Introduction
Pancreatic cancer is a life-threatening form of
cancer with a 90% mortality rate, ranking as the fourth leading
cause of cancer-associated mortality in China (1). At present, the primary form of
treatment for pancreatic cancer is surgery combined with
chemotherapy (2). Gemcitabine is
the first-line chemotherapeutic drug used clinically for the
treatment of pancreatic cancer and, although gemcitabine can
significantly inhibit the growth of pancreatic cancer, the
emergence of gemcitabine resistance in reduces the therapeutic
effect of gemcitabine, leading to shortened patient survival rates,
which for the majority is only a few months (3,4).
Therefore, clarifying the mechanism underlying gemcitabine
resistance is required to improve treatment. In order to enhance
the therapeutic effect of gemcitabine through the resensitization
of gemcitabine to pancreatic cancer, investigations have focused on
combination chemotherapy; however, few drugs have been screened,
indicating the requirement for a novel and efficient combination
drug.
Human equilibrative nucleoside transporter 1 (hENT1)
is a bidirectional channel, which is the carrier of pyrimidine
nucleotides, the gemcitabine, capecitabine and 5-fluorouracil may
bind to pyrimidine nucleotides and move into and out of the cell
(5). Previous studies have found
that hENT1 is important in the resistance of gemcitabine and in
patients treated with gemcitabine chemotherapy for pancreatic
cancer, as patients who have higher expression levels of hENT1 have
increased survival rates, compared with patients who have lower
expression levels of hENT1 (5,6).
These findings suggest that hENT1 may be a target of gemcitabine,
and selection of an hENT1 agonist may enhance the therapeutic
effect of gemcitabine. Previous investigations have revealed that
ribonucleoside diphosphate reductase 1 (RRM1) is a
gemcitabine-targeting molecule; when RRM1 was at expressed at a
lower level, patients with pancreatic cancer were more sensitive to
gemcitabine chemotherapy, and the expression level of RRM1 was
higher in gemcitabine-resistant patients (7–9).
Therefore, in order to inhibit gemcitabine resistance, use of an
hENT1 agonist can inhibit the expression of RRM1.
Epithelial to mesenchymal transition (EMT) describes
the process by which epithelial cells convert into cells with a
mesenchymal phenotype through specific pathways. EMT is important
in embryonic development, chronic inflammation, tissue remodeling,
cancer metastasis and several fibrotic diseases (10–12).
By EMT, epithelial cells lose cell polarity and lose connections
with the basement membrane in the epithelial phenotype, and obtain
higher migratory, invasive, anti-apoptotic and extracellular matrix
degradation capacities in the mesenchymal phenotype (10). It has been reported that EMT is
important in gemcitabine resistance in pancreatic cancer; in
gemcitabine-resistant pancreatic cancer, EMT is in the active state
(11,12). Therefore, the prevention of
gemcitabine-resistance-induced EMT may be an effective therapeutic
strategy.
Sclareolide is a sesquiterpene lactone and a natural
product derived from various plant sources, including Salvia
sclarea, Salvia yosgadensis and cigar tobacco (13,14).
At present, sclareolide is predominantly used for cosmetics and
weight-reduction products, with almost no applications in the
medical field. In the present study, whether sclareolide can
resensitize pancreatic cancer to gemcitabine was investigated.
Sclareolide promoted gemcitabine-induced cell death of pancreatic
cancer cells through apoptosis, the upregulated expression of
hENT1, downregulated expression of RRM1 and inhibition of the EMT
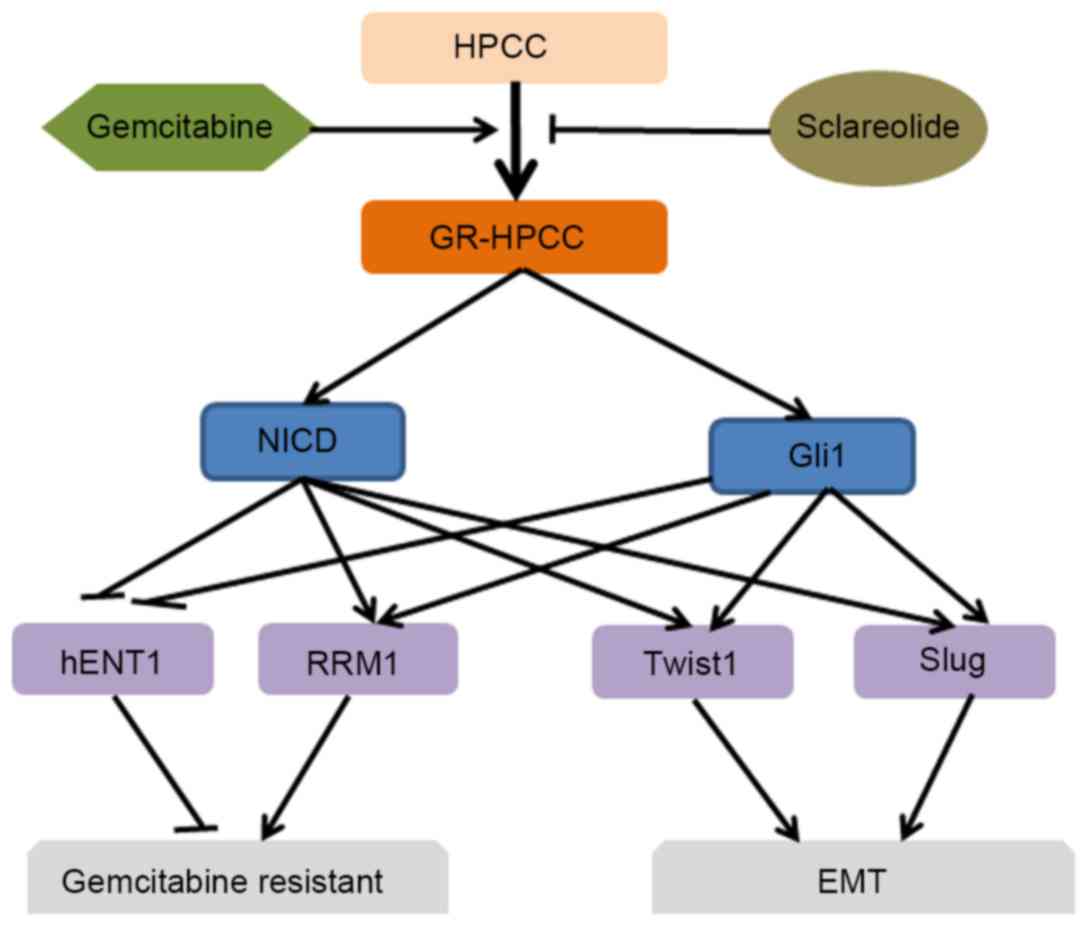
through the TWIST1/Slug pathway, which was mediated by NICD/Gli1
signals. The results of the in vivo experiments supported
the in vitro experiments. Therefore, sclareolide combined
with gemcitabine may provide a novel chemotherapeutic strategy for
gemcitabine-resistant pancreatic cancer.
