Introduction
The correction of malocclusion involves adjusting
tooth position via external tensile stresses generated by
orthodontic appliances, thus causing internal responses to these
forces (1,2). During such orthodontic treatments,
the periodontal tissues often sustain reversible injury (3). Full rectification of the dentition is
only achieved following extensive treatment, due to various factors
that may affect a positive outcome (4–6).
Despite the development of advanced orthodontic techniques and
appliances (7), the underlying
mechanisms of orthodontic tooth movement remain unclear.
A previous study has suggested an important role for
the periodontal ligament in promoting the movement of teeth and
dental implants during orthodontic treatment (8). A variant form of periosteum
interposes between the cementum and the alveolar bone; the
periodontal ligament consists of dense connective tissue arranged
in fiber bundles with the two ends buried in the cementum and the
alveolar bone wall socket. In addition to buffering and absorbing
external forces (9), the
periodontal ligament additionally serves a vital role in the
transduction of external forces to promote alveolar bone
mineralization and resorption, and repair of injured periodontal
tissues (10–12). Dangaria et al (13) demonstrated that stem cell colonies
in the periodontal ligament and the extracellular matrix may
promote regeneration of periodontal tissues.
A recent study identified periostin (PN) as a
critical extracellular matrix protein that functions to regulate
the steady state of the periodontal membrane (14). PN is additionally involved in
maintaining periodontal tissue integrity, tooth development,
external stimuli-induced repair and tissue regeneration, cell
adhesion, collagen cross-linking, and fiber formation (15). From a developmental perspective,
rat embryos exhibit PN expression on embryonic day 9.5, initially
in the first branchial arch, followed by the ectomesenchyme
(16), dental papilla cells, and
odontoblasts during the bell and hard tissue formation stages.
Following birth, PN expression is detected primarily in the
fibroblasts of the periodontal ligament and the osteoblasts of the
alveolar bone (17).
In the absence of a biting force, the periodontal
ligament of Wistar rats becomes weakened and exhibits structural
alterations, accompanied by a marked decrease in PN mRNA expression
levels after 24 h (18). In
PN-knockdown mice, X-ray and micro-computed tomography studies
demonstrated no marked alterations in the periodontal ligament
during tooth development and eruption, prior to the establishment
of normal occlusion, compared with healthy mice. However, in
PN-null mice with tooth eruption, significant damages occurred in
the periodontal tissue following exposure to a masticatory force
for three months (19), suggesting
that PN is essential for maintaining the integrity of the
periodontal membrane when a biting force is present.
PN is a multifunctional extracellular matrix protein
that may be induced by transforming growth factor-β (TGF-β), and
serves as a downstream factor of the latter to regulate the
synthesis and secretion of collagen in fibroblasts (20). PN secreted by airway epithelial
cells may activate TGF-β to promote airway fibrosis (21,22).
Norris et al (23)
demonstrated that in PN-knockout mice, the myocardial collagen has
a reduced denaturation temperature. Thus far, little is understood
concerning the role of PN in orthodontic periodontal ligament
remodeling, and of its association with TGF-β. The present study
aimed to investigate the role of PN in periodontal ligament
remodeling during orthodontic treatment, and the potential
underlying molecular mechanisms. This was achieved by detecting PN
expression levels under tensile stress conditions, in human
periodontal ligament cells (hPDLCs), a mouse model of orthodontic
tooth movement and in patients.
Materials and methods
Subjects
The present study was performed with approval from
the Southern Medical University Institutional Research Ethics
Committee (Guangzhou, China). Written informed consent was obtained
from 25 patients (male, n=13; female, n=12; age, 18.5±0.7) who
fulfilled the following inclusion criteria: Age, 12–25 years when
receiving orthodontic treatment; permanent dentition; normal
dentition (with the exception of the third molars); absence of
periodontal diseases, severe caries or hyperplasia of the tooth
root; absence of systemic diseases and no history of
anti-inflammatory agent or immunosuppressant use within 3 months
prior to enrollment; willingness to receive reduction tooth
extraction of the molars. All the patients received orthodontic
treatment in the Orthodontic Department of Nanfang Hospital,
Southern Medical University, between June 2012 and December 2013.
Of these patients, 10 with crowded dentition but without
orthodontic treatment prior to tooth extraction served as the
control group, and 15 with normal dentition undergoing orthodontic
treatment for 3–4 months constituted the experimental group. The
periodontal tissue of tooth roots was removed with a scalpel into a
1.5-ml centrifuge tube and stored at −80°C for further
experiments.
Animal experiments
All animal experiments were performed with the
approval of Southern Medical University Institutional Research
Ethics Committee. Specific pathogen-free male wild-type C57BL/6
mice (age, 4 weeks; weight, 20±3 g; n=35) were obtained from and
housed by the Experimental Animal Center of Southern Medical
University in standard laboratory conditions (25°C, 10–85% humidity
and a 12 h light/dark cycle [on at 7:15 a.m. and off at 7:15 p.m.])
with free access to food and water. The mice were randomized into
five groups, including a control group without external tensile
stress loading, and four experimental groups exposed to 20 g
external tensile stress loading, delivered using a conventional
orthodontic method (24) for 2, 4
or 6 days. Using a customized fixed oral traction device, a hook
was fixed under a microscope on the incisors of the mouse and a
ligature wire that delivered a 20 × g traction force was fixed on
the first molars of the mice, which were anesthetized with
intravenous sodium pentobarbital (1%, 35 mg/kg; cat. no. P3761;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) intravenously
injected, and surgical depth of anesthesia was deemed to be
achieved when the tail-pinch and pedal withdrawal reflexes were
lost (25). The coil spring was
kept constant for 2, 4 or 6 days, following which the upper molars
exposed to the traction force were removed. The periodontal tissue
of the tooth roots was removed with a scalpel and stored in a
1.5-ml centrifuge tube at −80°C for subsequent analysis.
