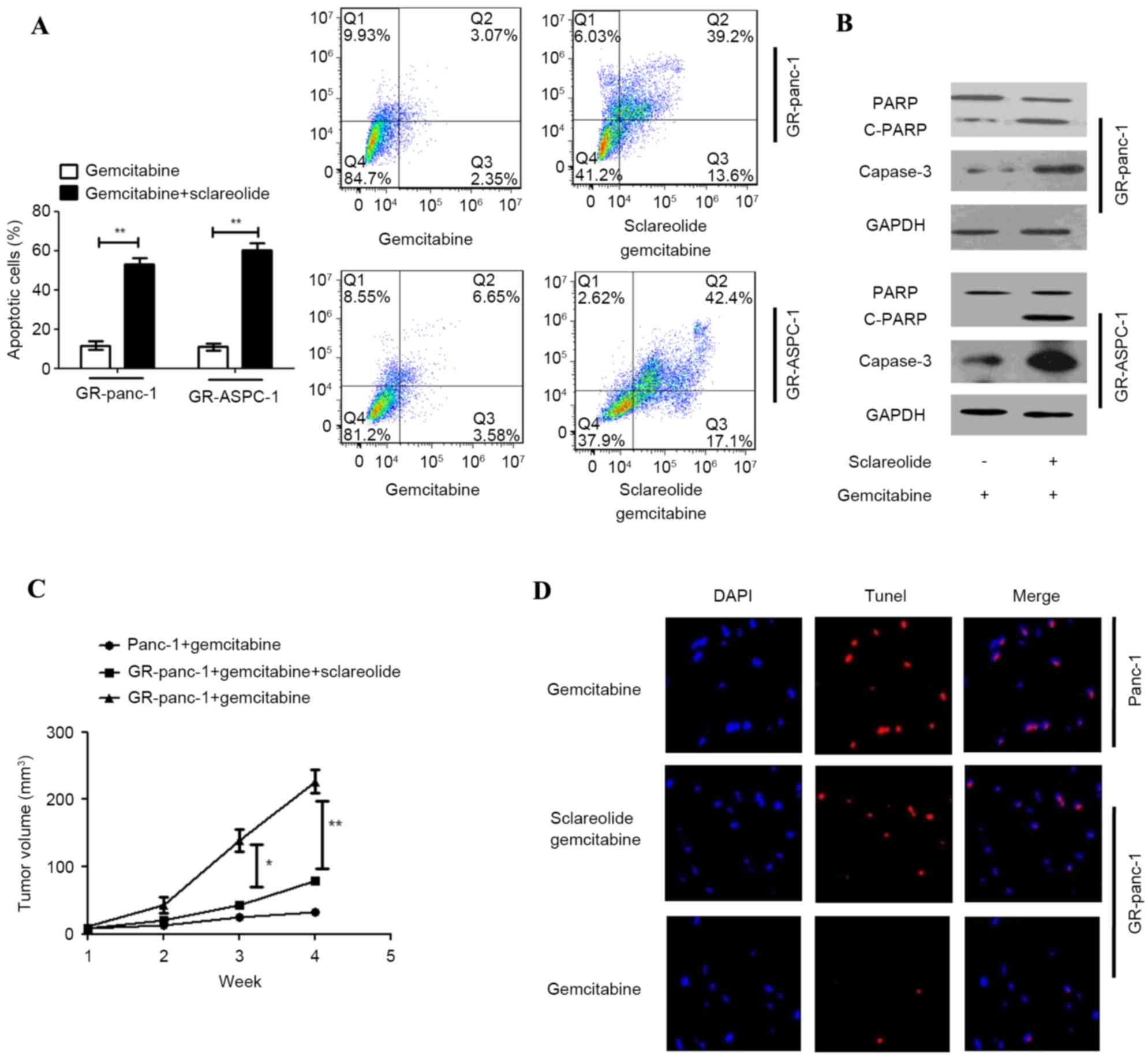
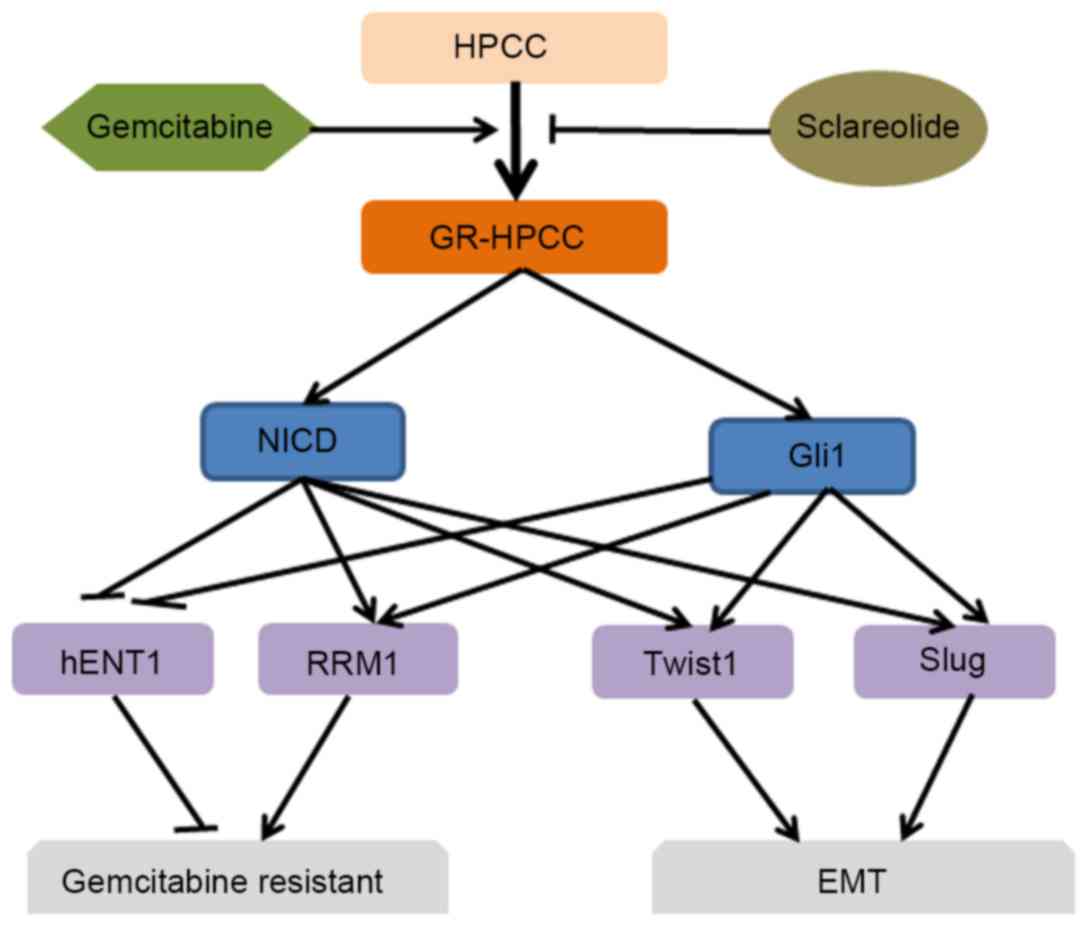
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seicean A, Petrusel L and Seicean R: New
targeted therapies in pancreatic cancer. World J Gastroenterol.
21:6127–6145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Massari F, Santoni M, Ciccarese C,
Brunelli M, Conti A, Santini D, Montironi R, Cascinu S and Tortora
G: Emerging concepts on drug resistance in bladder cancer:
Implications for future strategies. Crit Rev Oncol Hematol.
96:81–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Sousa Cavalcante L and Monteiro G:
Gemcitabine: Metabolism and molecular mechanisms of action,
sensitivity and chemoresistance in pancreatic cancer. Eur J
Pharmacol. 741:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nordh S, Ansari D and Andersson R: hENT1
expression is predictive of gemcitabine outcome in pancreatic
cancer: A systematic review. World J Gastroenterol. 20:8482–8490.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santini D, Schiavon G, Vincenzi B, Cass
CE, Vasile E, Manazza AD, Catalano V, Baldi GG, Lai R, Rizzo S, et
al: Human equilibrative nucleoside transporter 1 (hENT1) levels
predict response to gemcitabine in patients with biliary tract
cancer (BTC). Curr Cancer Drug Targets. 11:123–129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamba JK: Genetic factors influencing
cytarabine therapy. Pharmacogenomics. 10:1657–1674. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jordheim LP and Dumontet C: Do hENT1 and
RRM1 predict the clinical benefit of gemcitabine in pancreatic
cancer? Biomark Med. 7:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jordheim LP, Sève P, Trédan O and Dumontet
