Introduction
Aerobic organisms are exposed to reactive oxygen
species (ROS). Free radicals are essential at low levels as they
participate in various cellular processes, including signalling
pathways and defence against pathogens (1,2). ROS
are mainly produced endogenously by mitochondria or during the
‘oxidative burst’ of macrophages, but they can also be produced by
exogenous factors, such as environmental pollution, smoking and
ionizing or ultraviolet radiation. Aerobic organisms possess a
variety of antioxidant mechanisms to neutralise free radicals,
including enzymes, as well as non-enzymatic compounds (3,4).
When in excess, free radicals may interact to damage cellular
macromolecules, causing oxidative stress (5). This condition has been associated
with various pathological conditions, such as cancer, diabetes and
neurodegenerative diseases (6–8).
Over the past 30 years, particulate matter (PM) has
emerged as a key pollutant, with well-known effects on human
health. Only in 2012, there were approximately 3.7 million
premature deaths related to air pollution (9). There are studies that demonstrate the
implication of air pollutants in diseases of the cardiovascular
system (10,11), as well as of the respiratory system
(12). There are even signs of
genotoxicity, which ultimately can lead to lung cancer (13–15).
Some of these adverse health effects have been associated to
PM-induced oxidative stress (16).
PM promotes ROS generation through two different mechanisms: i) the
use of oxidative components adsorbed on the surface of the
particles, resulting in oxidation; and ii) PM generates ROS mainly
in pulmonary epithelial cells and macrophages. However, fine and
ultrafine particles have the ability to translocate into the
systemic circulation and eventually, other organs and tissues may
also be subjected to local inflammation associated with ROS
generation (10,17).
Since 2010, Greece faces a financial crisis with
significant repercussion on per capita growth domestic product.
This, in combination with the heavy taxation of oil used for
heating (diesel) has resulted in the overwhelming use of biomass
for domestic heating (18).
Smaller PM fractions have been associated with biomass burning
(19). The smaller PM fractions
have a greater oxidative potential per unit mass, as they have the
ability to adsorb more chemical substances, exhibiting a large
surface area per mass (20). In
addition, smaller particles are retained strongly by the lower
respiratory system, a phenomenon that is more evident in children
(21). Individual particle
deposition across the three main regions of the respiratory tract
depends on particle properties inter-individual physiology
differences (22). Wood smoke
particles are generally smaller than 1 µm and consist of several
toxic compounds such as PAHs, quinones and metals, that enhance the
particle-induced health effects (23).
Most commonly, PM10 and PM2.5 are measured as
indicators of air quality. Studies have shown that PM2.5 in
particular can lead to serious health issues, due to the small
aerodynamic diameter of these particles that allows them to reach
the alveoli, through the induction of oxidative stress,
inflammation and genotoxicity (24–27).
Based on the above, this study aimed to assess the
oxidative stress induced by exposure to urban PM and the beneficial
effect of consuming a highly antioxidant beverage, such as coffee.
Towards this aim, the detailed population exposure to PM during
wintertime, as well as the chemical composition and the
chemically-induced oxidative stress were analysed. Following the
chemical characterization of these samples, EA.hy926 cells were
exposed at PM2.5 concentrations to assess the cytotoxicity of
PM.
One main issue for the majority of PM-associated
studies appears to be the small sample quantity of PM obtained
through the filters of monitors, a possible prohibiting factor in
the experimental design (28).
Cell culture experiments require a substantial amount of extract in
order to obtain the desired concentration each time in the flask.
With the method described herein, the amount of used extract was
reduced to a minimum. Moreover, the possible protective effect of
food extracts, such as coffee, was examined.
Coffee is a very popular beverage due to its
pleasant characteristics (taste and aroma). Its worldwide annual
production exceeds 8 Mt, with an average daily consumption of 2.3
billion cups (29). As a beverage,
coffee is rich in polyphenols and abundant in chlorogenic acid
(CGA) (30). A number of studies
have investigated the quantity and the beneficial effects of CGA
(31–33) and the antioxidant properties of
coffee beans (34).
Materials and methods
Field measurements
PM2.5 measurements were carried out from the 10th to
22nd of December 2015 at two different sampling sites in the urban
area of Larissa, Greece, an urban background and a traffic site.
PM2.5 particle fractions were collected using low volume air
samplers (ENCO PM; TCR Tecora, Milan, Italy) equipped with PM2.5
sampling heads that meet the EN 14907 standards operating at a
flow-rate of 38 l/min. Sampling duration was 24 h. The inlet
sampling points were at a height of 10 m from the ground. PM2.5
samples were collected on PTFE membrane filters with PMP supporting
ring (Ø 47 mm, pore size 2 µm; Pall Life Sciences, Port Washington,
NY, USA), which are appropriate not only for gravimetric and
chemical analysis of PM, but also for genotoxicity tests. Filters
were weighed at least 3 times before and after the sampling on an
electronic microbalance with a sensitivity of ±1 µg and were stored
under controlled conditions of temperature (20–23°C) and relative
humidity (30–40%).
Chemical analysis on PM filters
PM2.5 ambient concentrations were obtained and
chemical analyses were conducted for black carbon content and the
elemental composition of the particles. Black carbon concentration
levels were estimated using a non-destructive analysis method
developed by a Magee Scientific SootScan™ Model OT21 Optical
Transmissometer. The elemental constituents of PM2.5, were
determined on one half of each 47 mm Teflon filter by ED-XRF using
an Epsilon 5 XRF instrument (PANalytical B.V., Eindhoven, The
Netherlands). For the calibration of the instrument, 27-µm-thin
standards were used. Corrections for instrument errors and the
effect of the matrix on the X-ray emission intensities were also
determined. Method detection limits were between 1 and 70
ng/cm2 for Na, Mg, Al, Si, S, Cl, K, Ca, Ti, V, Mn, Fe,
Ni, Cu, Zn and Pb. All samples were measured in duplicate according
to standard operating procedures.
DTT assay
The oxidative potential of PM2.5 was estimated
indirectly, on the basis of the rate of consumption of
dithiothreitol (DTT) over time. The PM2.5 samples were stored at
−20°C in the dark prior to analysis. One fourth of a filter with a
known mass of PM2.5 was added to a dark 4 ml vial and labelled.
Subsequently, 3 ml of 100 µΜ DTT in 0.1 M phosphate buffer were
added into the vial and the amount of DTT lost was measured (from 0
to 15 min) at 37°C. The vials were placed in a water bath and
shaken. At indicated time points, an aliquot of 0.5 ml of the
reaction mixture was removed and added to 0.5 ml of 10% TCA. TCA
was used in order to terminte the reaction. When all time points
were quenched, 50 µl of 10 mM DTNB (made in 0.1 M phosphate buffer,
pH 7.4) were added forming 2-nitro-5-thiobenzoic acid (TNB).
Subsequently, 2 ml of 0.4 M Tris-Base pH 8.9 with 20 mM EDTA were
added to chelate any transition metals. Light absorption was
measured at 412 nm, thus permitting us to quantify TNB, which has a
molar absorptivity of 14,150 M−1 cm−1. Linear
regression was used between the measurement time points and the DTT
loss in order to estimate the rate of DTT consumption. The results
were expressed in terms of pmol DTT/min per PM mass (µg) and volume
(m3) of air. As regards quality control samples, both
method blanks and positive control were prepared and analysed at
the same time as the unknown samples. Positive control samples
contained all the reagents with 16.1 µl of 9,10-phenanthrenequinone
(PQN). Blank and positive control (or PQN) were run in
triplicate.