Immunohistochemistry for PN
Following tensile stress loading for various time
periods, the five groups of mice were anesthetized with sodium
pentobarbital intravenously injected (1%, 80 mg/kg; cat. no. P376;
Sigma-Aldrich; Merck Millipore) and sacrificed by decapitation, and
the bone segment containing the first molar and periodontal tissues
was harvested. The direction of tensile stress was marked, and the
bone segments were fixed with formalin for 6 h and immersed in
decalcifying solution at 4°C for 3 weeks. The bone segments were
subsequently embedded in paraffin, and for each tissue sample, 3 µm
thick transverse sections of the upper molars were cut for
morphometric analysis. Immunohistochemical staining of the sections
was performed using mouse monoclonal anti-PN horseradish peroxidase
(HRP) antibody (1:300; cat. no. sc-398631; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). PN expression area and
intensity was determined using the labeled streptavidin-biotin
technique, as previously described (26). The SP-9000 kit was purchased from
ZSGB-BIO (cat. no. SP-9000; Beijing, China). Imaging analysis was
conducted using the Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Cultured hPDLCs and cyclic tensile
stress loading
To isolate hPDLCs, healthy wisdom teeth and
premolars freshly extracted from young adults for orthodontic
reasons were obtained. All the donors were informed of the purpose
of the study and written informed consent was given prior to
participation. hPDLCs were isolated from the extracted teeth by
explant culture combined with enzymatic digestion, as previously
described (27). The primary
hPDLCs were maintained in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C in a humidified atmosphere of 5% CO2. The cells
were dissociated from the surface of the culture flask with trypsin
solution [0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.),
0.1% glucose and citrate-saline buffer (pH 7.8)] and resuspended
for future experiments. The third-passage cells were cultured in a
mineralization medium (DMEM supplemented with 10% FBS, 0.1 µM
dexamethasome, 50 µM vitamin C and 10 mM β-phosphoglycerol) for 21
days. The cells were subsequently stained with Alizarin Red S to
visualize the formation of mineralized nodules, and
immunohistochemical staining for vimentin and keratin expression
was performed as previously described (26) to ensure that the cells originated
from the periodontal ligament instead of the epithelia. Following
verification, cells were exposed to cyclic tensile stress loading
with a 10% extent at a 0.5 Hz resonant frequency delivered from a
Flexcell® FX-5000 Tension system (28) for 12, 24 or 48 h. Cells cultured
without tensile stress loading under routine conditions served as
the control. In order to explore the mechanism of PN during
orthodontic periodontal ligament remodeling, hPDLCs were treated
with 5 ng/ml TGF-β (cat. no. 11412272001; Roche Applied Science,
Penzberg, Germany) for 12, 24 and 48 h (29).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the tensile stress-loaded hPDLCs,
periodontal tissues from the mouse model, and patient samples was
extracted using TRIzol (Takara Bio, Inc., Otsu, Japan) according to
the manufacturer's protocol. The RNA quality was determined using a
NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific, Inc.),
and the RNA samples were further analyzed if their absorbance ratio
(A260/A280) was within the range of
1.9–2.1, with a A260/A230 >2. The RNA was
reverse transcribed to cDNA using the RevertAidTM First Strand cDNA
Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.). Primers
(Table I; Thermo Fisher
Scientific, Inc.) were used to determine the gene expressions of
PN, TGF-β, type I collagen and α-smooth muscle actin (α-SMA) by
SYBR Green (cat. no. 172-5850; Bio-Rad Laboratories, Hercules, CA,
USA) RT-qPCR, and their mRNA expression levels were calculated
relative to that of GAPDH. The cycling conditions were as follows:
Predenaturation at 94°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 30 sec and annealing at 58°C for 30 sec.
mRNA expression levels were calculated based on the quantification
cycle (Cq) (30), and relative
expression levels were calculated as 2-[(Cq of target gene)-(Cq of
GAPDH)].
 | Table I.Primers for PN, TGF-β, type I
collagen, α-SMA and GAPDH. |
Table I.
Primers for PN, TGF-β, type I
collagen, α-SMA and GAPDH.
| Gene | Direction | Sequence
(5′-3′) |
|---|
| PN | F |
GAGACAAAGTGGCTTCCG |
|
| R |
CTGTCACCGTCACATCCT |
| TGF-β | F |
GCAACAATTCCTGGCGATAC |
|
| R |
AAGGCGAAAGCCCTCAAT |
| Type I | F |
GAGGGCCAAGACGAAGACATC |
| collagen |
|
|
|
| R |
CAGATCACGTCATCGCACAAC |
| α-SMA | F |
GGCATTCACGAGACCACCTAC |
|
| R |
CGACATGACGTTGTTGGCATAC |
| GAPDH | F |
ACAGTCAGCCGCATCTTCTT |
|
| R |
GACAAGCTTCCCGTTCTCAG |
Western blotting
The cells exposed to tensile stress loading were
analyzed for PN protein expression levels by western blotting.