C: The ribonucleotide reductase large subunit (RRM1) as a
predictive factor in patients with cancer. Lancet Oncol.
12:693–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaeffer DF, Assi K, Chan K, Buczkowski
AK, Chung SW, Scudamore CH, Weiss A, Salh B and Owen DA: Tumor
expression of integrin-linked kinase (ILK) correlates with the
expression of the E-cadherin repressor snail: An
immunohistochemical study in ductal pancreatic adenocarcinoma.
Virchows Arch. 456:261–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atta-ur-Rahman, Farooq A and Choudhary MI:
Microbial transformation of sclareolide. J Nat Prod. 60:1038–1040.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
González MA, Mancebo-Aracil J,
Tangarife-Castaño V, Agudelo-Goméz L, Zapata B, Mesa-Arango A and
Betancur-Galvis L: Synthesis and biological evaluation of
(+)-labdadienedial, derivatives and precursors from
(+)-sclareolide. Eur J Med Chem. 45:4403–4408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung HY and Yang J: Unraveling the TWIST
between EMT and cancer stemness. Cell Stem Cell. 16:1–2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian Y, Xu Y, Fu Q, Chang M, Wang Y, Shang
X, Wan C, Marymont JV and Dong Y: Notch inhibits chondrogenic
differentiation of mesenchymal progenitor cells by targeting
Twist1. Mol Cell Endocrinol. 403:30–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joost S, Almada LL, Rohnalter V, Holz PS,
Vrabel AM, Fernandez-Barrena MG, McWilliams RR, Krause M,
Fernandez-Zapico ME and Lauth M: GLI1 inhibition promotes
epithelial-to-mesenchymal transition in pancreatic cancer cells.
