Introduction
Anorectal malformations (ARMs) are common surgical
problem that affect 1/5,000 to 1/1,500 live births (1). Clinical manifestations include anal
stenosis, imperforate anus and persistent cloaca. Although numerous
technical advances have been made in the surgical treatment of
ARMs, certain patients continue to have postoperative anal
dysfunctions (1–5). Postoperative defecation problems may
occur as a result of maldeveloped pelvic floor muscles (PFMs),
abnormal innervation of PFMs, enteric nervous system developmental
disorders and sacral malformations (6–11).
Numerous efforts have been made to understand the mechanisms of
striated muscle complex (SMC) development in embryos. A previous
study demonstrated that dysregulated apoptosis may be one of the
fundamental factors leading to SMC maldevelopment in ARMs rats
(12). Another study revealed that
the spatiotemporal expression of Wnt5a was imbalanced during the
development of SMC in ARMs embryos (13). Despite the results of previous
studies, the mechanism of SMC development in ARMs remains to be
elucidated.
Wnt3a is an important member of the Wnt family of
signaling molecules, which has been demonstrated to promote
myoblast proliferation and differentiation (14,15).
Furthermore, primary myoblasts exhibited an increase in the number
of elongated myocytes and fused cells within 18 h of Wnt3a
treatment (16). A recent study
revealed that Wnt3a served an important role during hindgut
development, and the downregulation of Wnt3a may be associated, in
part, with the abnormal development of the terminal hindgut in ARMs
(17). However, it remains unclear
whether Wnt3a continues be involved in the development of the SMC
following the occurrence of ARMs. To provide insights into the
pattern of expression of Wnt3a and the possible role of Wnt3a
during SMC development, the present study examined the
spatiotemporal expression of Wnt3a in normal and ARM model rat
embryos.
Materials and methods
Animal model and tissue
collection
Mature Wistar rats of 10–12 weeks of age (250–300 g)
were obtained from the Experimental Animal Center, Shengjing
Hospital of China Medical University (Liaoning, China). The animals
were maintained in an temperature controlled environment (20–24°C),
humidity (50–70%) with a 9 h light/dark cycle. Solid laboratory
chow and water were available ad libitum. Ethical approval was
obtained from the China Medical University Animal Ethics Committee
prior to initiation of the study. A total of 55 time-mated pregnant
Wistar rats were gavage fed a single dose of either 1%
ethylenethiourea (ETU; 125 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to induce ARM (ARMs group) and 25 pregnant
Wistar rats were gavage fed an equal dose of saline (normal group)
at embryonic day 10 (E10); confirmed by E0-sperm in vaginal smear
after overnight mating. The pregnant rats were anesthetized with
pentobarbital sodium (40 mg/kg body weight; Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China). An incision was made in the
abdominal wall, and the uterine horn was exteriorized. The
resultant embryos were harvested by cesarean delivery on E17, E19
and E21. Following this, the pregnant rats were sacrificed with an
overdose of pentobarbitone sodium. The distribution of embryos used
for IHC, western blot and RT-qPCR in each group are presented in
Table I.
 | Table I.Distribution of embryos in the various
age and treatment groups. |
Table I.
Distribution of embryos in the various
age and treatment groups.
|
|
| Normal group | ARMs group |
|---|
|
|
|
|
|
|---|
| Age group | IHC | WB | RT-qPCR | IHC | WB | RT-qPCR |
|---|
| E17 | 17 | 20 | 21 | 21 | 26 | 25 |
| E19 | 12 | 18 | 16 | 18 | 24 | 23 |
| E21 | 11 | 16 | 12 | 17 | 22 | 22 |
| Total | 40 | 54 | 49 | 56 | 72 | 70 |
For immunohistochemical studies, normal and ARM
model rat embryos at various stages were fixed in 4%
paraformaldehyde/0.1 M PBS for 12 to 24 h, depending on their size.
Following fixation, embryos were embedded in paraffin in a routine
manner, sectioned sagittally (4 µm) and used for
immunohistochemical analysis. For western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses, SMCs were dissected under a light microscope and
immediately frozen and stored at −80°C. Since the SMC is thinner in
female fetal rats, only male fetuses were selected to use in this
study. Gender was determined by observing the gonad under a light
microscope; the testis has a characteristic ‘striped’ appearance,
whereas the ovary has a characteristic ‘spotty’ appearance.