PM extraction
The toxicity of ambient PM was determined according
to the following procedure: PM2.5 samples on filters were kept at
−20°C in the dark prior to use. For sample measurement half of each
PM-fraction filter (including the filter blank) was used for
cellular toxicity and the other half for elemental analysis. The
water-soluble components of PM were extracted from each filter half
by ultrasonic agitation in 900 µl distilled water at room
temperature in the dark. The extraction was carried out overnight.
The extracts were centrifuged at 3,409 × g for 1 min. The
supernatant was then filtered through a 0.22 µm polypropylene
filter into 1.5 ml polypropylene microcentrifuge tubes. Method
blanks were prepared using distilled water. Filter blanks were
treated identically to the actual samples. The samples from
different days were then pooled in a single sample of 1.65
µg/µl.
Preparation of extracts from coffee
beans
A total of 9 different coffee extracts were
prepared. Seven came from the variety of Coffea arabica
(Brazil and Decaf) and two from Coffea canephora (Robusta).
For the first variety (Brazil), we had extracts of green beans and
from 4 different roasting time points (R1:7 min; R2:6 min; R3:5
min; R4:4 min) at 215°C so as to examine the effects of various
roasting times on the activity.
For each sample, 10% w/v of ground (using mortar and
pestle) coffee in double distilled water was prepared.
Consequently, a 20-min sonication step (70% amplitude, 0.7 sec
cycle) and a 20-min stirring under moderate heat (35°C) were
carried out. The extract was separated from solid residues by
centrifuging each sample (7,000 × g, 10 min, 25°C). Finally, each
extract was aliquoted and kept at −80°C for future use.
XTT cytotoxicity assay
The XTT assay kit (Trevigen, Gaithersburg, MD, USA)
was used to assess cell viability. Briefly, EA.hy926 cells (kindly
provided by Profesor Koukoulis, University of Thessaly, Larissa,
Greece) were cultured in a 96-well plate in a 1×104
cells/well density in Dullbesco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (FBS). After 24 h, various
concentrations of PM2.5 extract in serum-free DMEM were
administered for 48 h. Subsequently, in each well 50 µl of XTT test
solution were added. The test solution was prepared by mixing 50 µl
XTT labelling reagent with 1 µl electron coupling reagent. Finally,
after 4 h of incubation, the absorbance of each well was measured
at 450 and 630 nm with the latter being a reference wavelength, in
a BioTek ELx800 microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). Serum-free DMEM was used as a negative control.
Additionally, PM2.5 extract concentration alone in serum-free DMEM
was tested at 450 nm. The percentage of viability was calculated
using the following formula: Viability (%) = [(ODcontrol
- ODsample)/ODcontrol] ×100, where
ODcontrol and ODsample indicate the optical
density of the negative control and the tested compounds,
respectively.
Assessment of DNA strand cleavage
The plasmid (pBluescript-SK+, Fermentas, Waltham,
MA, USA) DNA has a supercoiled conformation, but when a
single-strand break occurs, it loses that conformation and adapts
an open circular conformation. Based on this, the percentage of DNA
strand cleavage, as well as the protective activity of food
extracts was assessed. Firstly, 2 µl (4 µg/ml) of DNA was mixed
with different volumes of sterilised PBS and PM2.5 sample. That
way, a gradient of different concentrations of the PM2.5 samples
was created. The final volume of the reaction was 10 µl. The
samples were incubated for 45 min at 37°C. Subsequently, 3 µl of
loading buffer (Bromophenol Blue 0.25% + 30% Glycerol) was mixed to
terminate the reaction and the samples were loaded on an 0,8%
agarose gel. The samples were ran at 70 V for 55 min. Ethidium
bromide was used to stain the gel by suspending it in 12,5 µl of
ethidium bromide (10 mg/ml) and 250 ml of distilled water for 30
min. Consequently, the gel was washed with 250 ml distilled water
for 20 min. Results were obtained by exposing the gels to UV and
capturing a photo using MultiImage Light Cabinet (Alpha Innotech,
San Leandro, CA, USA). Finally, we used the Alpha View suite to
analyse the photos. When coffee extracts were introduced, the final
reaction volume was increased to 13 µl.
Reducing power assay
The reducing power of the extracts was determined
according to the protocol of Yen and Duh with some modifications
(35). Briefly, each extract was
dissolved in phosphate buffer (0.2 M, pH 6.6) at various
concentrations. A total of 250 µl of the sample solution were added
to 250 µl of potassium ferricyanide (1%) and incubated at 50°C for
20 min, followed by cooling on ice for an additional 5 min.
Consequently, 500 µl TCA (10%) were added and the samples were
centrifuged at 3,000 rpm for 10 min at 25°C. A total of 250 µl from
the supernatant were mixed with 250 µl deionised water, as well as
50 µl of ferric chloride (0.1%). The samples were incubated at room
temperature for 10 min and finally the absorbance was measured at
700 nm. All experiments were carried out in triplicate and at least
on two separate occasions.
Enzyme activity experiments
Polyphenolic compounds may absorb at the tested
wavelengths, possibly increasing the optical density of the samples
(even though, this was not the case for the currently tested
extracts). Therefore, control samples were prepared identically to
the test samples, without the extract. All initial reaction rates
were in the linear scale and were measured during the first 2 to 4
min of the reaction depending on the enzyme. Each assay was
performed in triplicate, and the optical density was measured using
a Hitachi U-1900 spectrophotometer (Hitachi, Tokyo, Japan).
Assessment of xanthine oxidase (XO)
activity
In order to determine XO activity and its
inhibition, the production of uric acid from xanthine was used. The
reaction mixture (with a final volume of 500 µl) comprised sodium
phosphate buffer (33 mM, pH 7.5), xanthine (4.8 µM), EDTA (0.1 mM)
and the coffee extract in various concentrations. Each reaction was
initiated by the addition of XO (43 mU) and the absorbance was
measured at 295 nm for 4 min. The specific activity of each extract
was measured by dividing the IC50 value (in µg of
polyphenols) to the amount of polyphenols per mg of coffee. The
IC50 value was determined as the extract's amount (in µg
of polyphenols) that inhibited XO activity by 50%, as monitored by
the decrease in uric acid production.
Assessment of CAT activity
The activity of catalase was determined using the
method described by Aebi (36). In
this assay, changes in the absorbance of H2O2
as it becomes decomposed by CAT are measured, allowing the
identification of potential inhibitors. Briefly, various coffee
extract concentrations were added in 4 µl of RBCL (diluted 1:40) in
sodium potassium phosphate (67 mM, pH 7.4), followed by incubation
at 37°C for 10 min. Consequently, H2O2 (0.6%)
was added and the absorbance was measured at 240 nm for 2 min.
Specific activity was determined as in the case of XO.
