzuki K, Minami T, Ikeda S, Tanaka S, Shimizu T, Enkhjargal B,
et al: Rho-Kinase inhibition during early cardiac development
causes arrhythmogenic right ventricular cardiomyopathy in mice.
Arterioscler Thromb Vasc Biol. 35:2172–2184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shimokawa H and Satoh K: 2015 ATVB Plenary
Lecture: Translational research on rho-kinase in cardiovascular
medicine. Arterioscler Thromb Vasc Biol. 35:1756–1769. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu XR, Liu Q, Chen GY, Hu Y, Sham JS and
Lin MJ: Down-regulation of TRPM8 in pulmonary arteries of pulmonary
hypertensive rats. Cell Physiol Biochem. 31:892–904. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Greenberg S: Vascular responses of the
perfused intestine to vasoactive agents during the development of
two-kidney, one-clip Goldblatt hypertension in dogs. Circ Res.
48:895–906. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Morishita R, Higaki J, Miyazaki M and
Ogihara T: Possible role of the vascular renin-angiotensin system
in hypertension and vascular hypertrophy. Hypertension. 19 Suppl
2:II62–II67. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cervenka L, Horácek V, Vanecková I,
Hubácek JA, Oliverio MI, Coffman TM and Navar LG: Essential role of
AT1A receptor in the development of 2K1C hypertension.
Hypertension. 40:735–741. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xie QY, Sun M, Yang TL and Sun ZL:
Losartan reduces monocyte chemoattractant protein-1 expression in
aortic tissues of 2K1C hypertensive rats. Int J Cardiol. 110:60–66.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Maxwell MH, Lupu AN, Viskoper RJ, Aravena
LA and Waks UA: Mechanisms of hypertension during the acute and
intermediate phases of the one-clip, two-kidney model in the dog.
Circ Res. 40(5 Suppl 1): I24–I28. 1977.PubMed/NCBI
|
|
69
|
Li DJ, Evans RG, Yang ZW, Song SW, Wang P,
Ma XJ, Liu C, Xi T, Su DF and Shen FM: Dysfunction of the
cholinergic anti-inflammatory pathway mediates organ damage in
hypertension. Hypertension. 57:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jamerson K, Weber MA, Bakris GL, Dahlöf B,
Pitt B, Shi V, Hester A, Gupte J, Gatlin M and Velazquez EJ:
ACCOMPLISH Trial Investigators: Benazepril plus amlodipine or
hydrochlorothiazide for hypertension in high-risk patients. N Engl
J Med. 359:2417–2428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jamerson KA, Devereux R, Bakris GL, Dahlöf
B, Pitt B, Velazquez EJ, Weir M, Kelly RY, Hua TA, Hester A and
Weber MA: Efficacy and duration of benazepril plus amlodipine or
hydrochlorothiazide on 24-h ambulatory systolic blood pressure
control. Hypertension. 57:174–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Valvo E, Casagrande P, Bedogna V, Antiga
L, Alberti D, Zamboni M, Perobelli L, Dal Santo F and Maschio G:
Systemic and renal effects of a new angiotensin converting enzyme
inhibitor, benazepril, in essential hypertension. J Hypertens.
8:991–995. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Balfour JA and Goa KL: Benazepril. A
review of its pharmacodynamic and pharmacokinetic properties, and
therapeutic efficacy in hypertension and congestive heart failure.
Drugs. 42:511–539. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mochel JP, Fink M, Peyrou M, Soubret A,
Giraudel JM and Danhof M: Pharmacokinetic/pharmacodynamic modeling
of renin-angiotensin aldosterone biomarkers following
angiotensin-converting enzyme (ACE) inhibition therapy with
benazepril in dogs. Pharm Res. 32:1931–1946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dikalov SI, Nazarewicz RR, Bikineyeva A,
Hilenski L, Lassègue B, Griendling KK, Harrison DG and Dikalova AE:
Nox2-induced production of mitochondrial superoxide in angiotensin
II-mediated endothelial oxidative stress and hypertension. Antioxid
Redox Signal. 20:281–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lu S, Xiang L, Clemmer JS, Mittwede PN and
Hester RL: Oxidative stress increases pulmonary vascular
permeability in diabetic rats through activation of transient
receptor potential melastatin 2 channels. Microcirculation.
21:754–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Palanivel R, Ganguly R, Turdi S, Xu A and
Sweeney G: Adiponectin stimulates Rho-mediated actin cytoskeleton
remodeling and glucose uptake via APPL1 in primary cardiomyocytes.
Metabolism. 63:1363–1373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Diaz MB, Herzig S and Vegiopoulos A:
Thermogenic adipocytes: From cells to physiology and medicine.
Metabolism. 63:1238–1249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sun L and Trajkovski M: MiR-27
orchestrates the transcriptional regulation of brown adipogenesis.
Metabolism. 63:272–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hondares E, Gallego-Escuredo JM, Flachs P,
Frontini A, Cereijo R, Goday A, Perugini J, Kopecky P, Giralt M,
Cinti S, et al: Fibroblast growth factor-21 is expressed in
neonatal and pheochromocytoma-induced adult human brown adipose
tissue. Metabolism. 63:312–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Boström PA, Fernández-Real JM and
Mantzoros C: Irisin in humans: Recent advances and questions for
future research. Metabolism. 63:178–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tsuchiya Y, Ando D, Takamatsu K and Goto
K: Resistance exercise induces a greater irisin response than
endurance exercise. Metabolism. 64:1042–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sharma AM, Janke J, Gorzelniak K, Engeli S
and Luft FC: Angiotensin blockade prevents type 2 diabetes by
formation of fat cells. Hypertension. 40:609–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Santos SH, Braga JF, Mario EG, Pôrto LC,
Rodrigues-Machado Mda G, Murari A, Botion LM, Alenina N, Bader M
and Santos RA: Improved lipid and glucose metabolism in transgenic
rats with increased circulating angiotensin-(1–7). Arterioscler
Thromb Vasc Biol. 30:953–961. 2010. View Article : Google Scholar : PubMed/NCBI
|