Briefly, the cells in each group were rinsed with
phosphate-buffered saline and lysed in radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) supplemented with protease inhibitors. The total protein was
quantified using a Bicinchoninic Acid assay kit (Pierce; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Equal amounts (30 µg) of total protein were separated by 10%
SDS-PAGE and subsequently transferred onto polyvinylidene
difluoride membranes. The membranes were blocked with 5% skimmed
milk in Tris-buffered saline containing Tween-20 (TBST) for 2 h at
room temperature, and subsequently washed three times (5 min each)
in TBST buffer. Following this, the membranes were incubated with
the following primary antibodies: Polyclonal mouse anti-human
anti-PN (cat. no. sc-398631; 1:500 dilution; Santa Cruz
Biotechnology, Inc.), polyclonal mouse anti-human anti-TGF-β (cat.
no. sc-130348; 1:500 dilution; Santa Cruz Biotechnology, Inc.),
mouse anti-type I collagen and mouse anti-α-SMA (cat. nos. sc-59772
and sc-53142, respectively; 1:500 dilution; Santa Cruz
Biotechnology, Inc.), with gentle agitation overnight at 4°C. The
membranes were subsequently incubated with goat anti-mouse
HRP-conjugated secondary antibodies (cat. no. A4416; 1:3,000
dilution; Sigma-Aldrich; Merck Millipore) for 45 min with gentle
rocking at room temperature prior to membrane washing with TBST
buffer. Mouse monoclonal anti-α-tubulin (cat. no. T9026; 1:1,000
dilution; Sigma-Aldrich; Merck Millipore) served as the loading
control. Chemiluminescence was detected using SuperSignal West
Femto Maximum Sensitivity Substrate (Pierce; Thermo Fisher
Scientific, Inc.) in a ChemiDocXRS system (Bio-Rad Laboratories,
Inc.) and Quantity One 1-D Analysis Software (version 1709600;
Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard error and
were analyzed with SPSS software version 13.0 (SPSS, Inc., Chicago,
IL, USA). Data were tested for normal distribution and/or
homogeneity variance prior to comparisons between groups and
analyses were performed by one-way analysis of variance. Least
significant difference or Dunnett's T3 post hoc tests were used to
compare the differences between the control and treatment groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Tensile stress load increases PN
expression levels in hPDLCs
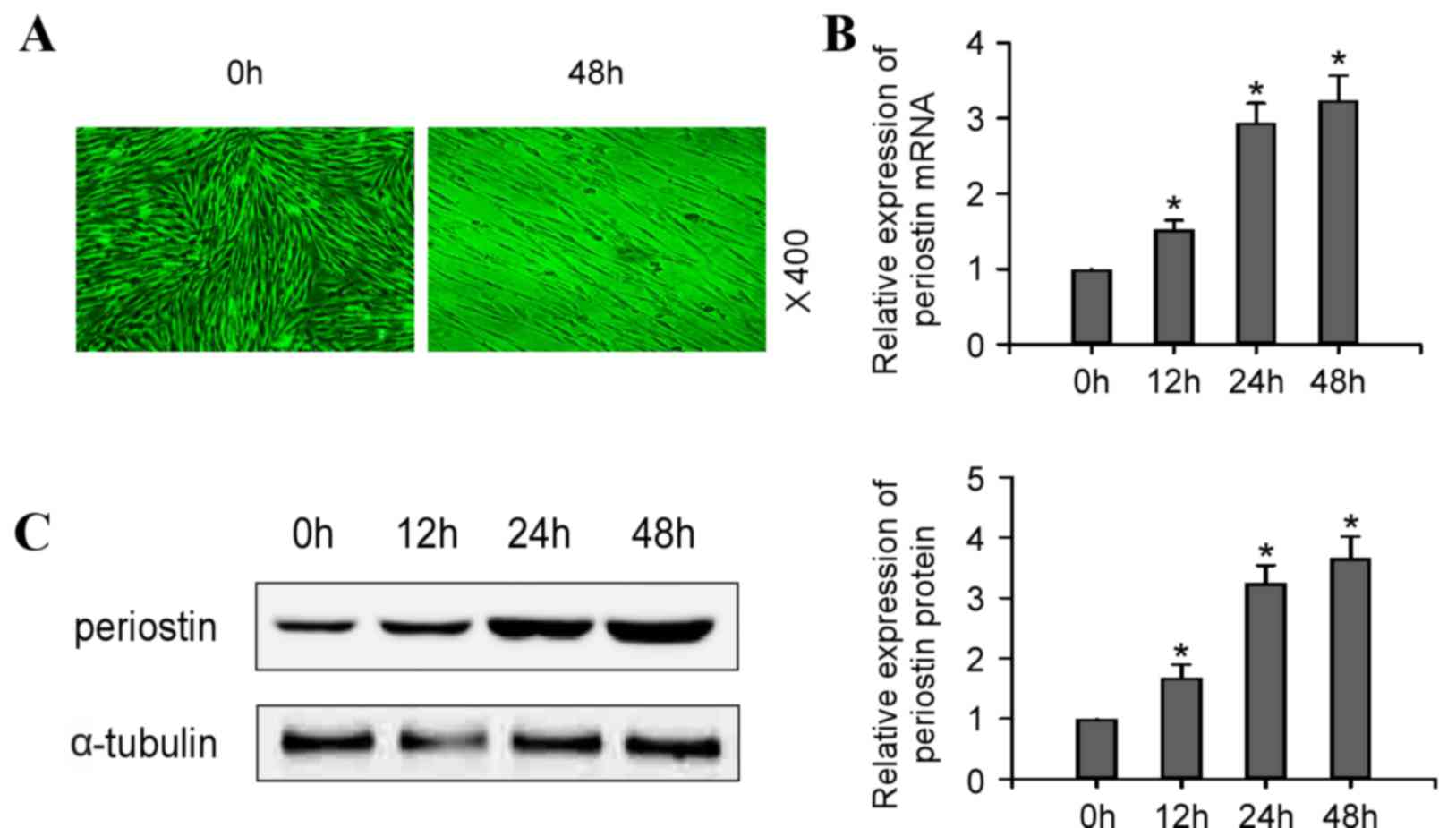
Compared with control cells, which demonstrated a
radial pattern of cell proliferation, hPDLCs exposed to cyclic
tensile stress of 10% extent at 0.5 Hz exhibited a fence-like and
parallel growth pattern, consistent with the direction of tensile
stress (Fig. 1A). To investigate
the role of PN in this process, mRNA (Fig. 1B; P<0.05) and protein (Fig. 1C; P<0.05) expression levels of
PN were analyzed in cells following tensile stress loading. RT-qPCR
and western blotting results revealed that cyclic tensile stress
induced a time-dependent increase of PN mRNA and protein expression
levels in hPDLCs.