Assessment of total superoxide
dismutase (SOD) activity
SOD activity was determined using the method of
Dieterich et al (37). In
this method, pyrogallol autoxidation caused by superoxide anions
present in the air can be inhibited by SOD. Therefore, a potential
inhibitor will decrease the ability of SOD to protect pyrogallol.
Briefly, the reaction mixture (final volume of 1 ml) included
Tris-HCl (0.04 mM, pH 8.2), diethylenetriaminepentaacetic acid
(DTPA, 0.08 mM), 30 µl of RBCL (diluted 1:10) and various
concentrations of the coffee extract. The mixture was incubated for
5 min at room temperature, followed by the initiation of the
reaction by the addition of pyrogallol (0.08 mM). The absorbance
was measured at 420 nm for 3 min. Control samples were prepared
identically to the test samples without the extracts. Due to the
fact that polyphenolic compounds are potential scavengers of
superoxide anion, the possible inhibitory effect of coffee on
pyrogallol autoxidation in the absence of SOD was examined
(38).
Statistical analysis
Statistical analyses were carried out using SPSS
software, version 20.0 (SPSS, Inc., Chicago, IL, USA). One-way
ANOVA was applied, along with Dunnett's test for multiple pairwise
comparisons. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
For the XTT cytotoxicity assay, a number of
different PM2.5 extract concentrations (10, 30, 60 and 80 µg per
100 µl well) were tested. Based on our results, PM2.5 ambient air
concentrations ranged from 39 to 168 µg/m3, with an
average of 105 (±46 SD) µg/m3. At the same time, the
observed DTT activity levels fall within the range of typical
levels identified in other similar studies (20–180 pmol/min/µg)
(39). PM2.5 is highly chemically
reactive as it can adsorb higher amounts of compounds, due to their
higher active surface (21). This
results in enhanced oxidative capacity, as well as higher
inflammatory potential and pulmonary deposition. It has also to be
noted that oxidative capacity has been associated with the metals
content such as Fe and Cu (40).
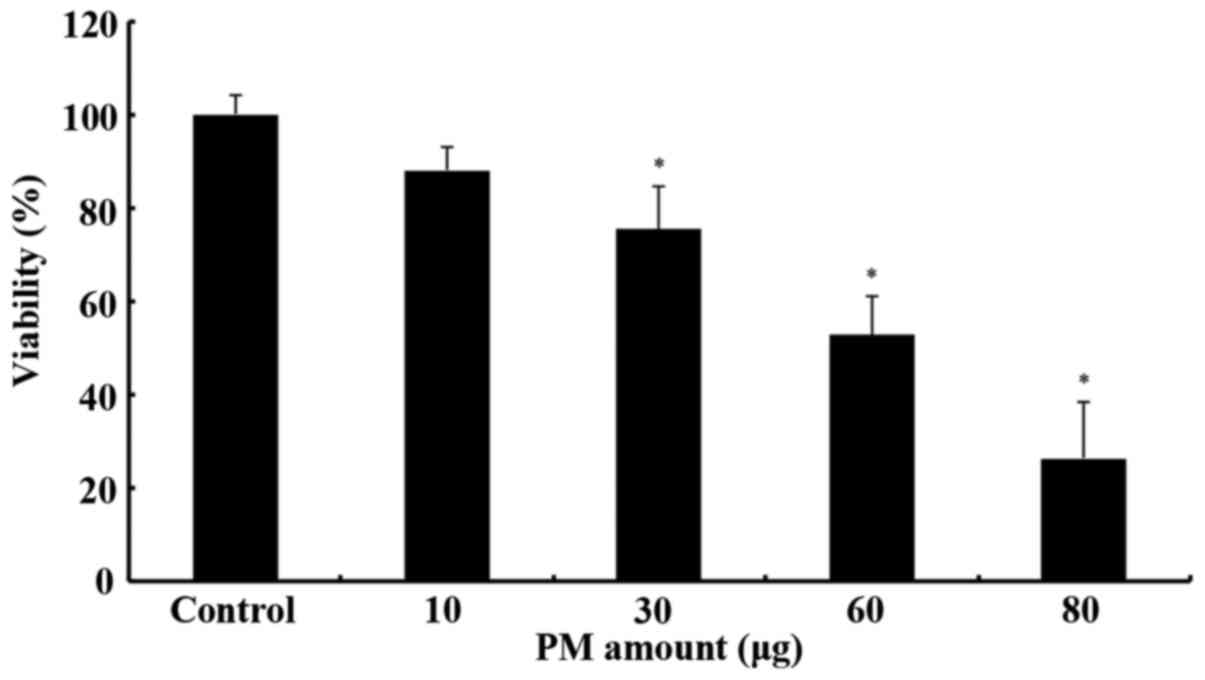
As shown in Fig. 1,
the PM extract caused a statistically significant decrease in cell
viability from the 30 µg concentration. Consequently, toxicity
levels increased dose-dependently up to the maximum concentration
used (80 µg). However, this assay required a relatively large
amount of the extract and therefore further analysis using cell
cultures was not possible. Instead, in order to investigate the
possible DNA-damaging potential of the PM extract, a plasmid
relaxation assay was developed, based on the assay of Chang et
al (41) with modifications,
including the replacement of AAPH (a peroxyl radical-forming
compound) with the PM extract.