Immunohistochemistry
Immunohistochemical staining was performed as
previously described (18). For
antigen retrieval, slides were incubated in boiling citrate buffer
(0.01 mol/l; pH 6.0) for 10 min and cooled to room temperature;
endogenous peroxidase activity was inhibited by incubation in 3%
H2O2 for 20 min at room temperature, and the
samples were blocked with 10% normal goat serum (ZSGB-BIO, Beijing,
China) to prevent nonspecific binding sites. Sections were
incubated overnight at 4°C with the primary rabbit anti-Wnt3a
polyclonal antibody (1:200; cat. no. 09–162; EMD Millipore,
Billerica, MA, USA). Following incubation, the primary antibody was
washed off, and the sections were incubated with biotinylated goat
anti-rabbit immunoglobulin G secondary antibody (catalog no.
sc-2054; 1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 15 min at room temperature. Immunoreactivity was visualized
with the addition of 3′,3-diaminobezidine (Merck KGaA); sections
were counterstained with hematoxylin for 10 sec at room
temperature. Specimens were mounted and images captured using a
Nikon E800 digitized microscope camera (Nikon Corporation, Tokyo,
Japan). Negative controls were performed by either omitting the
primary or secondary antibodies, or incubating with equivalent
concentrations of non-immune goat antiserum.
Protein preparation and western blot
analysis
Protein preparation was performed as described
previously (19). Total protein
was extracted from SMC samples collected from normal rat and ARM
model rat embryos by sonication in double-distilled H2O
containing protease inhibitors. Enhanced BCA Protein Assay Kit
(Beyotime Institute of Biotechnology, Shanghai, China) was applied
for protein quantification according to the manufacturer's
protocol. Protein extracts (50 µg) were heated at 90°C for 10 min,
size fractionated by 12% Bis-Tris SDS-PAGE (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and transferred to
polyvinylidene fluoride membranes (EMD Millipore). Membranes were
blocked with 5% fat-free milk in TBS at room temperature for 1 h
and incubated overnight at 4°C with primary antibodies against
Wnt3a (1:300) and β-actin (catalog no. 60008-1-Ig; 1:5,000;
ProteinTech Group, Inc., Chicago, IL, USA). The membrane was
subsequently incubated with a secondary antibody (1:2,000; catalog.
no. ZB-2301; ZSGB-BIO) for 1.5 h at room temperature), and the
immunostained bands were detected with a ProtoBlot II AP System
with a Stabilized Substrate (Promega Corporation, Madison, WI,
USA). Protein levels were normalized to β-actin.
RNA extraction and RT-qPCR
Total RNA was isolated from normal rat and ARM model
rat SMCs (~100 mg) using TRIzol (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The A260/A280 OD value of
the total RNA was between 1.8 and 2.0. The extracted RNA was
diluted to a concentration of 1 µg/µl, and aliquots were stored at
−80°C. Single-strand cDNA was reverse transcribed with the
PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's protocol. Primers used for
RT-qPCR included: Wnt3a, forward 5′-AGTCTCGTGGCTGGGTGGA-3′, and
reverse 5′-TTGGGCTCGCAGAAGTTAGG-3′; and β-actin (which was used as
an endogenous control), forward 5′-GGAGATTACTGCCCTGGCTCCTA-3′, and
reverse 5′-GACTCATCGTACTCCTGCTTGCTG-3′. RT-qPCR was performed in a
12.5 µl reaction volume, in triplicate for each specimen, using
SYBR Green PCR Master Mix (Takara Biotechnology Co., Ltd.) and a
LightCycler System (Roche Diagnostics GmbH, Mannheim, Germany).
qPCR cycling conditions were as follows: 10 min pre-denaturation at
95°C, followed by 40 cycles of 10 sec denaturation at 95°C, 60 sec
annealing at 60°C. qPCR results were analyzed with the LightCycler
1.5 System software. A dissociation procedure was performed to
generate a melting curve to confirm amplification specificity.
