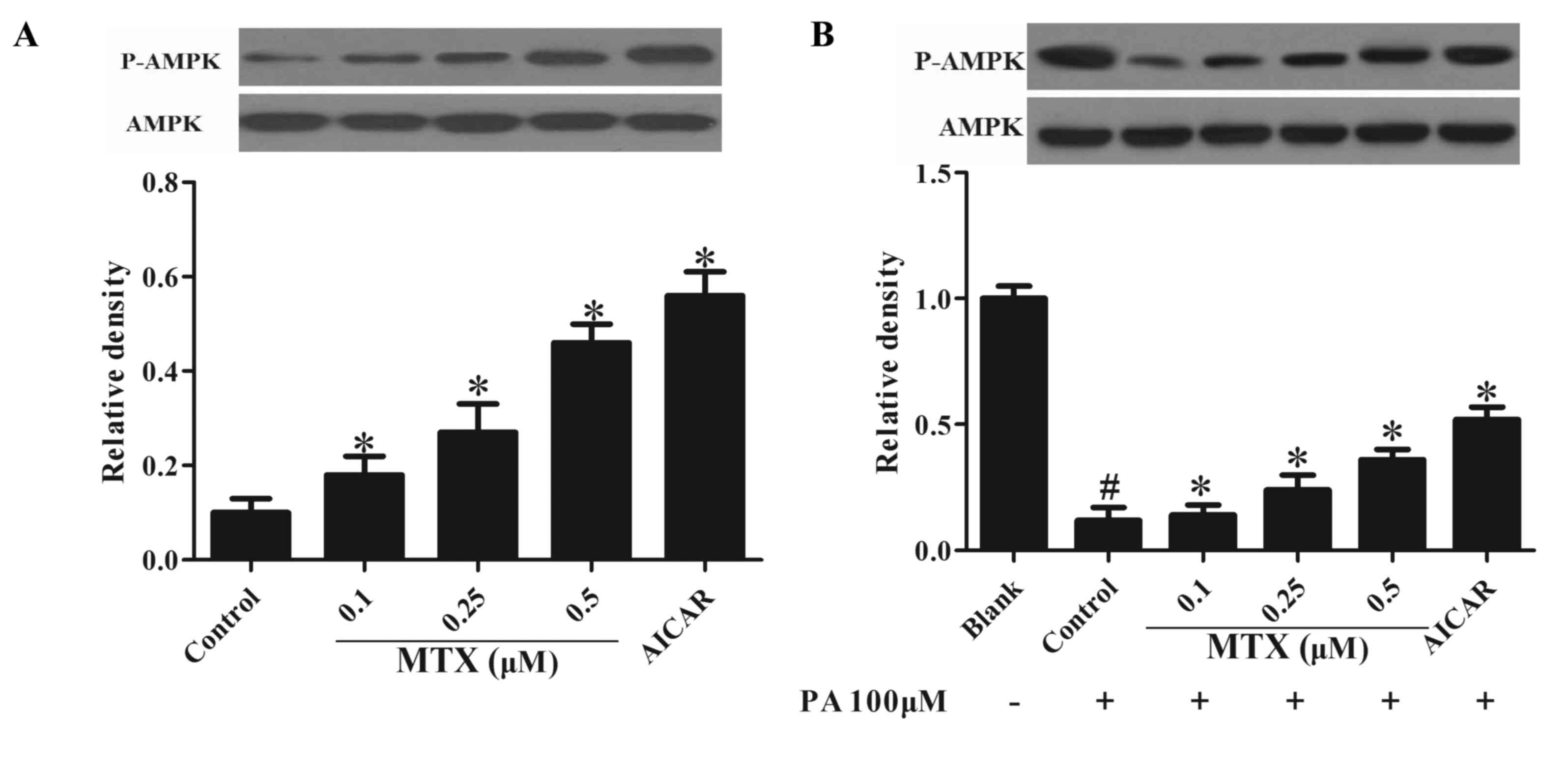