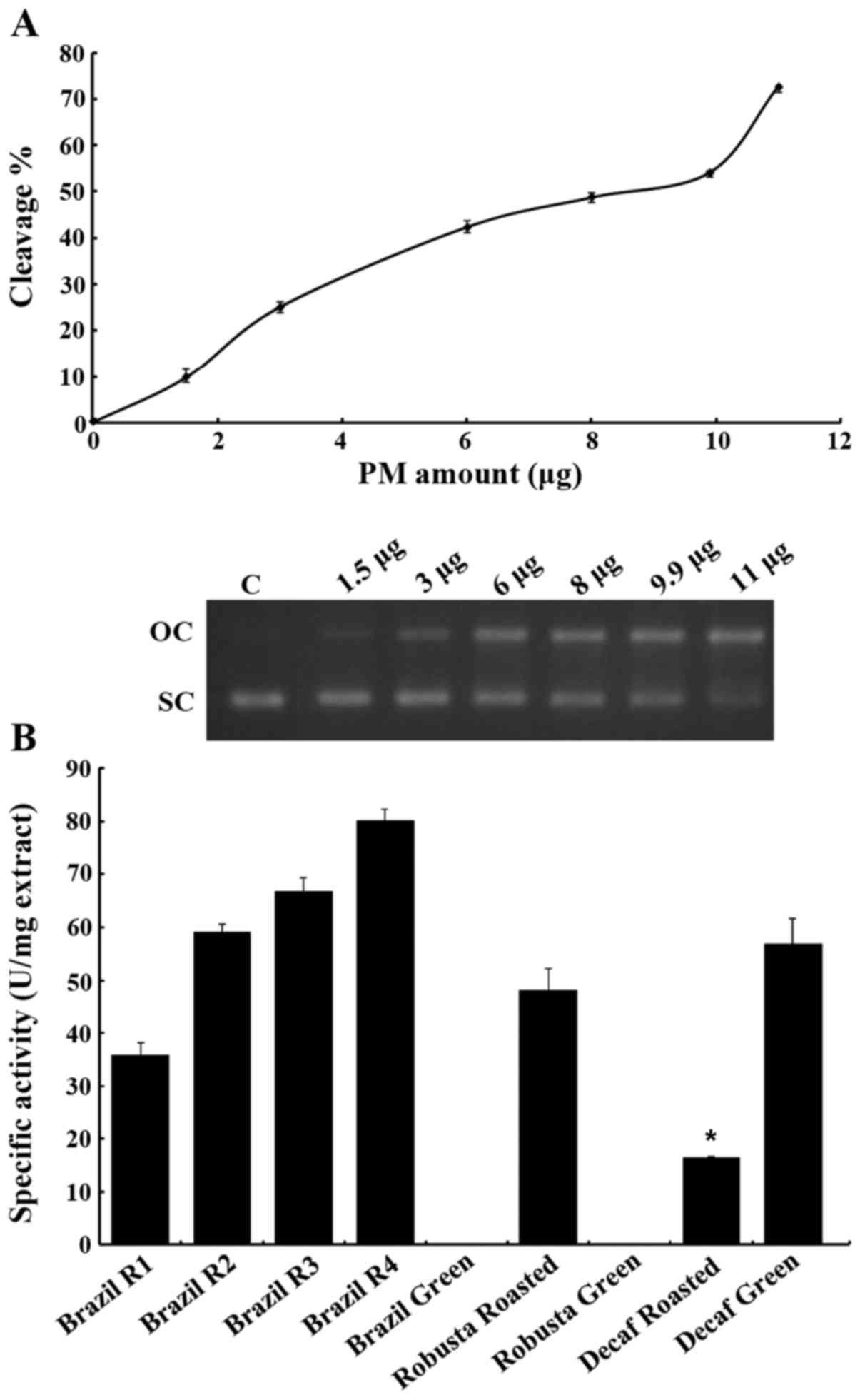
The effects on the plasmid DNA are shown in Fig. 2. The gradient of PM2.5
concentrations caused a greater percentage of DNA to migrate to the
upper zone of the gel that corresponds to the open conformation.
Unfortunately, PM particles have a quite complex composition and it
is difficult to assign the observations strictly to only one of the
components (13). It is also
well-known that transition metals adsorbed onto PM can generate ROS
through Fenton reactions (42). In
fact, we did observe a good concentration of Fe in the composition
analysis of our samples. Ferrous ions can generate HO•,
which can subsequently cause DNA breaks by attacking the backbone
and the bases. DNA is targeted by metal ions since it has an
electron-rich structure, resulting thus in the formation of stable
adducts (43).
Additionally, the presence of polycyclic aromatic
hydrocarbons (PAHs) may also be responsible for the DNA damage.
Studies have suggested that they can be metabolised from CYP450
enzymes and the products of this process can cause DNA damage as it
is know from the literature (44,45).
Furthermore, the existence of environmental persistent-free
radicals (EPFRs), such as semiquinones, on the surface of PM
particles is seemingly of high importance for initiating the
production of free radicals, particularly in cell-free conditions,
such as our assay, particularly without the addition of
H2O2 (46,47).
EPFRs were initially found to be occurring upon chemisorption of an
organic precursor to a redox metal site. This way the radical is
stabilised and bound onto the surface of the particle (46). In the literature, it has been
suggested that EPFRs are deprotonated in water and produce a
superoxide anion which consequently is dismutated to
H2O2, which can be used for the Fenton
reactions with metals to produce the very reactive hydroxyl radical
(46,48–52).
However, despite the fact that the amount of at
least 30 µg of PM extract that the XTT method requires is
relatively low, this corresponds to only 100 µl of medium per well.
The other cell-based methods would require 75 cm2
flasks, increasing the PM extract amount to at least 3,000 µg per
75 cm2 flask in order to assess its genotoxicity.
The amount of 11 µg PM extract (in 13 µl of the
total volume) was selected as it causes significant DNA cleavage
(~70% cleavage, whilst remaining a small amount compared to the
ones that the cell-based assays would require). When coffee
extracts were introduced to the mixture of the reaction, a
protective action on DNA was documented (Fig. 2B). In most occasions, coffee seems
to protect DNA from breaking quite efficiently. Of course different
extracts seem to achieve different degrees of protection, as is
shown in Fig. 2B. Two out of three
green bean coffee extracts (Brazil and Robusta) actually exhibited
no DNA-protecting activity. This could be due to the fact that
green beans are rich in small molecule antioxidants, which may act
in a pro-oxidative manner when in excess (53), while during roasting novel
antioxidant complexes are formed (e.g., melanoidins), which behave
in a different manner. As previously demonstrated, during the
roasting process, polyphenols can be incorporated into melanoidins
(54). Only the decaffeinated
green coffee extract showed the ability to protect DNA, while its
roasted form was actually less powerful with the current assay.
This could be due to the fact that the decaffeination process
(Swiss Water process) may interact in some way with the
antioxidants present in the beans. Interestingly enough, all of the
green bean extracts actually failed to protect the DNA even in the
absence of the PM2.5 extract. This is an intriguing result which
could be further investigated as to why some of these extracts
cause DNA damage and why during the presence of a pollutant they
may not. The extracts from Brazil as stated above were obtained
from beans with different roasting times. The results demonstrated
that the less the beans are roasted, the greater the protective
effects become. The R4, R3 and R2 extracts actually were the most
active ones among the tested extracts. Of note, the R1 extract,
which was roasted for a longer time period than the others, ranked
second to last. The activity of the roasted Decaf extract was
observed to be at least 2-fold lower than that of the other
extracts, thus ranking last out of the coffee extracts (apart from
the green bean extracts).
Briefly, the ability of PM extract to induce DNA
damage was exhibited using the aforementioned assay, as well as the
potential protective effect following the addition of coffee
extracts in the reaction mixture.
In addition, the antioxidant activity of these
coffee extracts was assessed using the Reducing Power assay, as
well as their potential inhibitory effect on XO. Furthermore, the
extracts were tested for potential inhibitory activities against
two antioxidant enzymes, namely catalase and SOD.
In the reducing power assay, the most potent extract
was the Robusta green sample, as it displayed the highest specific
activity (Fig. 3A). Specific
activity is a unit that was previously developed in order to
compare the activity of extracts, taking into consideration both
the amount of polyphenols and their respective activity by dividing
the amount of polyphenols required to reach the IC50
value to the amount of polyphenols contained per mg of ground
coffee (34). The total
polyphenolic content (TPC) of these coffee extracts has been
previously determined and was also used in the current analysis.
The TPC results are shown in Table
I. A higher value of specific activity corresponds to a more
potent extract. Therefore, the Robusta green extract displayed
22.52 units of specific activity, 35.2% higher than that of its
roasted counterpart (16.66). In the decaffeinated variety, the
green extract was more potent than the roasted one by 54.1%
(11.56–7.5 units). In the third variety, Brazil, in which four
different roasting degrees were examined, the green extract was the
least active, having 84.7, 76.9, 41 and 18.8%
(8.5–15.7/15.04/11.99/10.1 units) lower specific activity values
compared with the roasted samples, with the most active being the
less roasted one. It is noteworthy that the activity of the roasted
extracts diminishes over roasting time, an observation that is
frequent in the bibliography (34,55,56).