Cancer Res. 72:88–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gopalakrishnan N, Sivasithamparam ND and
Devaraj H: Synergistic association of Notch and NFκB signaling and
role of Notch signaling in modulating epithelial to mesenchymal
transition in colorectal adenocarcinoma. Biochimie. 107:310–318.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strimpakos AS, Syrigos KN and Saif MW:
Pharmacogenomics in pancreatic adenocarcinoma: New data and their
clinical implications. JOP. 14:359–362. 2013.PubMed/NCBI
|
|
22
|
Damaraju VL, Scriver T, Mowles D, Kuzma M,
Ryan AJ, Cass CE and Sawyer MB: Erlotinib, gefitinib, and
vandetanib inhibit human nucleoside transporters and protect cancer
cells from gemcitabine cytotoxicity. Clin Cancer Res. 20:176–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minami K, Shinsato Y, Yamamoto M,
Takahashi H, Zhang S, Nishizawa Y, Tabata S, Ikeda R, Kawahara K,
Tsujikawa K, et al: Ribonucleotide reductase is an effective target
to overcome gemcitabine resistance in gemcitabine-resistant
pancreatic cancer cells with dual resistant factors. J Pharmacol
Sci. 127:319–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie H, Jiang W, Jiang J, Wang Y, Kim R and
Liu X and Liu X: Predictive and prognostic roles of ribonucleotide
reductase M1 in resectable pancreaticadenocarcinoma. Cancer.
119:173–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Montero TD, Racordon D, Bravo L, Owen GI,
Bronfman ML and Leisewitz AV: PPARα and PPARγ regulate the
nucleoside transporter hENT1. Biochem Biophys Res Commun.
419:405–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koo JS and Kim H: Hypoxia-related protein
expression and its clinicopathologic implication in carcinoma of
unknown primary. Tumour Biol. 32:893–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tassone P, Di Martino MT, Ventura M,
Pietragalla A, Cucinotto I, Calimeri T, Bulotta A, Neri P, Caraglia
M and Tagliaferri P: Loss of BRCA1 function increases the antitumor
activity of cisplatin against human breast cancer xenografts in
vivo. Cancer Biol Ther. 8:648–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Khoueiry AB, Ramanathan RK, Yang DY,
Zhang W, Shibata S, Wright JJ, Gandara D and Lenz HJ: A randomized
phase II of gemcitabine and sorafenib versus sorafenib alone in
patients with metastatic pancreatic cancer. Invest New Drugs.
30:1175–1183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vena F, Causi EL, Rodriguez-Justo M,
Goodstal S, Hagemann T, Hartley JA and Hochhauser D: The MEK1/2
inhibitor Pimasertib enhances gemcitabine efficacy in pancreatic
cancer models by altering protein levels of ribonucleotide
reductase subunit-1 (RRM1). Clin Cancer Res. 21:5563–5577. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grishina IB: Mini-review: Does notch
promote or suppress cancer? New findings and old controversies. Am
J Clin Exp Urol. 3:24–27. 2015.PubMed/NCBI
|
|
31
|
Uzdensky AB, Demyanenko SV and Bibov MY:
Signal transduction in human cutaneous melanoma and target drugs.
Curr Cancer Drug Targets. 13:843–866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L and Leung PS: Use of herbal medicines
and natural products: An alternative approach to overcoming the
apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol.
53:224–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwanbeck R: The role of epigenetic
mechanisms in Notch signaling during development. J Cell Physiol.
230:969–981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gonnissen A, Isebaert S and Haustermans K:
Targeting the Hedgehog signaling pathway in cancer: Beyond
Smoothened. Oncotarget. 6:13899–13913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aberger F, Ruiz I and Altaba A:
Context-dependent signal integration by the GLI code: The oncogenic
load, pathways, modifiers and implications for cancer therapy.
Semin Cell Dev Biol. 33:93–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Merchant JL and Saqui-Salces M: Inhibition
of Hedgehog signaling in the gastrointestinal tract: Targeting the
cancer microenvironment. Cancer Treat Rev. 40:12–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Drenkhahn SK, Jackson GA, Slusarz A,
Slusarz A, Starkey NJ and Lubahn DB: Inhibition of hedgehog/Gli
signaling by botanicals: A review of compounds with potential
hedgehog pathway inhibitory activities. Curr Cancer Drug Targets.
13:580–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perrot CY, Javelaud D and Mauviel A:
Overlapping activities of TGF-β and Hedgehog signaling in cancer:
Therapeutic targets for cancer treatment. Pharmacol Ther.
137:183–199. 2013. View Article : Google Scholar : PubMed/NCBI
|