Materials and methods
Cell culture
Panc-1 and ASPC-1 human pancreatic cancer cells
(HPCCs) were obtained from the American Type Culture Collection
(Manassas, VA, USA). All cells were cultured in DMEM (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) with 10% fetal bovine
serum (FBS) (Hyclone; GE Healthcare Life Sciences), 2 Mm
L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin, at 5%
CO2 and 37°C. All cell culture reagents were purchased
from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The
Panc-1 and ASPC-1 cells (1×104) were exposed to
gemcitabine at serially increased concentrations, with an initial
concentration of 10 nM, to generate gemcitabine-resistant cells.
Following 2 weeks of adaptation, the concentration was doubled. The
cells were adapted to a final gemcitabine concentration of 640 nM,
termed gemcitabine-resistant (GR)-Panc-1 cells and GR-ASPC-1
cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA expression levels of hENT1 and RRM1
were determined using a previously described protocol (6,8).
Briefly, the total RNA of cell was extracted by TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the protocol.
Triplicates of each gene and each specimen were used, with GAPDH as
an internal standard. The single-strand cDNA for PCR template was
synthesized from 10 µg of total RNA by Illumina TotalPrep RNA
amplification kit (Ambion; Thermo Fisher Scientific, Inc.).
StepOne™ Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used in the RT-PCR assay. The RT-PCR was
performed with a total reaction volume of 20 µl, including 10 µl
Power SYBR Green PCR Master mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.), 5 pmol of forward and reverse primer
respectively and 2 µl of cDNA. Threshold cycle(Ct) was observed in
the amplification with 35 cycles of 1 min at 95°C, 1 min at 58°C,
and 1 min at 72°C. And the relative of mRNA expression levels was
calculated by the relative quantitation method 2−∆∆Cq
(15). The fold change to control
sample of each samples was calculated. Relative gene quantification
was performed using StepOne™ software 2.1 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following primers were used:
GAPDH, forward 5′-CGG AGT CAA CGG ATT TGG TCG TAT-3′ and reverse
5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′; hENT1, forward 5′-AGC AGG
CAA AGA GGA ATC TGG AGT-3′ and reverse 5′-GAA GGC AAA GGC AGC CAT
GAA GAA-3′; RRM1, forward 5′-CAT CCA CAT TGC TGA GCC TA-3′ and
reverse 5′-GAT TAG CCG CTG GTC TTG TC-3′.
Western blot analysis
For each sample, 5×106 cells were lysed
for 30 min in lysis buffer (Beyotime Institute of Biotechnology,
Beijing, China) on ice, and the debris was centrifuged at 12,000 ×
g for 12 min at 4°C. A BCA assay (Beyotime Institute of
Biotechnology) was used to determine the protein concentration,
following which 30 µg proteins were separated using 8–15% SDS-PAGE
and blotted onto a PVDF membrane. The membrane was blocked using
blocking solution of 5% nonfat dry milk in PBST (0.05% Tween20 in
PBS). The membrane was then incubated with primary antibodies for 2
h at 37°C at a 1:1,000 dilution, following which the PVDF membrane
was washed three times for 10 min in PBST. The membrane was then
incubated with secondary antibodies for 1 h at 37°C at a 1:10,000
dilution, following which the PVDF membrane was washed three times
for 10 min in PBST. All antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA), the details of
antibodies were followed: hENT1 (sc-48489, polyclonal, goats
anti-human), GAPDH (sc-293335, monoclonal, mouse anti-human), RRM1
(sc-22786, monoclonal, rabbit anti-human), Twist1 (sc-134136,
polyclonal, mouse anti-human), Slug (sc-166902, monoclonal, mouse
anti-human), E-cadherin (sc-33743, polyclonal, rabbit anti-human),
α-SMA (sc-53142, monoclonal, mouse anti-human), NICD (sc-74276,
monoclonal, mouse anti-human), Gli1 (sc-20687, polyclonal, rabbit
anti-human), PARP (sc-27034, polyclonal, goats anti-human), Capase3
(sc-1224, polyclonal, goats anti-human), goats IgG (sc-2419), mouse
IgG (sc-516176), rabbit IgG (sc-2794).
Cell viability assay
A CCK8 was used to assess cell viability. The cells
(104/well) were plated on a 96-well plate and attached
overnight, the cells were treated with the drugs for 24 h,
following which the medium was removed and the cells were washed
three times with PBS. Subsequently, 90 µl DMEM and 10 µl CCK8 were
added to each well, and incubated for 1.5 h at 37°C. A microplate
reader was used to measure the optical density values at 450
nm.
Trypan blue assay
To the adequately suspended treated cells, 0.4%
(w/v) Trypan blue solution was added, for which the volume ratio of
cell suspension to Trypan blue solution was 9:1. The cells were
counted under a microscope, with dead cells failing to exclude the
dye, and the death rate was calculated as follows: Total death
rate=(number of dead cells/total cells)x100%.
Transfection experiment
Transient transfection of cells with small
interfering (si)RNAs and plasmids were performed using
Lipofectamine 2000 (Invitrogen Life Technologies; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. At 36 h
post-transfection, the drugs were added to the cells, which were
collected following a 24 h period for western blot analysis. The
sequences of (si)RNAs were as follows: hENT1,
5′-AUGACAUUGUUGAAGAUGGCA-3′), Twist1, 5′-AAACAUUUGUUUUAAGGAGAA-3′);
Slug, 5′-ACUAAUGGGGCUUUCUGAGCC-3′); NICD,
(5′-UAAAGAGAGAAUAUCGUAGUC-3′); Gli1,
(5′-UUUCAUACACAGAUUCAGGCU-3′).
Cell invasion assay
A BioCoat Matrigel invasion chamber system (Corning
Incorporated, NY, USA) was used to determine cell invasion. In a
Transwell plate, the lower chamber was filled with culture medium
without cells, and the upper chamber was filled with cell
suspension (1×105) and medium containing 10% FBS. The
Transwell plate was incubated at 37°C for 24 h. The cells adherent
to upper chamber surface were removed, and the cells adherent to
lower chamber surface were stained with 4% paraformaldehyde for 15
min, rinsed with water and dried. Crystal violet was extracted with
50% ethanol containing 0.1 M sodium citrate, and the absorbance was
measured at 600 nm.
Detection of apoptosis
Following treatment, the cells were incubated with
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide
(PI; Beyotime Institute of Biotechnology) at room temperature for
15 min. A FACScan flow cytometer was used for analysis of the
apoptotic ratio.
Xenograft model
A total of 30 male 6-week-old BALB/c nude mice were
purchased from the Institute of Zoology, Chinese Academy of
Sciences (Beijing, China). All the mice were fed in the
specific-pathogen free environment, the plastic cage was sealed
with an air filter, animal isolators, air laminator, and air
laminar flow chamber were equipped, and the feeding environment
with temperature 24–28°C, relative humidity 50~60%, ventilation
required 10 to 15 times per hour, natural circadian light. and the
mice were given the sterilized food, and the water with bacitracin
(4 g/l) and neomycin (4 g/l). All animal experiments were performed
according to the guidelines of the Institutional Animal Care and
Use Committee of the Institute of Zoology, Chinese Academy of
Sciences. Each BALB/c nude mouse was subcutaneously inoculated with
5×106 Panc-1 or GR-Panc-1 cells in their right and left
hind footpads. At 2 days post-inoculation, the mice inoculated with
Panc-1 cells were administered with 10 mg/kg gemcitabine three
times every day via intraperitoneal injection. The mice inoculated
with GR-Panc-1 cells were administered with 10 mg/kg gemcitabine or
co-administered with 100 mg/kg sclareolide and 10 mg/kg gemcitabine
three times every day via intraperitoneal injection, with the
sclareolide injected 2 h prior to the gemcitabine. The sclareolide
and gemcitabine were dissolved in saline. The mice were sacrificed
by anesthesia with pentobarbital at 2, 3 and 4 weeks after the cell
inoculation, and the tumor volumes were measured.