The reducing power assay allows the determination of the potency of
a certain extract to reduce potassium ferricyanide
(Fe3+) to potassium ferrocyanide (Fe2+) by
offering an electron. Electron transfer is a major mechanism that
mediates free radical neutralization and therefore, the results
from the reducing power assay may provide information concerning
the antioxidant activity of a tested extract.
 | Table I.Total polyphenolic content of the
coffee extracts [adapted from Priftis et al, 2015 (34)]. |
Table I.
Total polyphenolic content of the
coffee extracts [adapted from Priftis et al, 2015 (34)].
| Coffee extract | TPC (mg GAE/g
coffee) |
|---|
| Brazil R1 | 29.61±1.03 |
| Brazil R2 | 35.26±2.01 |
| Brazil R3 | 45.28±3.50 |
| Brazil R4 | 42.55±4.05 |
| Brazil Green | 32.58±5.17 |
| Robusta
Roasted | 43.99±1.32 |
| Robusta Green | 52.71±3.11 |
| Decaf Roasted | 27.42±1.57 |
| Decaf Green | 41.40±4.04 |
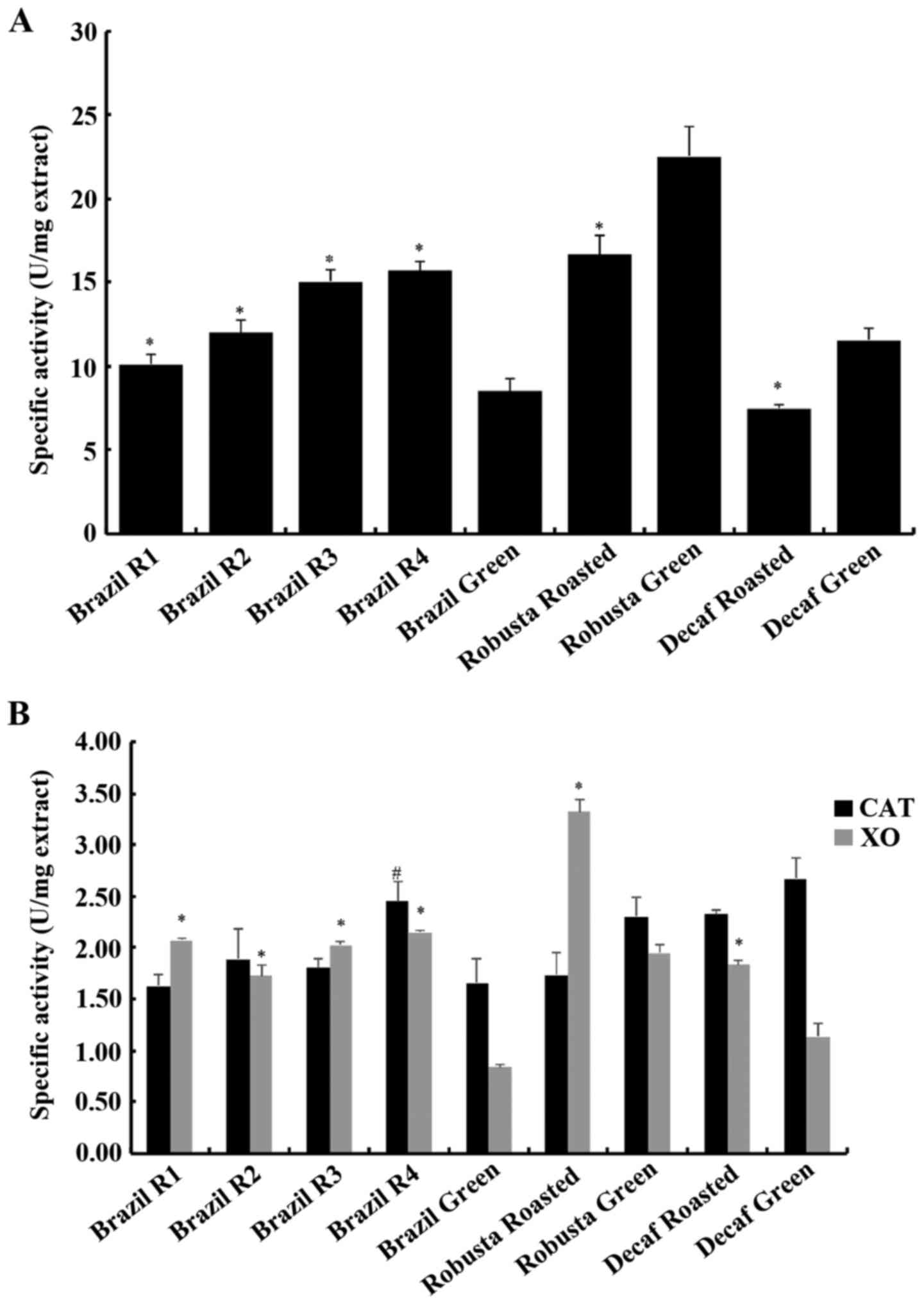
Following the determination of their antioxidant
capacity, the coffee extracts were examined as potential inhibitors
of ΧΟ, as shown in Fig. 3B. XO is
a flavoprotein comprising two identical 145 kDa subunits. It
possesses four redox centers that are aligned in an almost linear
fashion at the C-terminal 85-kDa molybdopterin-binding domain. The
active form of XO is a 290 kDa homodimer with each monomer being
able to catalyse independently (57). XO is a cytosolic enzyme present in
several mammalian tissues with the highest activity found in the
liver and the intestine (58).
However, XO can also be found extracellularly as it has an
extremely high affinity for the endothelium (at the nanomollar
scale) by binding to specific proteoglycans of the plasma membrane,
potentially leading to further oxidative damage (59).
XO derives from xanthine dehydrogenase (XDH) and
participates in purine degradation by metabolizing hypoxanthine to
xanthine and further to urate. As a part of its mechanism of
action, XO utilises molecular oxygen as the electron acceptor,
thereby leading to superoxide radical and hydrogen peroxide
production (60). However, it also
leads to the production of uric acid, a strong antioxidant that
accounts for >50% of the antioxidant capacity of plasma
(61). Consequently, this enzyme
has an equivocal role in the redox status, since it produces both
free radicals and uric acid. XO is a major contributor in free
radical production during exercise due to the ischemia-reperfusion
mechanism, but it has also been implicated in several diseases
including myocardial infarction, hypertension, atherosclerosis,
diabetes and cancer (57,62). In addition, excessive uric acid
production may lead to its crystallization and deposition in the
joints, connective tissue and the kidneys, a condition known as
gout and thus, XO inhibition may have therapeutic interest
(63).