Relative levels of gene expression were determined as ΔCq=(Cq
gene)-(Cq reference), and the fold change in gene expression was
calculated using the 2-ΔΔCq method (20).
Statistical analysis
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was
used for statistical analyses. The Student's t-test was used to
compare the levels of Wnt3a expression between the normal rat and
ARM model rat groups. All results were expressed as means ±
standard deviation, and P<0.05 was considered to indicate a
statistically significant difference.
Results
General observation
In the present study, no malformations were observed
in the 143 normal male rat embryos examined. A total of 198 ARM
model rat embryos were obtained from 432 ETU-treated male rat
embryos. All ETU-treated embryos exhibited a short or absent tail,
and 11 died in utero. Externally visible spinal bifida and/or
meningocele were also observed. The total incidence of ARMs
(manifesting as rectourethral fistula or common cloaca) in
ETU-treated embryos from E17 to E21 was 62.8%. As denervation may
affect the development of SMC (21), specimens with spina bifida aperta
were excluded.
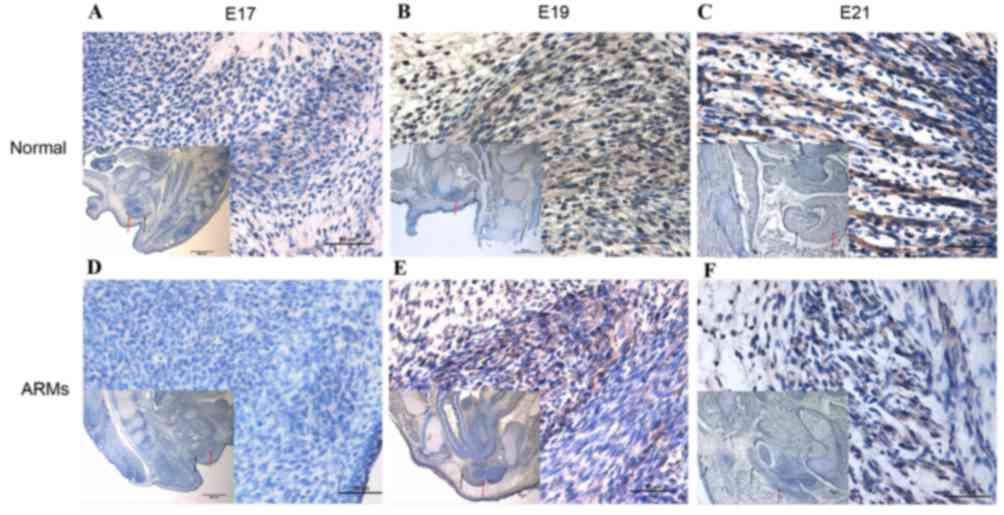
Immunohistochemical results
Wnt3a-positive cells were present in the SMC at E17
(Fig. 1A). At E19, the SMC was
very distinct, and the number of Wnt3a-positive cells that were
detected in the SMC and bulbocavernosus muscle increased (Fig. 1B). At E21, increasingly more
Wnt3a-positive stained cells were detected in the SMC (Fig. 1C). Results were varied in ARM model
rat embryos. At E17, sporadic positive-staining cells were detected
in the SMC (Fig. 1D). From E19,
although the number of Wnt3a-labeled cells in the SMC increased,
there were markedly fewer positive cells compared with normal rats
(Fig. 1E). Cells exhibiting Wnt3a
immunoreactivity increased gradually at E21 (Fig. 1F).