PN expression levels increase in mouse
periodontal ligaments during orthodontic tooth movement
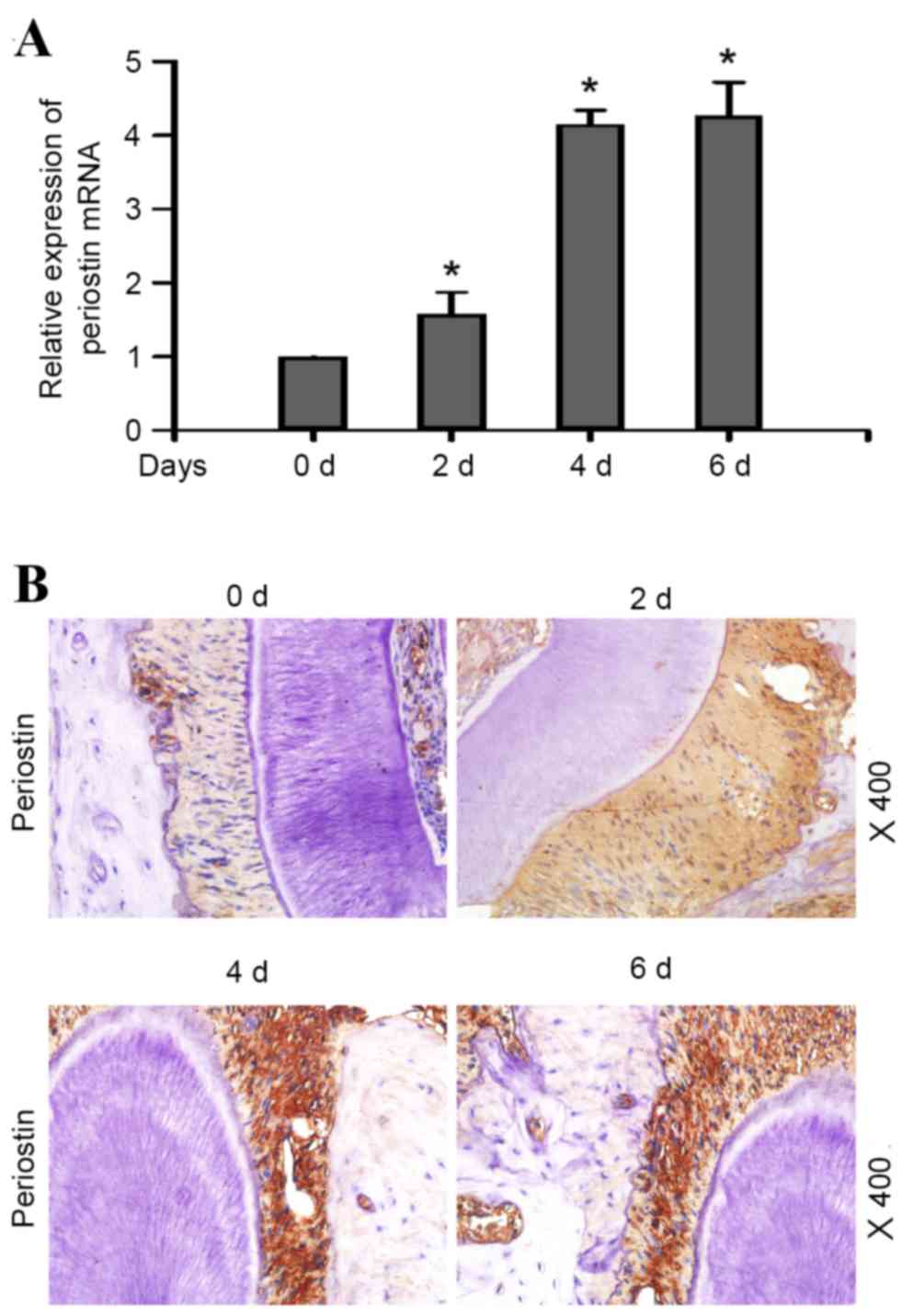
In a mouse model of orthodontic tooth movement, PN
mRNA expression levels in the periodontal ligament increased with
tensile stress loading in a time-dependent manner (Fig. 2A; P<0.05). Immunohistochemistry
revealed a diffuse pattern of PN distribution in the periodontal
ligament of healthy mice. Mice subjected to tensile stress loading
demonstrated a time-dependent increase in PN staining intensity in
the periodontal ligament during the first 4 days (Fig. 2B), whereas at 6 days, the staining
expression intensity had decreased, suggesting that PN was involved
in the entire process of periodontal ligament remodeling induced by
the external force, and that its expression levels increased as the
duration of the external force extended.