TUNEL assay
The tumors were immersed in 4% paraformaldehyde for
24 h, and were then dehydrated in 30% sucrose solution, following
which the tissues were paraffin-embedded for sectioning (10 µm).
The sections were treated using an in situ Cell Death
Detection kit (Roche Diagnostics, Indianapolis, IN, USA) according
to the manufacturer's protocol.
Statistical analysis
The data are represented as the mean ± standard
deviation from triplicate experiments. All the data were processed
by SPSS version 19.0 (IBM SPSS, Armonk, NY, USA). Two-way analysis
of variance was used to analyze the variance of different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
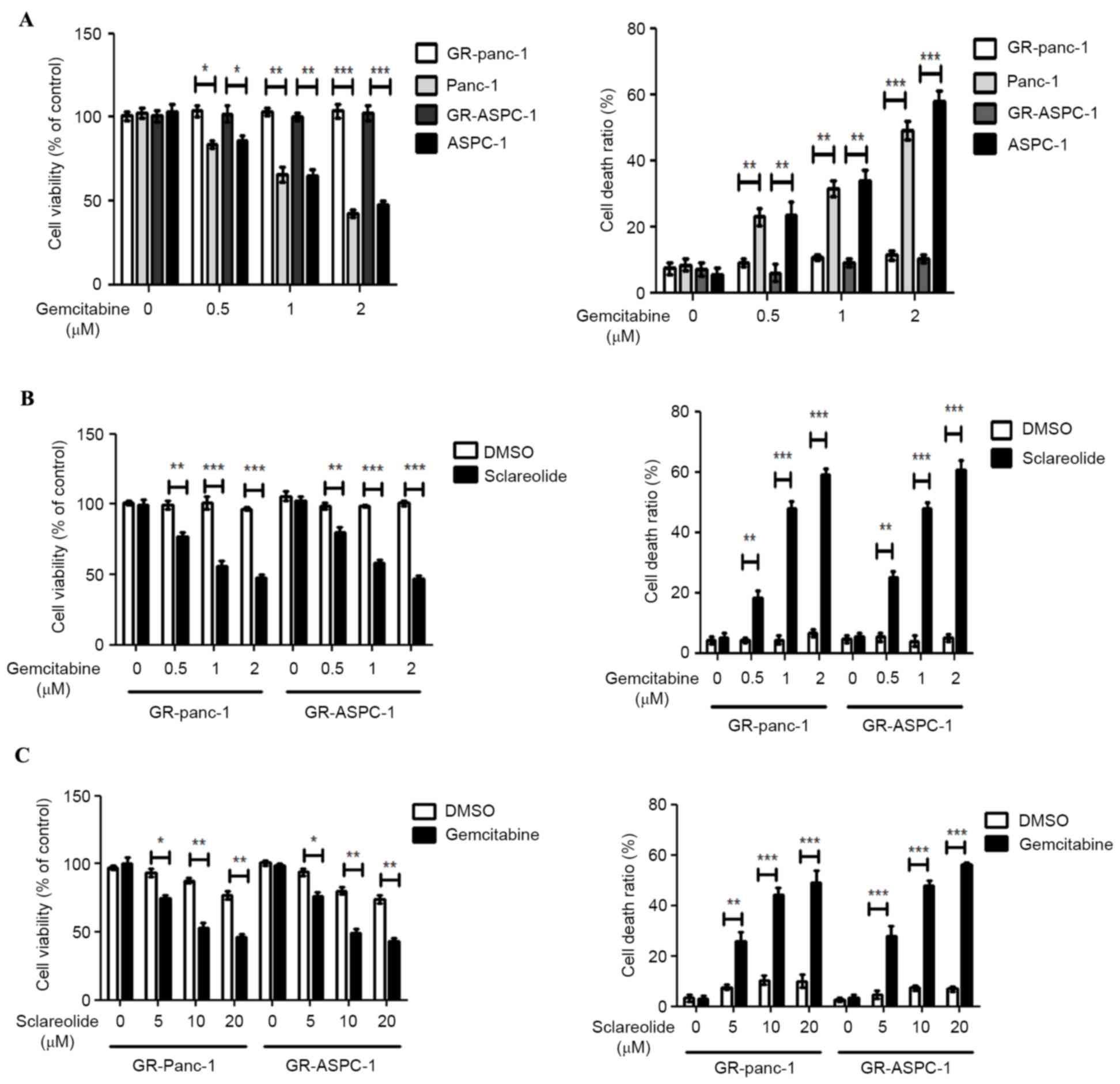
Sclareolide enhances
gemcitabine-induced GR-HPCC death
The GR-Panc-1 and GR-ASPC-1 cells were induced with
increasing concentrations of gemcitabine from 10 nm. The GR-HPCCs
(GR-Panc-1 and GR-ASPC-1 cells) and HPCCs (Panc-1 and ASPC-1 cells)
were treated with different concentrations of gemcitabine. No
significant alterations in cell viability or cell death ratio were
observed in the GR-HPCCs following exposure to increasing
gemcitabine concentrations. For the HPCCs, the cell viability
decreased and the cell death ratio increased with increased
gemcitabine concentrations (Fig.
1A). The GR-HPCCs were treated with different concentrations of
gemcitabine combined with 10 µM sclareolid. At a gemcitabine
concentration of 0.5 µM, the cell viability and cell death ratio
were significantly altered, compared with the cells treated with
gemcitabine alone. The optimal concentration of gemcitabine was 1
µM (Fig. 1B). Similarly, GR-HPCCs
were treated with different concentrations of sclareolide combined
with 1 µM gemcitabine. At a sclareolide concentration of 5 µM, the
cell viability and cell death ratio were significantly altered,
compared with the cells treated with gemcitabine alone, with an
optimal sclareolide concentration of 10 µM (Fig. 1C). These results indicated that 10
µM sclareolide enhanced gemcitabine-induced GR-HPCC death.