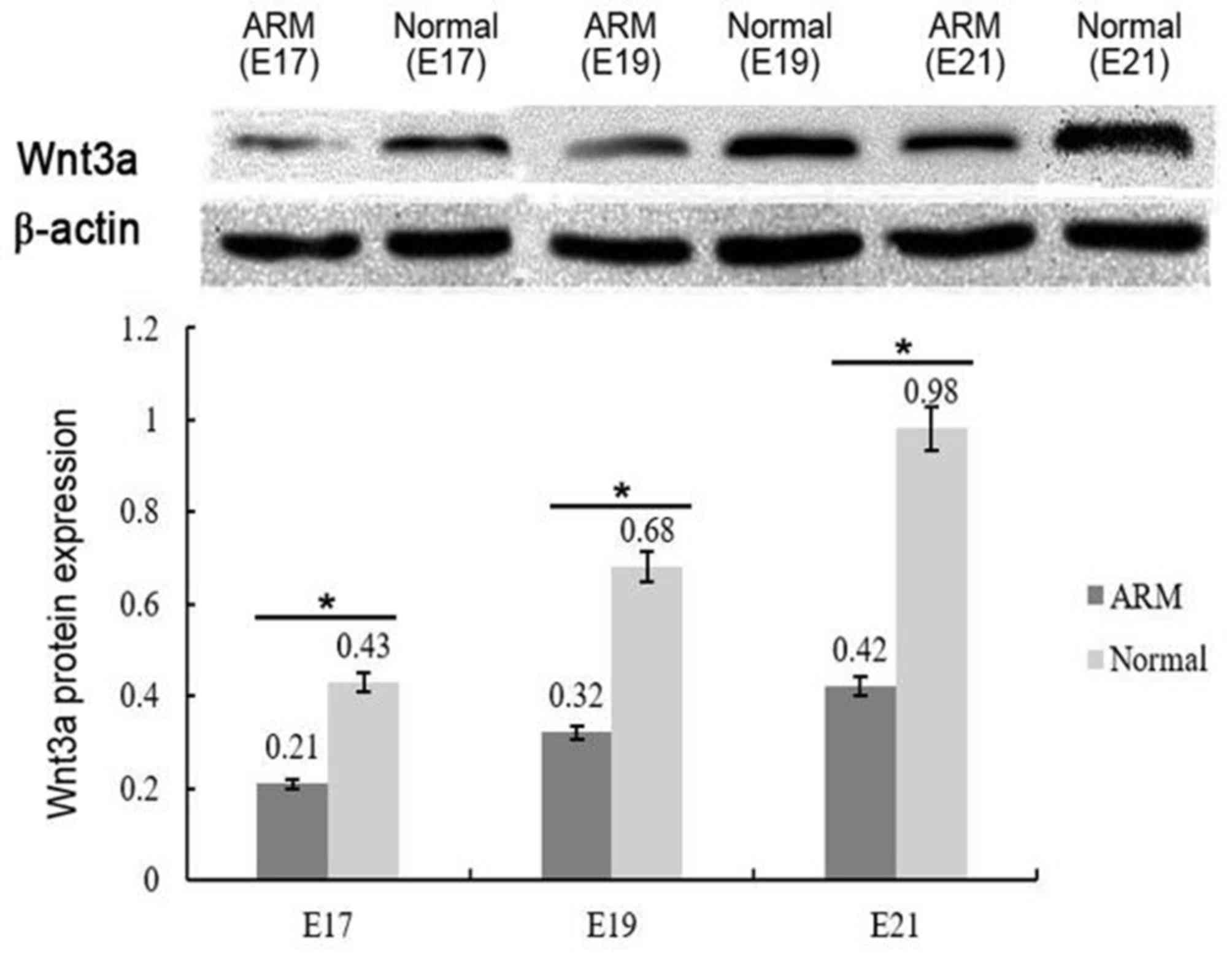
Western blot analysis
E17, 19 and 21 are key stages of SMC development.
Western blot analysis was performed to quantify the levels of Wnt3a
protein expression during SMC development from normal and ARM model
rats (Fig. 2). Wnt3a bands (~37
kDa) were normalized to β-actin. Although Wnt3a expression
gradually increased over time in both groups, the levels of Wnt3a
protein expression were significantly lower in the SMCs from ARM
model rats compared with the normal group (P<0.05): E17,
0.21±0.02 (ARMs group) compared with 0.43±0.02 (normal group); E19,
0.32±0.01 (ARMs group) compared with 0.68±0.04 (normal group); and
E21, 0.42±0.03 (ARMs group) compared with 0.98±0.05 (normal
group).