PN mRNA expression levels increase in
human periodontal ligament during orthodontic tooth movement
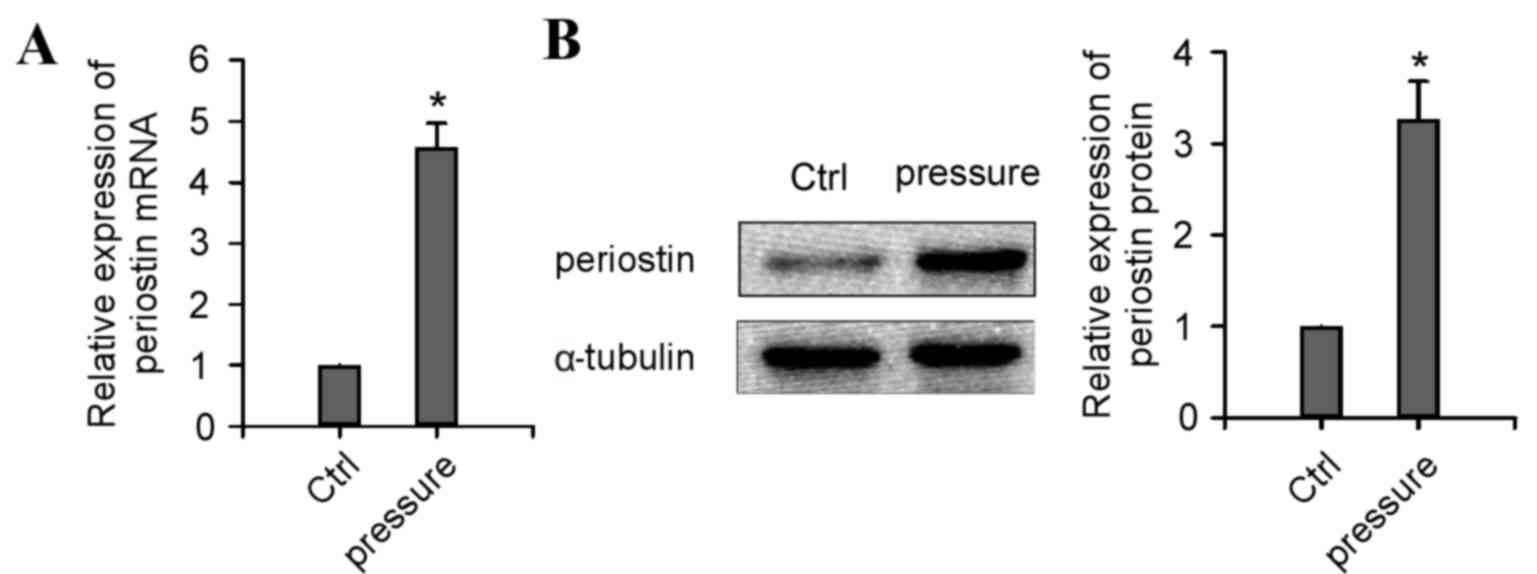
The 15 patients who received preceding orthodontic
treatment all exhibited significantly upregulated PN mRNA (Fig. 3A; P<0.05) and protein (Fig. 3B; P<0.05) expression levels in
the periodontal ligament of the extracted teeth, compared with
those who did not receive orthodontic treatment. This result
demonstrated an important role for PN in periodontal ligament
remodeling during orthodontic treatment.
TGF-β increases PN expression levels
and promotes type I collagen and α-SMA expression
Due to the association between PN and TGF-β, the
present study investigated whether the TGF-β signaling pathway
regulates PN expression levels in hPDLCs, and how PN regulates type
I collagen during periodontal ligament remodeling. It was
demonstrated that PN and TGF-β expression levels were upregulated
in parallel in hPDLCs exposed to cyclic tensile stress loading
(Fig. 4A; P<0.05). In addition,
hPDLCs cultured in the presence of TGF-β without tensile stress
loading exhibited a time-dependent increase in PN mRNA expression
levels (Fig. 4B; P<0.05), and
the expression of PN was in parallel with the protein and mRNA
expression of type I collagen and α-SMA in hPDLCs treated with
TGF-β (Fig. 4C; P<0.05). Taken
together, these results indicated that PN upregulated by TGF-β
might stimulate the expression of type I collagen and α-SMA, thus
contributing to periodontal ligament remodeling during orthodontic
treatment.
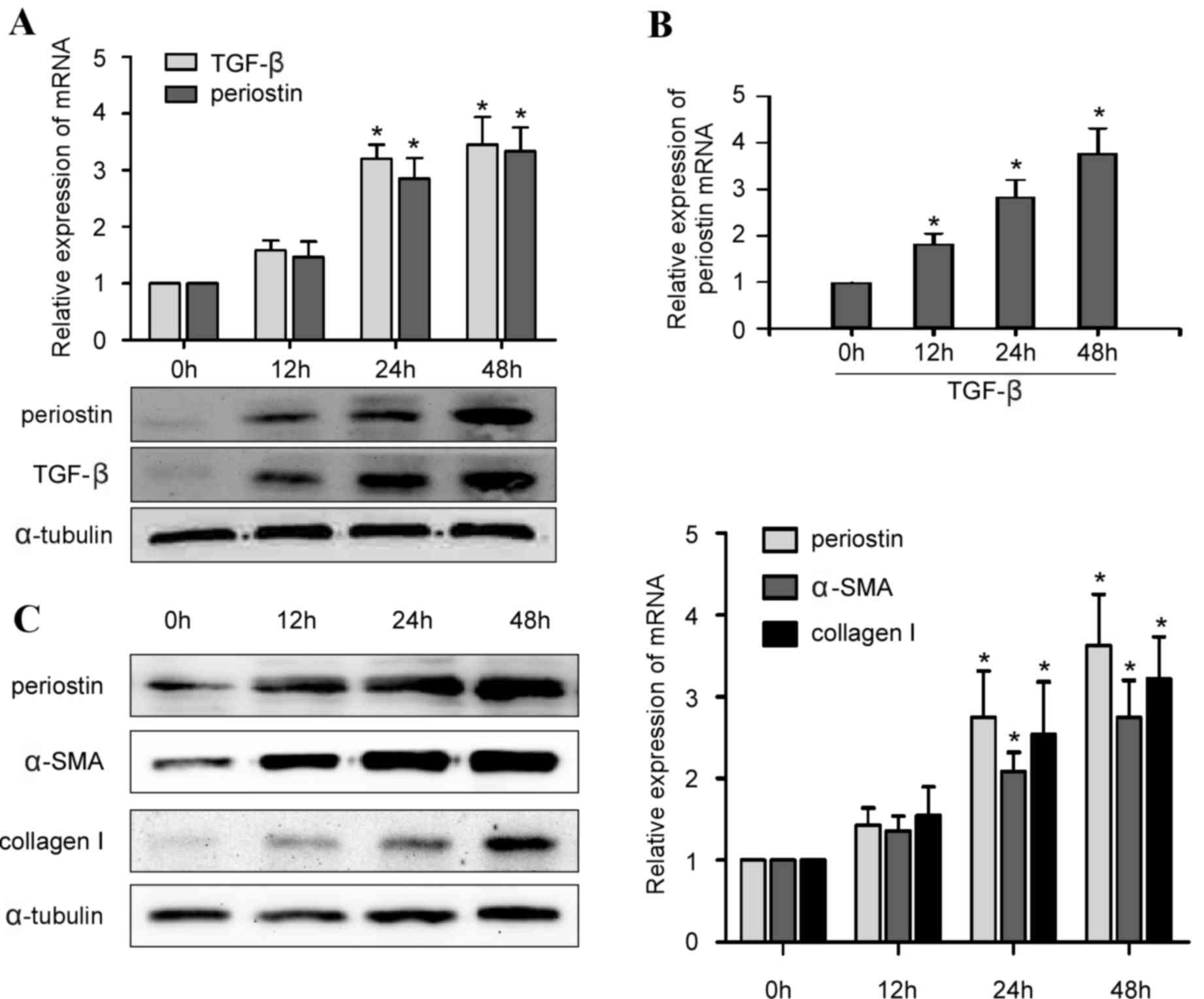 | Figure 4.Alterations in expression levels of
PN, TGF-β, type I collagen and α-SMA over time in hPDLCs. (A)
Reverse transcription-quantitative polymerase chain reaction
quantification and representative western blot images of PN and
TGF-β mRNA and protein expression levels in hPDLCs, respectively,
upon exposure to 0, 12, 24 or 48 h external tensile stress. (B) PN
mRNA expression levels in hPDLCs were upregulated by TGF-β in a
time-dependent manner. (C) Type I collagen and α-SMA protein and
mRNA expression levels increased with PN expression levels in
hPDLCs treated with 5 ng/ml TGF-β, in a time-dependent manner. Data
are presented as the mean ± standard error. *P<0.05 vs. 0 h. PN,
periostin; TGF-β, transforming growth factor-β, α-SMA, α-smooth
muscle actin; hPLCs, human periodontal ligament cells. |
Discussion
As extracellular matrix proteins have been
identified to serve important functions in multiple systems of the
human body, PN has been increasingly investigated (31–36).
An anti-PN antibody has been developed for use in cancer treatment
(37), and recombinant PN
constructs have been assessed in mice to induce angiogenesis
(38), and to stimulate myocardial
regeneration in large mammals (39). Previous studies have demonstrated a
criticial role of PN as a tissue biomarker in the periodontal