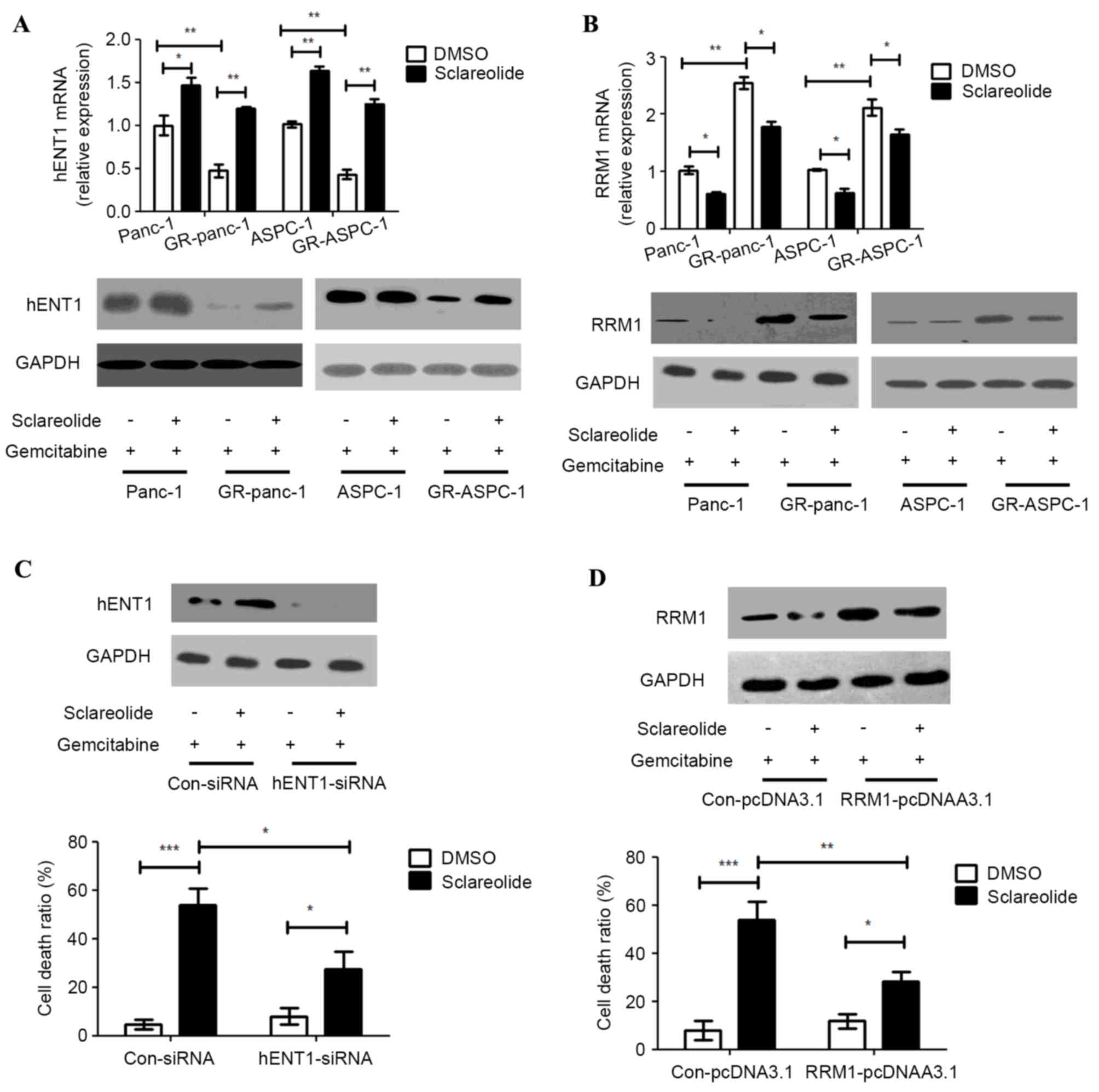
Sclareolide upregulates the expression
of hENT1 and downregulates the expression of RRM1 in GR-HPCCs
Several studies have reported that hENT1 and RRM1
are important in the treatment of pancreatic cancer with
gemcitabine (5,6,8).
HENT1 is a transporter of gemcitabine, and RRM1 is a target of
gemcitabine. Higher expression levels of hENT1 and lower expression
levels of RRM1 may assist in the treatment of pancreatic cancer
with gemcitabine. The mRNA and protein expression levels of hENT1
were lower in the GR-HPCCs, compared with the HPCCs, and the mRNA
and protein expression levels of RRM1 were higher in the GR-HPCCs,
compared with the HPCCs. This suggested that hENT1 and RRM1 were
important in gemcitabine resistance (Fig. 2A and B). The HPCCs and GR-HPCCs
were treated with gemcitabine combined with sclareolide, and the
results showed that sclareolide upregulated the mRNA and protein
expression levels of hENT1 and downregulated the mRNA and protein
expression levels of RRM1 (Fig. 1A and
B). Knocking down hENT1 by transfection of the GR-Panc-1 cells
with siRNA resulted in a reduction in the cell death ratio induced
by gemcitabine combined with sclareolide, compared with the cells
transfected with control siRNA (Fig.
2C). The overexpression of RRM1 also inhibited the cell death
ratio induced by gemcitabine combined with sclareolide in the
GR-Panc-1 cells (Fig. 2D). These
results showed that sclareolide enhanced gemcitabine-induced
GR-HPCC death through targeting hENT1 and RRM1.
Sclareolide suppresses the EMT
phenotype in GR-HPCC
Previous studies have reported that EMT is in the
active state in gemcitabine-resistant pancreatic cancer. In order
to confirm whether sclareolide can affect the EMT in GR-HPCCs, the
epithelial cell marker, E-cadherin, and mesenchymal cell marker,
α-smooth muscle actin (SMA) were detected in the HPCCs and
GR-HPCCs, and the expression levels of TWIST1 and Slug were
examined. The HPCCs and GR-HPCCs were treated with gemcitabine
alone or combined with sclareolide, the results showed that the
expression level of E-cadherin was lower in the GR-HPCCs, compared
with the HPCCs, and the expression of α-SMA was higher, indicating
that EMT was in the active state in the GR-HPCCs. In addition, the
expression levels of TWIST1 and Slug were higher in the GR-HPCCs,
compared with the HPCCs, and all alterations were inhibited by
sclareolide (Fig. 3A). Several
studies have reported that TWIST1 and Slug are mediators of EMT as
transcriptional factors, and the expression levels of TWIST1 and
Slug have been found to be higher in several cancer EMT phenotypes
(16,17). In the present study, on detecting
the GR-HPCC EMT phenotype through knocking down TWIST1 and Slug by
siRNA in the GR-Panc-1 cells, the expression of α-SMA was inhibited
and the expression of E-cadherin was enhanced. In addition, the
expression level of hENT1 was inhibited and that of RRM1 was
enhanced (Fig. 3B and C).
Additionally, the cell death ratio was increased in GR-Panc-1 cells
induced by gemcitabine following the knock down of TWIST1 and Slug
(Fig. 3D and E). The invasive
ability of the GR-HPCCs was more marked, compared with that of the
HPCCs, and sclareolide suppressed the invasive ability of the
GR-HPCCs (Fig. 3F). These results
indicated that sclareolide suppressed the GR-HPCC EMT phenotype and
resensitized GR-HPCCs to gemcitabine through the TWIST1 and Slug
pathway.