RT-qPCR analysis
The levels of Wnt3a mRNA expression were normalized
to β-actin from the same specimen. Consistent with the results
obtained from western blotting, the levels of Wnt3a mRNA expression
increased over time in both the normal group and the ARMs group.
Similarly, the levels of Wnt3a mRNA were significantly higher in
the normal group (2.38, 3.23 and 4.06 fold, at E17, E19 and E21,
respectively) compared with embryos in the ARMs group (P<0.05;
Table II).
 | Table II.Relative levels of Wnt3a mRNA
expression. |
Table II.
Relative levels of Wnt3a mRNA
expression.
| Group | Wnt3a Cq value | β-actin Cq value | ∆Cq | ∆∆Cq | Fold change (vs.
corresponding ARMs group) |
|---|
| A17 | 26.57±1.34 | 23.91±1.72 | 2.66 | 0.00 | 1.00 |
| N17 | 25.45±1.58 | 24.04±1.23 | 1.41 | −1.25 | 2.38 |
| A19 | 25.98±1.43 | 23.74±1.61 | 2.24 | 0.00 | 1.00 |
| N19 | 24.46±1.65 | 23.91±1.31 | 0.55 | −1.69 | 3.23 |
| A21 | 25.43±1.25 | 23.52±1.68 | 1.91 | 0.00 | 1.00 |
| N21 | 23.79±1.65 | 23.68±1.21 | −0.11 | −2.02 | 4.06 |
Discussion
Dysregulation of the stem cell signaling network due
to epigenetic and genetic alterations may lead to congenital
abnormalities (22–24). The Wnt signaling cascade has been
demonstrated to have a certain amount of crosstalk with other
signaling pathways, including hedgehog, bone morphogenetic protein
(BMP)/transforming growth factor β, fibroblast growth factor and
Notch, which together constitute the stem-cell signaling network
(25–27). Wnt3a has been demonstrated to serve
a crucial role in muscle development, providing positional cues to
specify the myotomes and inducing Myogenic regulatory factor (MRFs)
expression (28–30). Wnt3a has also been revealed to
serve a role during the development of ARMs (17); however, the functions of Wnt3a
during SMC development with or without the concurrent development
of ARMs remain to be elucidated. The present study investigated the
potential role of Wnt3a during SMC development by examining the
spatiotemporal expression patterns of Wnt3a mRNA and protein in
normal and ARM model rat SMCs at different embryonic developmental
stages.
In normal rats, Wnt3a-positive stained cells were
localized in the SMC and gradually increased from E17 to E21; at
E21, Wnt3a-positive cells were also detected in the bulbocavernosus
muscle. By comparison, although Wnt3a expression in ARM model
embryos also increased from E17 to E21, the levels of Wnt3a
expression were lower compared with normal embryos. These results
indicate that there was a relative spatiotemporal imbalance between
the normal and ARM model embryos during the SMC development. Our
previous studies have demonstrated that the crucial period for SMC
morphogenesis is from E17 to E19, and original skeletal muscle
fibers gradually fuse into mature skeletal muscle fibers after E19
(31). Based on the results of the
present study, the increase in Wnt3a expression in normal embryos
occurs at a critical time during SMC development (E17-E21); the
increasing levels of Wnt3a expression indicates that Wnt3a may be
important for SMC development. Conversely, the decreased level of
Wnt3a expression in ARM model rat embryos may affect the
conformation of the original skeletal muscle fibers, resulting in
the maldevelopment of the SMC during this essential stage of
development.
The genetics of ARM development is extremely
complex, and morphological changes of the SMC take place after the
occurrence of abnormal anorectum in rats with ARMs (31). The present study hypothesized that
maldevelopment of the rectum influenced the development of the SMC
to a certain extent. A previous study emphasized the importance of
the rectum in the development of the sphincter (32). This is corroborated by the results
of the present study, which demonstrated that the spatiotemporal
expression of Wnt3a was imbalanced during the development of SMC in
ARMs embryos; a trend consistent with that demonstrated in terminal
hindgut development (17), which
suggests that the rectum may be crucial in the development of SMC.