ligament (15,40); however, the precise underlying
mechanisms of its effects and its potential value in clinical
orthodontic treatment remain to be clarified.
In the present study, the function of PN during
orthodontic treatment was investigated by assessing PN expression
levels in response to external tensile stress load in vitro
and in vivo. These results supported the hypothesis that PN
serves an important role in periodontal remodeling during
orthodontic tooth movement. Notably, results from patient samples
further demonstrated the potential clinical applications of PN.
Wen et al (41) demonstrated that TGF-β significantly
increased PN mRNA expression levels in periodontal ligament
fibroblasts, and that this effect was attenuated by focal adhesion
kinase (FAK) inhibitors; in FAK knockout fibroblasts, no PN mRNA
was detected even following cyclic stress stimulation. PN may
additionally be chemotactic for myocardial fibroblasts via
αV-integrin-induced phosphorylation of FAK and protein kinase B
(42,43). The present study revealed that in
hPDLCs exposed to tensile stress, PN and TGF-β expression levels
increased in parallel. These results suggested that the
TGF-β/FAK-dependent pathway may participate in the regulation of PN
in the periodontal ligament.
PN has previously been demonstrated to promote
angiogenesis by upregulating matrix metalloproteinase-2 mediated by
αVβ3 integrin/extracellular signal-regulated kinase signaling and
vascular endothelial growth factor; the latter was additionally
revealed to be present in human periodontal ligaments (44). Under stress conditions, PN may bind
to a Notchl precursor that inhibits cell apoptosis by upregulating
B-cell lymphoma-extra large (45).
TGF-β has been reported to regulate the mRNA expression of type I
collagen and α-SMA in human PDL cells (29). Based on these observations, it is
possible to speculate that as a downstream target of TGF-β, PN
regulates type I collagen and α-SMA expression to affect the
mechanical properties of the periodontal ligament and serves as a
marker of periodontal ligament remodeling (46,47).
These findings indicate the versatile functions of PN in
periodontal ligament remodeling and in numerous other disease
conditions, including proliferative vitreoretinopathy (48) and certain cancers (49). However, the complex mechanisms
underlying its functions remain to be further investigated.
In conclusion, the present study demonstrated that
PN serves a crucial role in periodontal ligament remodeling during
orthodontic treatment, and may be associated with TGF-β, type I
collagen and α-SMA expression. These results suggested the value of
PN as a potential therapeutic target for the treatment of
malocclusion.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81371137) and the Natural Science Foundation of Guangdong Province
(Guangzhou, China; grant no. 2015A030310140).