According to the results, all coffee extracts
displayed inhibitory activity against XO, with the most potent
inhibitor being the roasted Robusta sample, exhibiting 3.32 units
of specific activity (corresponding to an IC50 value of
300 µg/ml) and the least potent being the Brazil green extract with
0.84 units of specific activity, corresponding to an
IC50 value of 1,193 µg/ml. In the Brazil variety, the
roasting process increased the inhibitory activity of coffee, as
all four roasted samples had higher specific activity values. In
more detail, the less roasted sample (R4) was the most potent
inhibitor, exhibiting 2.15 units of specific activity
(IC50 at 465 µg/ml), R3 had 2.03 units, R2 1.73 units
and the more roasted sample (R1) displayed 2.07 units of specific
activity. In the Robusta variety, the green extract was less potent
than the roasted one, as it had 1.95 units of specific activity
(IC50 at 512.6 µg/ml). As for the decaffeinated variety,
again roasting boosted its inhibitory effect by increasing the
specific activity from 1.14 (IC50 at 877.9 µg/ml) to
1.84 (IC50 at 544.2 µg/ml). Therefore, all coffee
extracts resulted in XO inhibition and interestingly, roasting had
an activating effect, increasing the inhibitory effect for each of
the three tested coffee varieties.
The coffee extracts were also tested for their
ability to inhibit SOD, which is an antioxidant enzyme. There are
many SOD isoforms that all catalyse the reduction of superoxide
anion to hydrogen peroxide. According to the results, no effect of
either coffee sample was observed on SOD activity (data not shown).
In vivo studies of coffee consumption in rats found either
no effect or an increase on SOD activity (64,65).
Therefore, coffee may not affect the activity of this particular
enzyme but could possibly alter its expression levels.
Finally, coffee extracts were tested for their
ability to inhibit the activity of catalase (Fig. 3B). This enzyme catalyses the
neutralization of hydrogen peroxide to oxygen and water. It is one
of the fastest enzymes known to day and is considered one of the
most important intracellular antioxidant mechanisms (66). All coffee extracts displayed an
inhibition of catalase activity with the most potent being the R4
sample from the Brazil variety that had 2.45 units of specific
activity (IC50 at 408.1 µg/ml). In all nine tested
samples, the specific activity values ranged from 1.62–2.15 units
(with the former having an IC50 value at 615.5 µg/ml).
In contrast to the XO activity assay, roasting did not affect the
ability of coffee to interfere with catalase activity, apart from
the R4 sample which displayed a significantly higher inhibitory
effect compared to its green counterpart. In addition, caffeine
depletion did not affect this assay. Furthermore, no differences
were observed between the Coffea arabica and Coffea
canephora varieties. The inhibition of catalase has been
observed before by plant polyphenols as in the case of tea
catechins (67). Despite the
currently shown inhibitory effect, in in vivo studies coffee
supplementation has been shown to improve the catalase system in
rat liver (68). Therefore,
further examination on the role of coffee on this enzyme is
required.
The concomitant inhibition of both XO (a
ROS-producing enzyme) and CAT (a ROS detoxifying enzyme) by coffee
is an important finding, shedding light on its potential mechanism
of action. It has been reported that CGA lactones, present in
roasted coffee may inhibit XO (69). However, the effects of
bioavailability and metabolism need to be taken into consideration,
as coffee constituents (>1,000 different compounds) need to be
absorbed and pass through the liver before entering the
bloodstream. CGAs, the main polyphenolic compound found in coffee
exhibit high levels of bioavailability (~30%) compared to other
phenolics (70).
To conclude, PM displayed genotoxic activity as
shown in the currently used plasmid relaxation assay. The advantage
of this assay is the miniscule amount of PM extract required to
obtain reliable results. This activity can be attributed to the
transition metals and quinones that are present in the extract. The
genotoxic activity of PM however, can be prevented through
antioxidant mechanisms. In the current study, coffee extracts from
three varieties (one Coffea canephora and two Coffea
arabica of which one was decaffeinated) were examined. The
roasted samples exhibited an inhibitory effect on the PM-induced
plasmid relaxation as they had shown before in a AAPH-induced
plasmid relaxation assay (34).
The antioxidant activity of these coffee extracts was determined
using the reducing power assay, as well as examining their effect
on XO, SOD and catalase activity.
The current study deals with the evaluation of an
assay based on plasmid relaxation for assessing the toxicity of
ambient air PM and the antioxidant potential of a typical beverage
such as coffee. Given the widespread exposure of the human
population to ambient air particles of varying composition,
aerodynamic characteristics and, consequently, toxicity it is
important for the scientific community to have at bay integrated
tools that can capture not only the toxic potency of the particles
but also the protective potential of potential interventions such
as the uptake of food additives. The joint evaluation of the
antioxidant capacity of typically consumed food items, such as
coffee against the oxidative potential of ubiquitous environmental
stressors such as ambient air particulates could be an example in
case of the new assay efficiency. This is of particular importance
when dealing with population exposure in socioeconomically deprived
areas, where environmental degradation is more evident, due to
unsustainable environmental management or energy poverty. The
results of this demonstrated that the plasmid relaxation assay
developed herein manages to provide robust results on both
oxidative stress and genotoxicity induced by exposure to typical
ambient air fine particles found in cities. In addition, the assay
allowed us to evaluate efficiently the antioxidant and thus
protective potential of different coffee bean extracts. These
results may be used as the basis for development of guidance
regarding the type of coffee bean (both before and after toasting)
that would provide the highest protection to population susceptible
individuals exposed to PM with high genotoxic potency.
Based on our results, the plasmid relaxation assay
developed and tested herein may prove to be a cost-effective manner
for assessing the oxidative potential of environmental stressors,
as well as for quantifying the antioxidant capacity and the
protective action against the damage caused to DNA by food
additives and other protective xenobiotics. The results obtained
may be used to set the ground for the provision of guidelines
promoting consumer behaviour that aims towards public health
protection.
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
TPC
|
total polyphenolic content
|
|
CGA
|
chlorogenic acid
|
|
PM
|
particulate matter
|
|
XO
|
xanthine oxidase
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
References
|
1
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elias RJ, Kellerby SS and Decker EA:
Antioxidant activity of proteins and peptides. Crit Rev Food Sci
Nutr. 48:430–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halliwell B: Free Radicals and Other
Reactive Species in DiseaseeLS. John Wiley & Sons, Ltd.;
Chichester, UK: 2001
|
|
6
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rochette L, Zeller M, Cottin Y and Vergely
C: Diabetes, oxidative stress and therapeutic strategies. Biochim
Biophys Acta. 1840:2709–2729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Wang W, Li L, Perry G, Lee HG and
Zhu X: Oxidative stress and mitochondrial dysfunction in
Alzheimer's disease. Biochim Biophys Acta. 1842:1240–1247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
World Health Organisation (WHO), . Media
centre. Ambient (outdoor) air quality and health. WHO; Geneva: pp.
1–7. 2014
|
|
10
|
Brook RD, Rajagopalan S, CA III Pope,
Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV,
Mittleman MA, et al: American Heart Association Council on
Epidemiology and Prevention, Council on the Kidney in
Cardiovascular Disease, and Council on Nutrition, Physical Activity
and Metabolism: Particulate matter air pollution and cardiovascular
disease: An update to the scientific statement from the American
Heart Association. Circulation. 121:2331–2378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franklin BA, Brook R and Arden Pope C III:
Air pollution and cardiovascular disease. Curr Probl Cardiol.