Tanaka et al (15)
hypothesized that Wnt signaling through β-catenin may act as a
molecular switch to regulate the transition from cell proliferation
to myogenic differentiation. In the present study, the levels of
Wnt3a expression during the development of SMC in ARM model rat
embryos was lower than in normal rat embryos of the same
gestational age. The present study hypothesized that the
downregulation of Wnt3a expression inhibited the transition of
myogenic progenitor cells from proliferation to myogenic
differentiation, which caused a delay in SMC maturation.
Subsequently, considerable amounts of connective tissue infiltrated
the intermuscular bundles, which resulted in the malformation of
the SMC in ARMs rats.
The present study demonstrated that the
spatiotemporal expression of Wnt3a was imbalanced during the
embryonic development of the SMC in ARM model rats, which may
contribute to poor SMC development. In conclusion, results from the
present study, combined with results from previous studies, suggest
that Wnt3a is extremely important for terminal hindgut and SMC
development in ARMs rat embryos. As numerous signaling molecules
have been shown to be expressed and function during different
phases of SMC development, the present study was unable to
substantiate whether Wnt3a expression was the initial event that
lead to SMC malformation. Further studies are required to elucidate
the other signaling pathways that are involved in regulating SMC
formation during embryonic development and to clarify the
underlying molecular mechanisms mediating the maldevelopment of
SMC. Understanding these mechanisms may help to establish potential
therapeutic strategies to reduce skeletal muscle wasting and
maintain physiologic function.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170334 and
81270436).
References
|
1
|
Peña A, Guardino K, Tovilla JM, Levitt MA,
Rodriguez G and Torres R: Bowel management for fecal incontinence
in patients with anorectal malformations. J Pediatr Surg.
33:133–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levitt MA and Peña A: Outcomes from the
correction of anorectal malformations. Curr Opin Pediatr.
17:394–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sonnino RE, Reinberg O, Bensoussan AL,
Laberge JM and Blanchard H: Gracilis muscle transposition for anal
incontinence in children: Long-term follow-up. J Pediatr Surg.
26:1219–1223. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai Y, Yuan Z and Wang W, Zhao Y, Wang H
and Wang W: Quality of life for children with fecal incontinence
after surgically corrected anorectal malformation. J Pediatr Surg.
35:462–464. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rintala RJ and Lindahl H: Is normal bowel
function possible after repair of intermediate and high anorectal
malformations? J Pediatr Surg. 30:491–494. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rintala R: Postoperative internal
sphincter function in anorectal malformation: A manometric study.
Pediatr Surg Int. 5:127–130. 1990. View Article : Google Scholar
|
|
7
|
Li L, Li Z, Wang LY and Xiao FD: Anorectal
anomaly: Neuropathological changes in the sacral spinal cord. J
Pediatr Surg. 28:880–885. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan Z, Bai Y, Zhang Z, Ji S, Li Z and
Wang W: Neural electrophysiological studies on the external anal
sphincter in children with anorectal malformation. J Pediatr Surg.
35:1052–1057. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernández-Fraga X, Azpiroz F and
Malagelada JR: Significance of pelvic floor muscles in anal
incontinence. Gastroenterology. 123:1441–1450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan ZW, Lui VC and Tam PK: Deficient
motor innervation of the sphincter mechanism in fetal rats with
anorectal malformation: A quantitative study by fluorogold
retrograde tracing. J Pediatr Surg. 38:1383–1388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia H, Zhang K, Zhang S, Yuan Z, Bai Y and
Wang W: Quantitative analysis of sacral parasympathetic nucleus
innervating the rectum in rats with anorectal malformation. J
Pediatr Surg. 42:1544–1548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen QJ, Jia HM, Zhang SW, Bai YZ, Yuan ZW
and Wang WL: Apoptosis during the development of pelvic floor
muscle in anorectal malformation rats. J Pediatr Surg.
44:1884–1891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mi J, Chen D, Ren X, Jia H, Gao H and Wang
W: Spatiotemporal expression of Wnt5a during the development of the
striated muscle complex in rats with anorectal malformations. Int J
Clin Exp Pathol. 7:1997–2005. 2014.PubMed/NCBI
|
|
14
|
Pansters NA, Velden JL vander, Kelders MC,
Laeremans H, Schols AM and Langen RC: Segregation of myoblast
fusion and muscle-specific gene expression by distinct
ligand-dependent inactivation of GSK-3β. Cell Mol Life Sci.