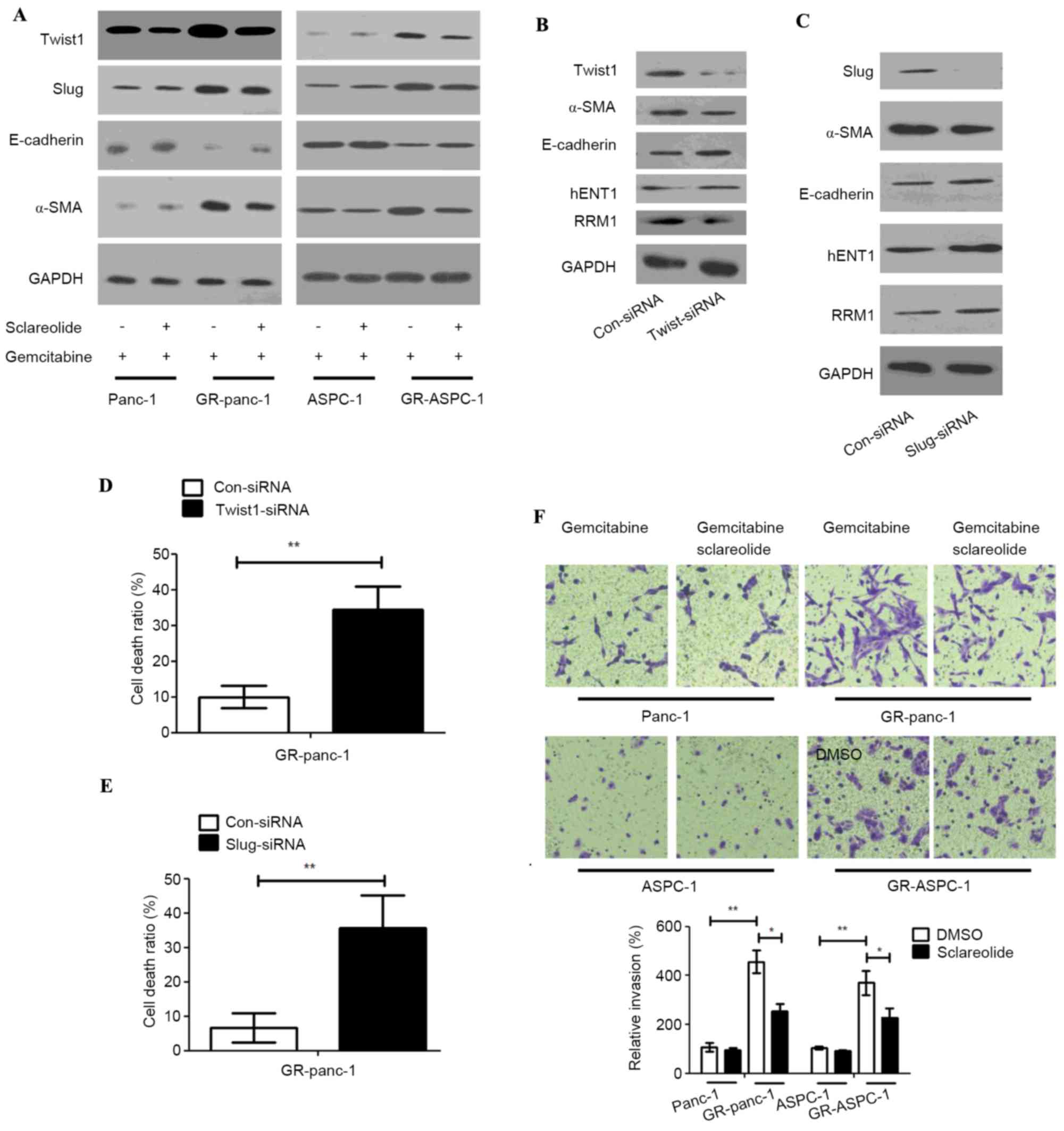 | Figure 3.Sclareolide suppresses the EMT
phenotype of GR-HPCC through TWIST1 and Slug signals, and recovers
the activation of TWIST1 and Slug in GR-HPCCs. HPCCs and GR-HPCCs
were treated with 1 µm gemcitabine alone or with 10 µm sclareolide
(2 h pretreatment) and 1 µm gemcitabine for 24 h. (A) Sclareolide
recovered the activation of TWIST1 and Slug in GR-HPCCs. GR-Panc-1
cells were transfected with control siRNA, (B) TWIST1-siRNA or (C)
Slug-siRNA, and expression levels of hENT, RRM1, α-SMA and
E-cadherin were analyzed. Cell death ratios were analyzed in the
(D) TWIST1-siRNA and (E) Slug-siRNA cells using Trypan blue. (F)
Sclareolide suppressed the invasive ability of GR-HPCCs, HPCCs and
GR-HPCCs treated with 1 µm gemcitabine alone or with 10 µm
sclareolide (2 h pretreatment) for 24 h. Magnification, ×400.
*P<0.05 and **P<0.01. GR-HPCCs, gemcitabine-resistant human
pancreatic cancer cells; hENT1, human equilibrative nucleoside
transporter 1; RRM1, ribonucleoside diphosphate reductase 1; α-SMA,
α-smooth muscle actin; siRNA, small interfering RNA. |
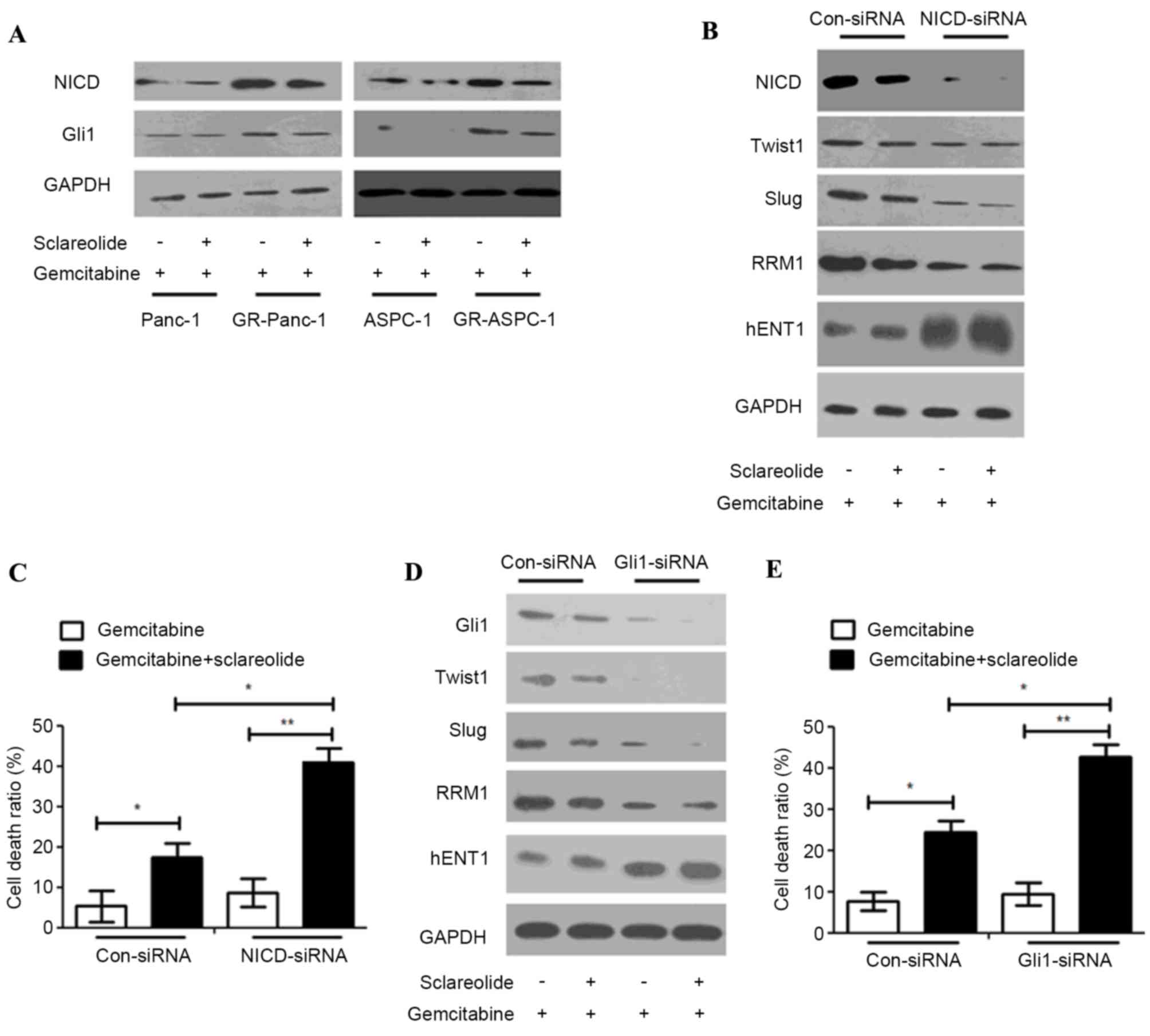
Sclareolide resensitizes GR-HPCsC to
gemcitabine through inhibiting NICD/Gli1 signals
Several studies have reported that NICD and Gli1 can
mediate TWIST1 and Slug (18–20).