40:207–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arbex MA, Ude P Santos, Martins LC,
Saldiva PHN, Pereira LAA and Braga ALF: Air pollution and the
respiratory system. J Bras Pneumol. 38:643–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borgie M, Ledoux F, Verdin A, Cazier F,
Greige H, Shirali P, Courcot D and Dagher Z: Genotoxic and
epigenotoxic effects of fine particulate matter from rural and
urban sites in Lebanon on human bronchial epithelial cells. Environ
Res. 136:352–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Billet S, Abbas I, Le Goff J, Verdin A,
André V, Lafargue PE, Hachimi A, Cazier F, Sichel F, Shirali P, et
al: Genotoxic potential of Polycyclic Aromatic Hydrocarbons-coated
onto airborne Particulate Matter (PM 2.5) in human lung epithelial
A549 cells. Cancer Lett. 270:144–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golokhvast KS, Chernyshev VV, Chaika VV,
Ugay SM, Zelinskaya EV, Tsatsakis AM, Karakitsios SP and
Sarigiannis DA: Size-segregated emissions and metal content of
vehicle-emitted particles as a function of mileage: Implications to
population exposure. Environ Res. 142:479–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen LC and Lippmann M: Effects of metals
within ambient air particulate matter (PM) on human health. Inhal
Toxicol. 21:1–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka M, Takano H, Fujitani Y, Hirano S,
Ichinose T, Shimada A and Inoue KI: Effects of exposure to
nanoparticle-rich diesel exhaust on 8-OHdG synthesis in the mouse
asthmatic lung. Exp Ther Med. 6:703–706. 2013.PubMed/NCBI
|
|
18
|
Sarigiannis DΑ, Karakitsios SP and
Kermenidou MV: Health impact and monetary cost of exposure to
particulate matter emitted from biomass burning in large cities.
Sci Total Environ. 524–525, 319–330. 2015.
|
|
19
|
Sarigiannis DΑ, Karakitsios SP, Kermenidou
M, Nikolaki S, Zikopoulos D, Semelidis S, Papagiannakis A and
Tzimou R: Total exposure to airborne particulate matter in cities:
The effect of biomass combustion. Sci Total Environ. 493:795–805.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarigiannis D, Kyriakou S, Kermenidou M
and Karakitsios S: The reactive oxidative potential from biomass
emitted particulate matter (PM10, PM2.5 & PM1) and its impact
on human health. Proceedings of the 18th International Symposium on
Environmental Pollution and its Impact on Life in the Mediterranean
Region. Mediterranean Scientific Association of Environmental
Protection, Crete. 2015.
|
|
21
|
Sarigiannis DΑ, Karakitsios SP, Zikopoulos
D, Nikolaki S and Kermenidou M: Lung cancer risk from PAHs emitted
from biomass combustion. Environ Res. 137:147–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Albuquerque-Silva I, Vecellio L, Durand M,
Avet J, Le Pennec D, De Monte M, Montharu J, Diot P, Cottier M,
Dubois F, et al: Particle deposition in a child respiratory tract
model: In vivo regional deposition of fine and ultrafine aerosols
in baboons. PLoS One. 9:e954562014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bølling A Kocbach, Pagels J, Yttri KE,
Barregard L, Sallsten G, Schwarze PE and Boman C: Health effects of
residential wood smoke particles: The importance of combustion
conditions and physicochemical particle properties. Part Fibre
Toxicol. 6:292009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cachon BF, Firmin S, Verdin A, Ayi-Fanou
L, Billet S, Cazier F, Martin PJ, Aissi F, Courcot D, Sanni A, et
al: Proinflammatory effects and oxidative stress within human
bronchial epithelial cells exposed to atmospheric particulate
matter (PM(2.5) and PM(>2.5)) collected from Cotonou, Benin.
Environ Pollut. 185:340–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lodovici M and Bigagli E: Oxidative stress
and air pollution exposure. J Toxicol. 2011:4870742011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zakharenko AM, Engin AB, Chernyshev VV,
Chaika VV, Ugay SM, Rezaee R, Karimi G, Drozd VA, Nikitina AV,
Solomennik SF, et al: Basophil mediated pro-allergic inflammation
in vehicle-emitted particles exposure. Environ Res. 152:308–314.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Golokhvast K, Vitkina T, Gvozdenko T,
Kolosov V, Yankova V, Kondratieva E, Gorkavaya A, Nazarenko A,
Chaika V, Romanova T, et al: Impact of Atmospheric Microparticles
on the Development of Oxidative Stress in Healthy City/Industrial
Seaport Residents. Oxid Med Cell Longev. 2015:4121732015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boisa N, Entwistle J and Dean JR: A new
simple, low-cost approach for generation of the PM10 fraction from
soil and related materials: Application to human health risk
assessment. Anal Chim Acta. 852:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higdon JV and Frei B: Coffee and health: A
review of recent human research. Crit Rev Food Sci Nutr.
46:101–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murthy PS and Naidu MM: Recovery of
Phenolic Antioxidants and Functional Compounds from Coffee Industry
By-Products. Food Bioprocess Technol. 5:897–903. 2012. View Article : Google Scholar
|
|
31
|
Sato Y, Itagaki S, Kurokawa T, Ogura J,
Kobayashi M, Hirano T, Sugawara M and Iseki K: In vitro and in vivo
antioxidant properties of chlorogenic acid and caffeic acid. Int J
Pharm. 403:136–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu JG, Hu QP and Liu Y: Antioxidant and
DNA-protective activities of chlorogenic acid isomers. J Agric Food
Chem. 60:11625–11630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henry-Vitrac C, Ibarra A, Roller M,
Mérillon JM and Vitrac X: Contribution of chlorogenic acids to the
inhibition of human hepatic glucose-6-phosphatase activity in vitro
by Svetol, a standardized decaffeinated green coffee extract. J
Agric Food Chem. 58:4141–4144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Priftis A, Stagos D, Konstantinopoulos K,
Tsitsimpikou C, Spandidos DA, Tsatsakis AM, Tzatzarakis MN and
Kouretas D: Comparison of antioxidant activity between green and
roasted coffee beans using molecular methods. Mol Med Rep.
12:7293–7302. 2015.PubMed/NCBI
|
|
35
|
Yen GC and Duh DP: Scavenging Effect of
Methanolic Extracts of Peanut Hulls on Free-Radical and
Active-Oxygen Species. J Agric Food Chem. 42:629–632. 1994.
View Article : Google Scholar
|
|
36
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dieterich S, Bieligk U, Beulich K,
Hasenfuss G and Prestle J: Gene expression of antioxidative enzymes
in the human heart: Increased expression of catalase in the
end-stage failing heart. Circulation. 101:33–39. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cos P, Ying L, Calomme M, Hu JP, Cimanga
K, Van Poel B, Pieters L, Vlietinck AJ and Vanden Berghe D:
Structure-activity relationship and classification of flavonoids as
inhibitors of xanthine oxidase and superoxide scavengers. J Nat
Prod. 61:71–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Velali E, Papachristou E, Pantazaki A,
Choli-Papadopoulou T, Planou S, Kouras A, Manoli E, Besis A, Voutsa
D and Samara C: Redox activity and in vitro bioactivity of the
water-soluble fraction of urban particulate matter in relation to
particle size and chemical composition. Environ Pollut. 208(Pt B):
774–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Terzano C, Di Stefano F, Conti V, Graziani
E and Petroianni A: Air pollution ultrafine particles: Toxicity
beyond the lung. Eur Rev Med Pharmacol Sci. 14:809–821.