68:523–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka S, Terada K and Nohno T: Canonical
Wnt signaling is involved in switching from cell proliferation to
myogenic differentiation of mouse myoblast cells. J Mol Signal.
6:12–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhuang L, Hulin JA, Gromova A, Nguyen TD
Tran, Yu RT, Liddle C, Downes M, Evans RM, Makarenkova HP and Meech
R: Barx2 and Pax7 have antagonistic functions in regulation of Wnt
signaling and satellite cell differentiation. Stem Cells.
32:1661–1673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren X, Mi J, Jia H, Gao H, Bai Y and Wang
W: Reduced Wnt3a expression correlates with poor development of the
hindgut in rats with anorectal malformations. Exp Mol Pathol.
99:81–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pluznick JL, Wei P, Grimm PR and Sansom
SC: BK-{beta1} subunit: Immunolocalization in the mammalian
connecting tubule and its role in the kaliuretic response to volume
expansion. Am J Physiol Renal Physiol. 288:846–854. 2005.
View Article : Google Scholar
|
|
19
|
Mandhan P, Quan QB, Beasley S and Sullivan
M: Sonic hedgehog, BMP4 and Hox genes in the development of
anorectal malformations in ethylenethiourea-exposed fetal rats. J
Pediatr Surg. 41:2041–2045. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adhihetty PJ, O'Leary MF, Chabi B, Wicks
KL and Hood DA: Effect of denervation on mitochondrially mediated
apoptosis in skeletal muscle. J Appl Physiol (1985). 102:1143–1151.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katoh M: Dysregulation of stem cell
signaling network due to germline mutation, SNP, Helicobacter
pylori infection, epigenetic change and genetic alteration in
gastric cancer. Cancer Biol Ther. 6:832–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katoh M: Cancer genomics and genetics of
FGFR2 (Review). Int J Oncol. 33:233–237. 2008.PubMed/NCBI
|
|
24
|
Katoh Y and Katoh M: Hedgehog signaling,
epithelial-to-mesenchymal transition and miRNA (review). Int J Mol
Med. 22:271–275. 2008.PubMed/NCBI
|
|
25
|
Garciadiego-Cazares D, Rosales C, Katoh M
and Chimal-Monroy J: Coordination of chondrocyte differentiation
and joint formation by alpha5beta1 integrin in the developing
appendicular skeleton. Development. 131:4735–4742. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katoh M: WNT signaling in stem cell
biology and regenerative medicine. Curr Drug Targets. 9:565–570.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bailey J, Singh PK and Hollingsworth MA:
Cancer metastasis facilitated by developmental pathways: Sonic
hedgehog, Notch, and bone morphogenetic proteins. J Cell Biochem.
102:829–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tajbakhsh S, Borello U, Vivarelli E, Kelly
R, Papkoff J, Duprez D, Buckingham M and Cossu G: Differential
activation of Myf5 and MyoD by different Wnts in explants of mouse
paraxial mesoderm and the later activation of myogenesis in the
absence of Myf5. Development. 125:4155–4162. 1998.PubMed/NCBI
|
|
29
|
Takata H, Terada K, Oka H, Sunada Y,
Moriguchi T and Nohno T: Involvement of Wnt4 signaling during
myogenic proliferation and differentiation of skeletal muscle. Dev
Dyn. 236:2800–2807. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsivitse S: Notch and Wnt signaling,
physiological stimuli and postnatal myogenesis. Int J Biol Sci.
6:268–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang SW, Bai YZ, Zhang SC, Wang DJ, Zhang
T, Zhang D and Wang WL: Embryonic development of the striated
muscle complex in rats with anorectal malformations. J Pediatr
Surg. 43:1452–1458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bourdelat D and Barbet JP: Morphological
differentiation of the anorectal sphincter in the human embryo and
fetus. Chir Pediatr. 31:12–17. 1990.(In French). PubMed/NCBI
|