Glossary
Abbreviations
Abbreviations:
|
PN
|
periostin
|
|
hPDLC
|
human periodontal ligament cell
|
|
FAK
|
focal adhesion kinase
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Rody WJ Jr, King GJ and Gu G: Osteoclast
recruitment to sites of compression in orthodontic tooth movement.
Am J Orthod Dentofacial Orthop. 120:477–489. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wise GE and King GJ: Mechanisms of tooth
eruption and orthodontic tooth movement. J Dent Res. 87:414–434.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rafiuddin S, Yg PK, Biswas S, Prabhu SS,
Bm C and Mp R: Iatrogenic damage to the periodontium caused by
orthodontic treatment procedures: An overview. Open Dent J.
9:228–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prasad RV, Chincholi S, V D, Sirajuddin S,
Biswas S, Prabhu SS and Mp R: Iatrogenic factors affecting the
periodontium: An overview. Open Dent J. 9:208–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan FS, Moles DR, Shute JT, Clarke A and
Cunningham SJ: Social anxiety in orthognathic patients. Int J Oral
Maxillofac Surg. 45:19–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pachêco-Pereira C, Abreu LG, Dick BD, De
Luca Canto G, Paiva SM and Flores-Mir C: Patient satisfaction after
orthodontic treatment combined with orthognathic surgery: A
systematic review. Angle Orthod. 86:495–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Zhu Y, Long H, Zhou Y, Jian F, Ye
N, Gao M and Lai W: The effectiveness of the Herbst appliance for
patients with Class II malocclusion: A meta-analysis. Eur J Orthod.
38:324–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Romanos GE, Asnani KP, Hingorani D and
Deshmukh VL: PERIOSTIN: Role in formation and maintenance of dental
tissues. J Cell Physiol. 229:1–5. 2014.PubMed/NCBI
|
|
9
|
Beertsen W, McCulloch CA and Sodek J: The
periodontal ligament: A unique, multifunctional connective tissue.
Periodontology 2000. 13:20–40. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jessop HL, Rawlinson SC, Pitsillides AA
and Lanyon LE: Mechanical strain and fluid movement both activate
extracellular regulated kinase (ERK) in osteoblast-like cells but
via different signaling pathways. Bone. 31:186–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McAllister TN and Frangos JA: Steady and
transient fluid shear stress stimulate NO release in osteoblasts
through distinct biochemical pathways. J Bone Miner Res.
14:930–936. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bakker AD, Soejima K, Klein-Nulend J and
Burger EH: The production of nitric oxide and prostaglandin E(2) by
primary bone cells is shear stress dependent. J Biomech.
34:671–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dangaria SJ, Ito Y, Walker C, Druzinsky R,
Luan X and Diekwisch TG: Extracellular matrix-mediated
differentiation of periodontal progenitor cells. Differentiation.
78:79–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Padial-Molina M, Volk SL and Rios HF:
Preliminary insight into the periostin leverage during periodontal
tissue healing. J Clin Periodontol. Jul 23–2015.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Padial-Molina M, Volk SL, Rodriguez JC,
Marchesan JT, Galindo-Moreno P and Rios HF: Tumor necrosis factor-α
and Porphyromonas gingivalis lipopolysaccharides decrease periostin
in human periodontal ligament fibroblasts. J Periodontol.
84:694–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kruzynska-Frejtag A, Wang J, Maeda M,
Rogers R, Krug E, Hoffman S, Markwald RR and Conway SJ: Periostin
is expressed within the developing teeth at the sites of
epithelial-mesenchymal interaction. Dev Dyn. 229:857–868. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilde J, Yokozeki M, Terai K, Kudo A and
Moriyama K: The divergent expression of periostin mRNA in the
periodontal ligament during experimental tooth movement. Cell
Tissue Res. 312:345–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi JW, Arai C, Ishikawa M, Shimoda S and
Nakamura Y: Fiber system degradation, and periostin and connective
tissue growth factor level reduction, in the periodontal ligament
of teeth in the absence of masticatory load. J Periodontal Res.
46:513–521. 2011.PubMed/NCBI
|
|
19
|
Rios HF, Ma D, Xie Y, Giannobile WV,
Bonewald LF, Conway SJ and Feng JQ: Periostin is essential for the
integrity and function of the periodontal ligament during occlusal
loading in mice. J Periodontol. 79:1480–1490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stambolic V and Woodgett JR: Functional
distinctions of protein kinase B/Akt isoforms defined by their
influence on cell migration. Trends Cell Biol. 16:461–466. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sidhu SS, Yuan S, Innes AL, Kerr S,
Woodruff PG, Hou L, Muller SJ and Fahy JV: Roles of epithelial
cell-derived periostin in TGF-beta activation, collagen production,
and collagen gel elasticity in asthma. Proc Natl Acad Sci USA.
107:14170–14175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon ED, Sidhu SS, Wang ZE, Woodruff PG,
Yuan S, Solon MC, Conway SJ, Huang X, Locksley RM and Fahy JV: A
protective role for periostin and TGF-β in IgE-mediated allergy and
airway hyperresponsiveness. Clin Exp Allergy. 42:144–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Norris RA, Borg TK, Butcher JT, Baudino
TA, Banerjee I and Markwald RR: Neonatal and adult cardiovascular
pathophysiological remodeling and repair: developmental role of
periostin. Annals of the New York Academy of Sciences. 1123:30–40.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alobeid A, Dirk C, Reimann S, El-Bialy T,
Jäger A and Bourauel C: Mechanical properties of different esthetic
and conventional orthodontic wires in bending tests: An in vitro
study. J Orofac Orthop. Dec 9–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
25
|
Burnside WM, Flecknell PA, Cameron AI and
Thomas AA: A comparison of medetomidine and its active enantiomer
dexmedetomidine when administered with ketamine in mice. BMC Vet
Res. 9:482013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin M, Izumi S and Nakane PK: Multilayer
peroxidase-labeled antibody method: Comparison with labeled
streptavidin-biotin method, avidin-biotin-peroxidase complex
method, and peroxidase-antiperoxidase method. J Clin Lab Anal.