In order to fully understand the upstream mediators of TWIST1 and
SLUG, the expression levels of NICD and Gli1 were detected in HPCCs
and GR-HPCCs, which were treated with gemcitabine alone or combined
with sclareolide. The results showed that the expression levels of
NICD and Gli1 in the GR-HPCCs were higher, compared with those in
the HPCCs, and sclareolide inhibited the expression of NICD and
Gli1 (Fig. 4A). In the GR-Panc-1
cells, the expression levels of TWIST1, Slug and RRM1 were
inhibited, and the expression of hENT1 was enhanced. Treatment with
gemcitabine combined with sclareolide induced an increase in the
GR-HPCC death ratio when NICD and Gli1 were knocked down by siRNA
(Fig. 4B-E). These results
suggested that sclareolide resensitized the GR-HPCCs to gemcitabine
through the NICD/Gli1 pathway.
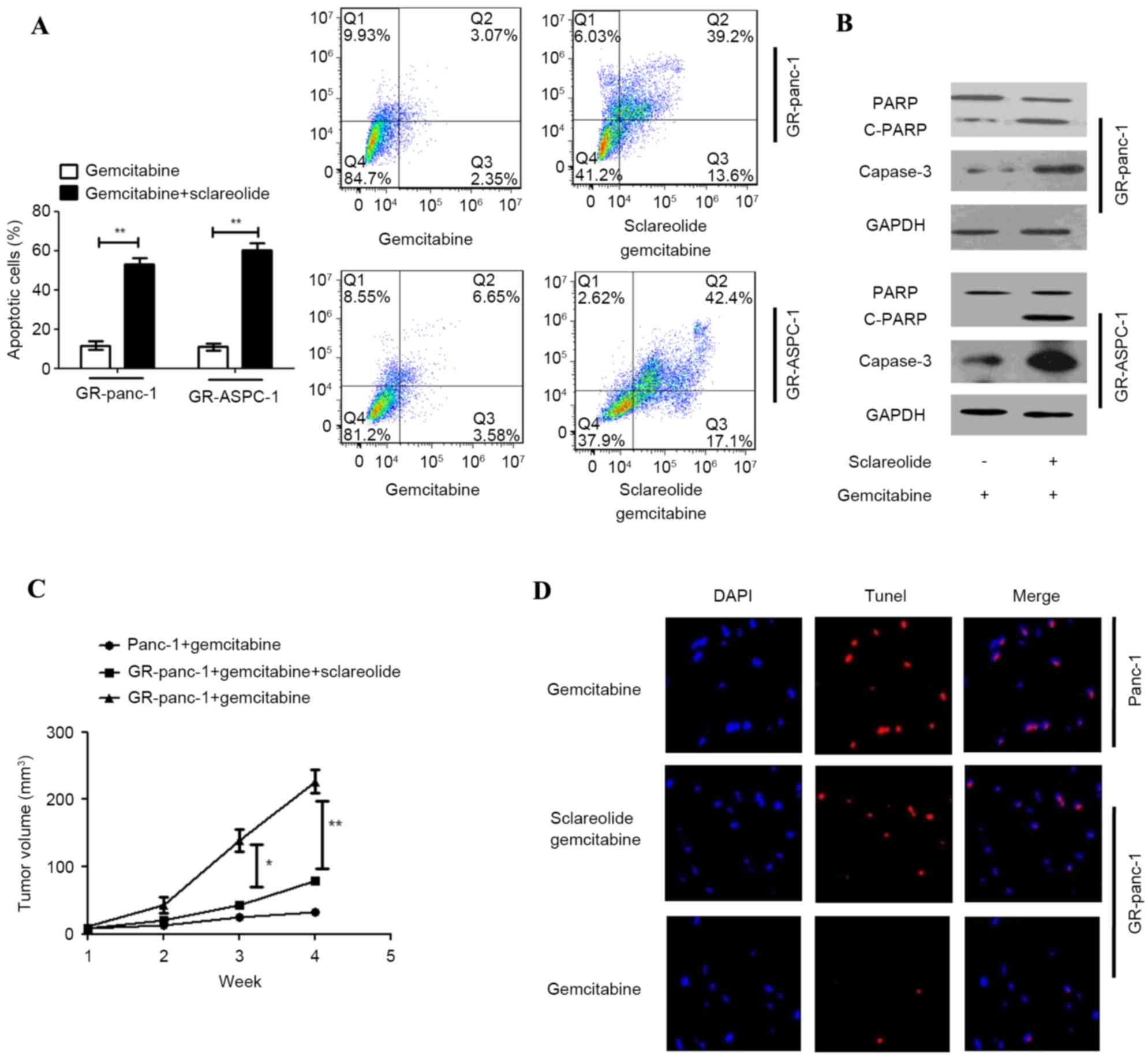
Sclareolide suppresses
gemcitabine-resistant pancreatic tumor growth through
apoptosis
In order to clarify the mechanism of
sclareolide-stimulated gemcitabine-induced cell death, the
apoptosis of GR-HPCCs treated with gemcitabine alone or with
sclareolide was detected using Annexin V-FITC and PI. Additionally,
the markers of apoptosis, poly (ADP-ribose) polymerase (PARP) and
Capase-3 were detected. When the GR-HPCCs were treated with
gemcitabine and sclareolide, the apoptotic ratio was higher, and
the expression levels of PARP and Capase-3 were higher, compared
with the GR-HPCCs treated with gemcitabine alone (Fig. 5A and B). These results showed that
the mechanism of sclareolide-stimulated gemcitabine-induced cell
death involved stimulating apoptosis. In order to confirm the
effect in vivo, nude mice were used to establish a
pancreatic tumor xenograft model with Panc-1 or GR-Panc-1 cells to
examine the effect of co-treatment with gemcitabine and
sclareolide. Through intraperitoneal injection, gemcitabine and
sclareolide were administered three times each day, with the
sclareolide administered 2 h prior to gemcitabine administration.