2010.PubMed/NCBI
|
|
41
|
Chang ST, Wu JH, Wang SY, Kang PL, Yang NS
and Shyur LF: Antioxidant activity of extracts from Acacia confusa
bark and heartwood. J Agric Food Chem. 49:3420–3424. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Valavanidis A, Vlahoyianni T and Fiotakis
K: Comparative study of the formation of oxidative damage marker
8-hydroxy-2′-deoxyguanosine (8-OHdG) adduct from the nucleoside
2′-deoxyguanosine by transition metals and suspensions of
particulate matter in relation to metal content and redox
reactivity. Free Radic Res. 39:1071–1081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Imlay JA, Chin SM and Linn S: Toxic DNA
damage by hydrogen peroxide through the Fenton reaction in vivo and
in vitro. Science. 240:640–642. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Longhin E, Pezzolato E, Mantecca P, Holme
JA, Franzetti A, Camatini M and Gualtieri M: Season linked
responses to fine and quasi-ultrafine Milan PM in cultured cells.
Toxicol In Vitro. 27:551–559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Delfino RJ, Staimer N, Tjoa T, Arhami M,
Polidori A, Gillen DL, Kleinman MT, Schauer JJ and Sioutas C:
Association of biomarkers of systemic inflammation with organic
components and source tracers in quasi-ultrafine particles. Environ
Health Perspect. 118:756–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gehling W, Khachatryan L and Dellinger B:
Hydroxyl radical generation from environmentally persistent free
radicals (EPFRs) in PM2.5. Environ Sci Technol. 48:4266–4272. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Farias MS, Pich CT, Kviecinski MR, Bucker
NC, Felipe KB, Da Silva FO, Günther TM, Correia JF, Ríos D, Benites
J, et al: Substituted 3-acyl-2-phenylamino-1,4-naphthoquinones
intercalate into DNA and cause genotoxicity through the increased
generation of reactive oxygen species culminating in cell death.
Mol Med Rep. 10:405–410. 2014.PubMed/NCBI
|
|
48
|
Dellinger B, Pryor WA, Cueto R, Squadrito
GL, Hegde V and Deutsch WA: Role of free radicals in the toxicity
of airborne fine particulate matter. Chem Res Toxicol.
14:1371–1377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Squadrito GL, Cueto R, Dellinger B and
Pryor WA: Quinoid redox cycling as a mechanism for sustained free
radical generation by inhaled airborne particulate matter. Free
Radic Biol Med. 31:1132–1138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alaghmand M and Blough NV:
Source-dependent variation in hydroxyl radical production by
airborne particulate matter. Environ Sci Technol. 41:2364–2370.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Valavanidis A, Fiotakis K, Bakeas E and
Vlahogianni T: Electron paramagnetic resonance study of the
generation of reactive oxygen species catalysed by transition
metals and quinoid redox cycling by inhalable ambient particulate
matter. Redox Rep. 10:37–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Khachatryan L, Vejerano E, Lomnicki S and
Dellinger B: Environmentally persistent free radicals (EPFRs). 1.
Generation of reactive oxygen species in aqueous solutions. Environ
Sci Technol. 45:8559–8566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bouayed J and Bohn T: Exogenous
antioxidants - Double-edged swords in cellular redox state: Health
beneficial effects at physiologic doses versus deleterious effects
at high doses. Oxid Med Cell Longev. 3:228–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Perrone D, Farah A and Donangelo CM:
Influence of coffee roasting on the incorporation of phenolic
compounds into melanoidins and their relationship with antioxidant
activity of the brew. J Agric Food Chem. 60:4265–4275. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Smrke S, Opitz SEW, Vovk I and Yeretzian
C: How does roasting affect the antioxidants of a coffee brew?
Exploring the antioxidant capacity of coffee via on-line
antioxidant assays coupled with size exclusion chromatography. Food
Funct. 4:1082–1092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bakuradze T, Lang R, Hofmann T, Stiebitz
H, Bytof G, Lantz I, Baum M, Eisenbrand G and Janzowski C:
Antioxidant effectiveness of coffee extracts and selected
constituents in cell-free systems and human colon cell lines. Mol
Nutr Food Res. 54:1734–1743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Borges F, Fernandes E and Roleira F:
Progress towards the discovery of xanthine oxidase inhibitors. Curr
Med Chem. 9:195–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Krenitsky TA, Spector T and Hall WW:
Xanthine oxidase from human liver: Purification and
characterization. Arch Biochem Biophys. 247:108–119. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Houston M, Estevez A, Chumley P, Aslan M,
Marklund S, Parks DA and Freeman BA: Binding of xanthine oxidase to
vascular endothelium. Kinetic characterization and oxidative
impairment of nitric oxide-dependent signaling. J Biol Chem.
274:4985–4994. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Choi EY, Stockert AL, Leimkühler S and
Hille R: Studies on the mechanism of action of xanthine oxidase. J
Inorg Biochem. 98:841–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
de Oliveira EP and Burini RC: High plasma
uric acid concentration: Causes and consequences. Diabetol Metab
Syndr. 4:122012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gomez-Cabrera MC, Borrás C, Pallardó FV,
Sastre J, Ji LL and Viña J: Decreasing xanthine oxidase-mediated
oxidative stress prevents useful cellular adaptations to exercise
in rats. J Physiol. 567:113–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Day RO, Kamel B, Kannangara DRW, Williams
KM and Graham GG: Xanthine oxidoreductase and its inhibitors:
Relevance for gout. Clin Sci (Lond). 130:2167–2180. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Viana ALM, Fonseca M, Meireles EL, Duarte
SM, Rodrigues MR and Paula FB: Effects of the consumption of
caffeinated and decaffeinated instant coffee beverages on oxidative
stress induced by strenuous exercise in rats. Plant Foods Hum Nutr.
67:82–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Abreu RV, Silva-Oliveira EM, Moraes MFD,
Pereira GS and Moraes-Santos T: Chronic coffee and caffeine
ingestion effects on the cognitive function and antioxidant system
of rat brains. Pharmacol Biochem Behav. 99:659–664. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pal S, Dey SK and Saha C: Inhibition of
catalase by tea catechins in free and cellular state: a biophysical
approach. PLoS One. 9:e1024602014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
De Magalhães CS, Takarada JE, Carvalho NC,
do Carvalho DC, De Andrade FL, Ferreira EB, Luccas PO and Azevedo
L: The Coffee Protective Effect on Catalase System in the
Preneoplastic Induced Rat Liver. J Chem. 2016:85703212016.
View Article : Google Scholar
|
|
69
|
Honda S, Miura Y, Masuda A and Masuda T:
Identification of crypto- and neochlorogenic lactones as potent
xanthine oxidase inhibitors in roasted coffee beans. Biosci
Biotechnol Biochem. 78:2110–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Farah A, Monteiro M, Donangelo CMLS and
Lafay S: Chlorogenic acids from green coffee extract are highly
bioavailable in humans. J Nutr. 138:2309–2315. 2008. View Article : Google Scholar : PubMed/NCBI
|