9:424–430. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silverio-Ruiz KG, Martinez AE, Garlet GP,
Barbosa CF, Silva JS, Cicarelli RM, Valentini SR, Abi-Rached RS and
Junior CR: Opposite effects of bFGF and TGF-beta on collagen
metabolism by human periodontal ligament fibroblasts. Cytokine.
39:130–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei FL, Wang JH, Ding G, Yang SY, Li Y, Hu
YJ and Wang SL: Mechanical force-induced specific MicroRNA
expression in human periodontal ligament stem cells. Cells Tissues
Organs. 199:353–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe T, Yasue A and Tanaka E:
Hypoxia-inducible factor-1α is required for transforming growth
factor-β1-induced type I collagen, periostin and α-smooth muscle
actin expression in human periodontal ligament cells. Arch Oral
Biol. 59:595–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takayama I, Kii I and Kudo A: Expression,
purification and characterization of soluble recombinant periostin
protein produced by Escherichia coli. J Biochem. 146:713–723. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daines SM, Wang Y and Orlandi RR:
Periostin and osteopontin are overexpressed in chronically inflamed
sinuses. Int Forum Allergy Rhinol. 1:101–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dobreva MP, Lhoest L, Pereira PN, Umans L,
Camus A, de Sousa Lopes SM Chuva and Zwijsen A: Periostin as a
biomarker of the amniotic membrane. Stem Cells Int.
2012:9871852012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Liu J, Wang Z, Huang Y, Liu W, Zhu
X, Cai Y, Fang X, Lin S, Yuan L and Ouyang G: Periostin contributes
to the acquisition of multipotent stem cell-like properties in
human mammary epithelial cells and breast cancer cells. PLoS One.
8:e729622013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arima K, Ohta S, Takagi A, Shiraishi H,
Masuoka M, Ontsuka K, Suto H, Suzuki S, Yamamoto K, Ogawa M, et al:
Periostin contributes to epidermal hyperplasia in psoriasis common
to atopic dermatitis. Allergol Int. 64:41–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sorocos K, Kostoulias X, Cullen-McEwen L,
Hart AH, Bertram JF and Caruana G: Expression patterns and roles of
periostin during kidney and ureter development. J Urol.
186:1537–1544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kyutoku M, Taniyama Y, Katsuragi N,
Shimizu H, Kunugiza Y, Iekushi K, Koibuchi N, Sanada F, Oshita Y
and Morishita R: Role of periostin in cancer progression and
metastasis: Inhibition of breast cancer progression and metastasis
by anti-periostin antibody in a murine model. Int J Mol Med.
28:181–186. 2011.PubMed/NCBI
|
|
38
|
Kim BR, Jang IH, Shin SH, Kwon YW, Heo SC,
Choi EJ, Lee JS and Kim JH: Therapeutic angiogenesis in a murine
model of limb ischemia by recombinant periostin and its fasciclin I
domain. Biochim Biophys Acta. 1842:1324–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ladage D, Yaniz-Galende E, Rapti K,
Ishikawa K, Tilemann L, Shapiro S, Takewa Y, Muller-Ehmsen J,
Schwarz M, Garcia MJ, et al: Stimulating myocardial regeneration
with periostin Peptide in large mammals improves function
post-myocardial infarction but increases myocardial fibrosis. PLoS
One. 8:e596562013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wen W, Chau E, Jackson-Boeters L, Elliott
C, Daley TD and Hamilton DW: TGF-ss1 and FAK regulate periostin
expression in PDL fibroblasts. Journal of Dental Research.
89:1439–1443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hakuno D, Takahashi T, Lammerding J and
Lee RT: Focal adhesion kinase signaling regulates cardiogenesis of
embryonic stem cells. J Biol Chem. 280:39534–39544. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Watanabe T, Yasue A, Fujihara S and Tanaka
E: PERIOSTIN regulates MMP-2 expression via the αvβ3 integrin/ERK
pathway in human periodontal ligament cells. Arch Oral Biol.
57:52–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tanabe H, Takayama I, Nishiyama T,
Shimazaki M, Kii I, Li M, Amizuka N, Katsube K and Kudo A:
Periostin associates with Notch1 precursor to maintain Notch1
expression under a stress condition in mouse cells. PLoS One.
5:e122342010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kaku M and Yamauchi M: Mechano-regulation
of collagen biosynthesis in periodontal ligament. J Prosthodont
Res. 58:193–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Olson C, Uribe F, Kalajzic Z, Utreja A,
Nanda R, Rowe D and Wadhwa S: Orthodontic tooth movement causes
decreased promoter expression of collagen type 1, bone sialoprotein
and alpha-smooth muscle actin in the periodontal ligament. Orthod
Craniofac Res. 15:52–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ishikawa K: Periostin in the pathogenesis
of proliferative vitreoretinopathy. Nippon Ganka Gakkai Zasshi.
119:772–780. 2015.(In Japanese). PubMed/NCBI
|
|
49
|
Qin X, Yan M, Zhang J, Wang X, Shen Z, Lv
Z, Li Z, Wei W and Chen W: TGFβ3-mediated induction of Periostin
facilitates head and neck cancer growth and is associated with
metastasis. Sci Rep. 6:205872016. View Article : Google Scholar : PubMed/NCBI
|