As expected, following administration with gemcitabine, the volume
of tumors was lower in the nude mice xenografted with Panc-1 cells,
compared with the nude mice xenografted with GR-Panc-1 cells. In
the nude mice xenografted with GR-Panc-1 cells, the volume of
tumors was lower following the co-administration of gemcitabine and
sclareolide, compared with that following administration of
gemcitabine alone (Fig. 5C). The
results of the TUNEL assay showed that co-administrating
gemcitabine and sclareolide induced apoptosis and inhibited tumor
growth in the xenografted GR-Panc-1 model (Fig. 5D).
Discussion
In the present study, whether sclareolide promotes
gemcitabine-induced pancreatic cancer cell death was investigated
in GR-HPCCs and GR-HPCCs with an EMT phenotype. The expression
levels of hENT1 were lower, and those of RRM1 were higher in the
GR-HPCCs, compared with the HPCCs. Following treatment of the
GR-HPCCs with sclareolide and gemcitabine, the altered expression
levels of hENT1 and RRM1 were altered, altering the HPCC state via
TWIST1/Slug signaling, mediated by the NICD/Gli1 pathway (Fig. 6). It was also found that
sclareolide enhanced gemcitabine-induced pancreatic cancer cell
death through stimulating apoptosis. In xenografted pancreatic
tumors using GR-Panc-1 cells, the co-administration of sclareolide
and gemcitabine significantly suppressed tumor growth, compared
with gemcitabine alone.
It has been previously reported that hENT1 is a
transporter of gemcitabine into and out of the cell, and patients
with a higher expression of hENT1 have increased survival rates,
compared with those with a lower expression of hENT1, particularly
in patients with gemcitabine-resistant pancreatic cancer (21,22).
RRM1, as a target of gemcitabine is expressed at a high level in
patients with gemcitabine-resistant pancreatic cancer, and lower
expression levels of RRM1 lead to increased survival rates in
patients with gemcitabine-resistant pancreatic cancer (23,24).
It has been reported that EMT is in the active state in
gemcitabine-resistant pancreatic cancer, and inhibiting EMT can
suppress the growth of pancreatic cancer (10,12).
Although hENT1 and RRM1 have been found to be associated with
gemcitabine-resistant pancreatic cancer, the molecular mechanism
remains to be elucidated. Previous reports have stated that Notch
is involved in mediating hENT1 and RRM1, with PPARα and PPARγ
mediating hENT1, AKT, phosphoinositide 3-kinase, RAS/ERT and
mitogen-activated protein kinase kinase 1/2 mediating RRM1
(25–29). The present study investigated
whether the EMT transcriptional factors, TWIST1 and Slug, can
suppress the expression of hENT1 and enhance the expression of RRM1
in GR-HPCCs. Sclareolide reversed the mediating effects of TWIST1
and Slug on hENT1 and RRM1 through the inhibition of TWIST1 and
Slug. In addition, sclareolide inhibited the EMT phenotype in
GR-HPCCs treated with gemcitabine. The activation of the Notch
signaling pathway involves three steps, involving the S1, S2 and S3
proteolytic cleavage sites. Following γ-secretase cleavage at the
S3 site, releasing the soluble NICD, NICD transfers to the nucleus
and interacts with the DNA binding protein CBF1/suppressor of
Hairless/Lag1, recruits mastermind-like and histone
acetyltransferase P300/cAMP response sequence binding protein
(CREB) binding protein to activate target gene expression (30–35).
The Hh pathway is one of the developmental pathways, involving
canonical and non-canonical signaling, and several studies have
focused on the potential of targeting Hh signaling as an anticancer
strategy, in which targeting Hh signaling downstream of Smoothened
(SMO) is important. Activation of GLI transcription factors can
promote the transcription of Hh target genes (36,37).
GLI1 is one of the members of the GLI transcription factor family,
with an exclusively full-length transcriptional activator (38,39).
GLI1 is upstream in the signaling pathway, thus targeting GLI1 can
be useful in tumors harboring mutations of SMO or mediation
downstream of SMO (36). GLI1 is
also activated in several important oncogenic pathways, which can
inhibit more upstream members of the Hh pathway (37,39,40).
In the present study, it was found that NICD and GLI1 were involved
in the EMT phenotype of GR-HPCC mediation, and mediated the
expression of TWIST1 and Slug, which were mediators of hENT1 and
RRM1. hENT1 and RRM1 were important in resensitizing
gemcitabine-resistant pancreatic cancer to gemcitabine. Although
NICD and GLI1 led to significant alterations in HPCCs and GR-HPCCs,
sclareolide reversed the alterations in GR-HPCCs when co-treated
with gemcitabine, and contributed to gemcitabine-induced cell death
in GR-HPCCs, resensitizing GR-HPCCs to gemcitabine. In order to
clarify the mechanism underlying sclareolide-enhanced
gemcitabine-induced GR-HPCC death, apoptosis was detected in
GR-HPCCs, and the results showed that sclareolide enhanced
gemcitabine-induced GR-HPCC death through the activation of
apoptosis. Using a tumor xenograft model to evaluate the effect of
sclareolide and gemcitabine co-treatment in vivo, the
present study demonstrated that single administration of
gemcitabine suppressed tumor growth in the gemcitabine-sensitive
cell xenograft model, however, it did not suppress tumor growth in
the gemcitabine-resistant cell xenografted model. When
co-administered with sclareolide and gemcitabine, the tumor growth
was significantly inhibited in the gemcitabine-resistant cell
xenografted model. The results obtained using the xenograft model
supported the in vitro results. Furthermore, in situ
apoptosis detection revealed that sclareolide and gemcitabine
co-treatment in the gemcitabine-sensitive cell xenografted model
led to the suppression of tumor growth through the activation of
apoptosis.
In conclusion, the present study demonstrated that
NICD/GLI1-TWIST1/Slug mediated the EMT phenotype of GR-HPCCs, and
they are a novel mechanism of gemcitabine-resistance in pancreatic
cancer. In addition, the present study indicated that sclareolide
resensitized GR-HPCCs to gemcitabine through the NICD and GLI1
pathway, targeting hENT1 and RRM1 signals, inhibiting the
gemcitabine-induced EMT phenotype and enhancing gemcitabine-induced
GR-HPCC death through the activation of apoptosis. Gemcitabine
combined with sclareolide may be a novel and efficient therapeutic
strategy for patients with gemcitabine-resistant pancreatic
cancer.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant no. 81472753).
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial to mesenchymal
transition
|
|
GR-HPCC
|
gemcitabine-resistant human pancreatic
cancer cell
|
|
HPCC
|
human pancreatic cancer cell
|
|
hENT1
|
human equilibrative nucleoside
transporter 1
|
|
RRM1
|
ribonucleoside diphosphate reductase
1
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seicean A, Petrusel L and Seicean R: New
targeted therapies in pancreatic cancer. World J Gastroenterol.
21:6127–6145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Massari F, Santoni M, Ciccarese C,
Brunelli M, Conti A, Santini D, Montironi R, Cascinu S and Tortora
G: Emerging concepts on drug resistance in bladder cancer:
Implications for future strategies. Crit Rev Oncol Hematol.
96:81–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Sousa Cavalcante L and Monteiro G:
Gemcitabine: Metabolism and molecular mechanisms of action,
sensitivity and chemoresistance in pancreatic cancer. Eur J
Pharmacol. 741:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordh S, Ansari D and Andersson R: hENT1
expression is predictive of gemcitabine outcome in pancreatic
cancer: A systematic review. World J Gastroenterol. 20:8482–8490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santini D, Schiavon G, Vincenzi B, Cass
CE, Vasile E, Manazza AD, Catalano V, Baldi GG, Lai R, Rizzo S, et
al: Human equilibrative nucleoside transporter 1 (hENT1) levels
predict response to gemcitabine in patients with biliary tract
cancer (BTC). Curr Cancer Drug Targets. 11:123–129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamba JK: Genetic factors influencing
cytarabine therapy. Pharmacogenomics. 10:1657–1674. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jordheim LP and Dumontet C: Do hENT1 and
RRM1 predict the clinical benefit of gemcitabine in pancreatic
cancer? Biomark Med. 7:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jordheim LP, Sève P, Trédan O and Dumontet
C: The ribonucleotide reductase large subunit (RRM1) as a
predictive factor in patients with cancer. Lancet Oncol.
12:693–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaeffer DF, Assi K, Chan K, Buczkowski
AK, Chung SW, Scudamore CH, Weiss A, Salh B and Owen DA: Tumor
expression of integrin-linked kinase (ILK) correlates with the
expression of the E-cadherin repressor snail: An
immunohistochemical study in ductal pancreatic adenocarcinoma.
Virchows Arch. 456:261–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atta-ur-Rahman, Farooq A and Choudhary MI:
Microbial transformation of sclareolide. J Nat Prod. 60:1038–1040.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
González MA, Mancebo-Aracil J,
Tangarife-Castaño V, Agudelo-Goméz L, Zapata B, Mesa-Arango A and
Betancur-Galvis L: Synthesis and biological evaluation of
(+)-labdadienedial, derivatives and precursors from
(+)-sclareolide. Eur J Med Chem. 45:4403–4408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung HY and Yang J: Unraveling the TWIST
between EMT and cancer stemness. Cell Stem Cell. 16:1–2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian Y, Xu Y, Fu Q, Chang M, Wang Y, Shang
X, Wan C, Marymont JV and Dong Y: Notch inhibits chondrogenic
differentiation of mesenchymal progenitor cells by targeting
Twist1. Mol Cell Endocrinol. 403:30–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joost S, Almada LL, Rohnalter V, Holz PS,
Vrabel AM, Fernandez-Barrena MG, McWilliams RR, Krause M,
Fernandez-Zapico ME and Lauth M: GLI1 inhibition promotes
epithelial-to-mesenchymal transition in pancreatic cancer cells.
Cancer Res. 72:88–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gopalakrishnan N, Sivasithamparam ND and
Devaraj H: Synergistic association of Notch and NFκB signaling and
role of Notch signaling in modulating epithelial to mesenchymal
transition in colorectal adenocarcinoma. Biochimie. 107:310–318.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strimpakos AS, Syrigos KN and Saif MW:
Pharmacogenomics in pancreatic adenocarcinoma: New data and their
clinical implications. JOP. 14:359–362. 2013.PubMed/NCBI
|
|
22
|
Damaraju VL, Scriver T, Mowles D, Kuzma M,
Ryan AJ, Cass CE and Sawyer MB: Erlotinib, gefitinib, and
vandetanib inhibit human nucleoside transporters and protect cancer
cells from gemcitabine cytotoxicity. Clin Cancer Res. 20:176–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minami K, Shinsato Y, Yamamoto M,
Takahashi H, Zhang S, Nishizawa Y, Tabata S, Ikeda R, Kawahara K,
Tsujikawa K, et al: Ribonucleotide reductase is an effective target
to overcome gemcitabine resistance in gemcitabine-resistant
pancreatic cancer cells with dual resistant factors. J Pharmacol
Sci. 127:319–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie H, Jiang W, Jiang J, Wang Y, Kim R and
Liu X and Liu X: Predictive and prognostic roles of ribonucleotide
reductase M1 in resectable pancreaticadenocarcinoma. Cancer.
119:173–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Montero TD, Racordon D, Bravo L, Owen GI,
Bronfman ML and Leisewitz AV: PPARα and PPARγ regulate the
nucleoside transporter hENT1. Biochem Biophys Res Commun.
419:405–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koo JS and Kim H: Hypoxia-related protein
expression and its clinicopathologic implication in carcinoma of
unknown primary. Tumour Biol. 32:893–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tassone P, Di Martino MT, Ventura M,
Pietragalla A, Cucinotto I, Calimeri T, Bulotta A, Neri P, Caraglia
M and Tagliaferri P: Loss of BRCA1 function increases the antitumor
activity of cisplatin against human breast cancer xenografts in
vivo. Cancer Biol Ther. 8:648–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Khoueiry AB, Ramanathan RK, Yang DY,
Zhang W, Shibata S, Wright JJ, Gandara D and Lenz HJ: A randomized
phase II of gemcitabine and sorafenib versus sorafenib alone in
patients with metastatic pancreatic cancer. Invest New Drugs.
30:1175–1183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vena F, Causi EL, Rodriguez-Justo M,
Goodstal S, Hagemann T, Hartley JA and Hochhauser D: The MEK1/2
inhibitor Pimasertib enhances gemcitabine efficacy in pancreatic
cancer models by altering protein levels of ribonucleotide
reductase subunit-1 (RRM1). Clin Cancer Res. 21:5563–5577. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grishina IB: Mini-review: Does notch
promote or suppress cancer? New findings and old controversies. Am
J Clin Exp Urol. 3:24–27. 2015.PubMed/NCBI
|
|
31
|
Uzdensky AB, Demyanenko SV and Bibov MY:
Signal transduction in human cutaneous melanoma and target drugs.
Curr Cancer Drug Targets. 13:843–866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L and Leung PS: Use of herbal medicines
and natural products: An alternative approach to overcoming the
apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol.
53:224–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwanbeck R: The role of epigenetic
mechanisms in Notch signaling during development. J Cell Physiol.
230:969–981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gonnissen A, Isebaert S and Haustermans K:
Targeting the Hedgehog signaling pathway in cancer: Beyond
Smoothened. Oncotarget. 6:13899–13913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aberger F, Ruiz I and Altaba A:
Context-dependent signal integration by the GLI code: The oncogenic
load, pathways, modifiers and implications for cancer therapy.
Semin Cell Dev Biol. 33:93–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Merchant JL and Saqui-Salces M: Inhibition
of Hedgehog signaling in the gastrointestinal tract: Targeting the
cancer microenvironment. Cancer Treat Rev. 40:12–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Drenkhahn SK, Jackson GA, Slusarz A,
Slusarz A, Starkey NJ and Lubahn DB: Inhibition of hedgehog/Gli
signaling by botanicals: A review of compounds with potential
hedgehog pathway inhibitory activities. Curr Cancer Drug Targets.
13:580–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perrot CY, Javelaud D and Mauviel A:
Overlapping activities of TGF-β and Hedgehog signaling in cancer:
Therapeutic targets for cancer treatment. Pharmacol Ther.
137:183–199. 2013. View Article : Google Scholar : PubMed/NCBI
|