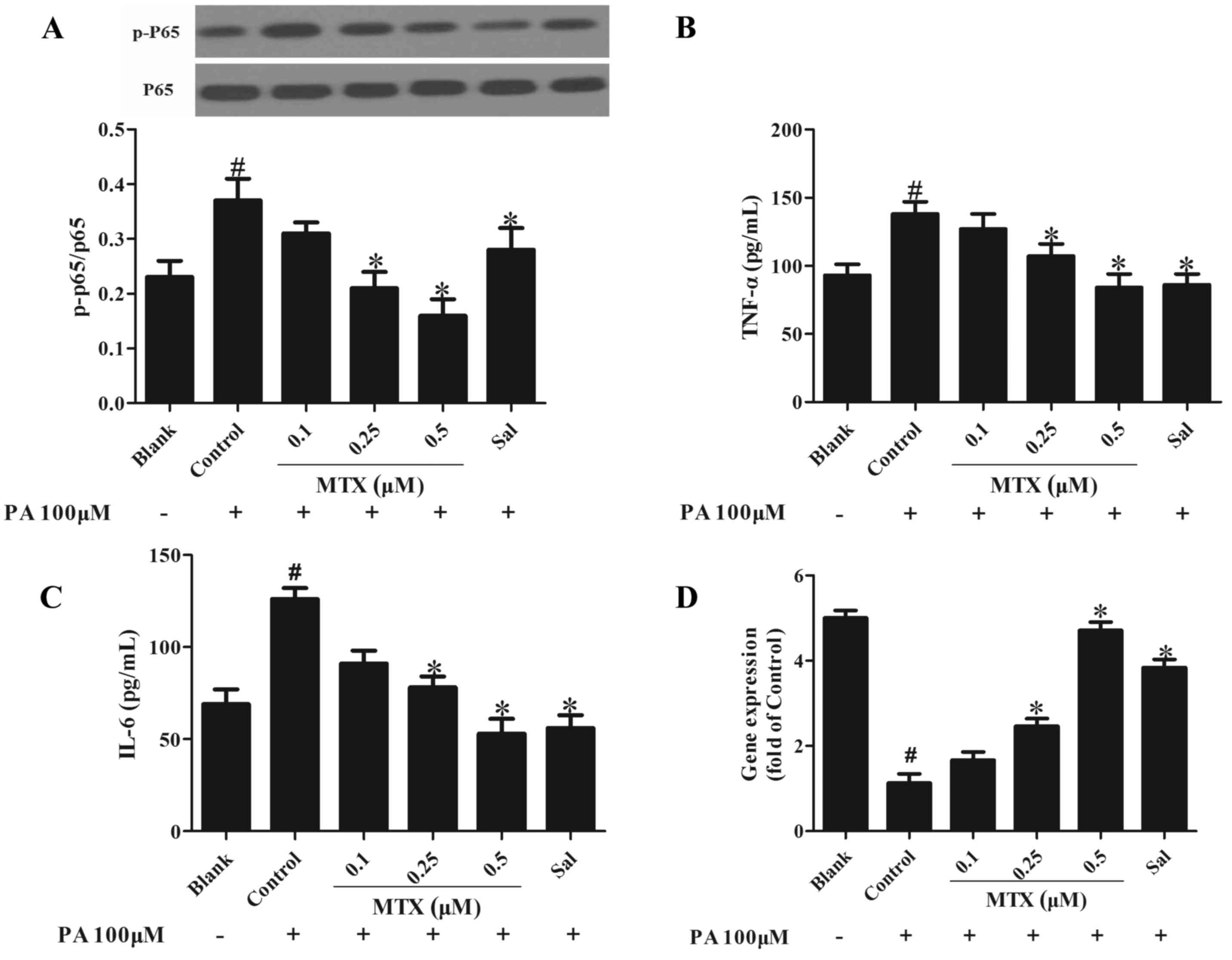
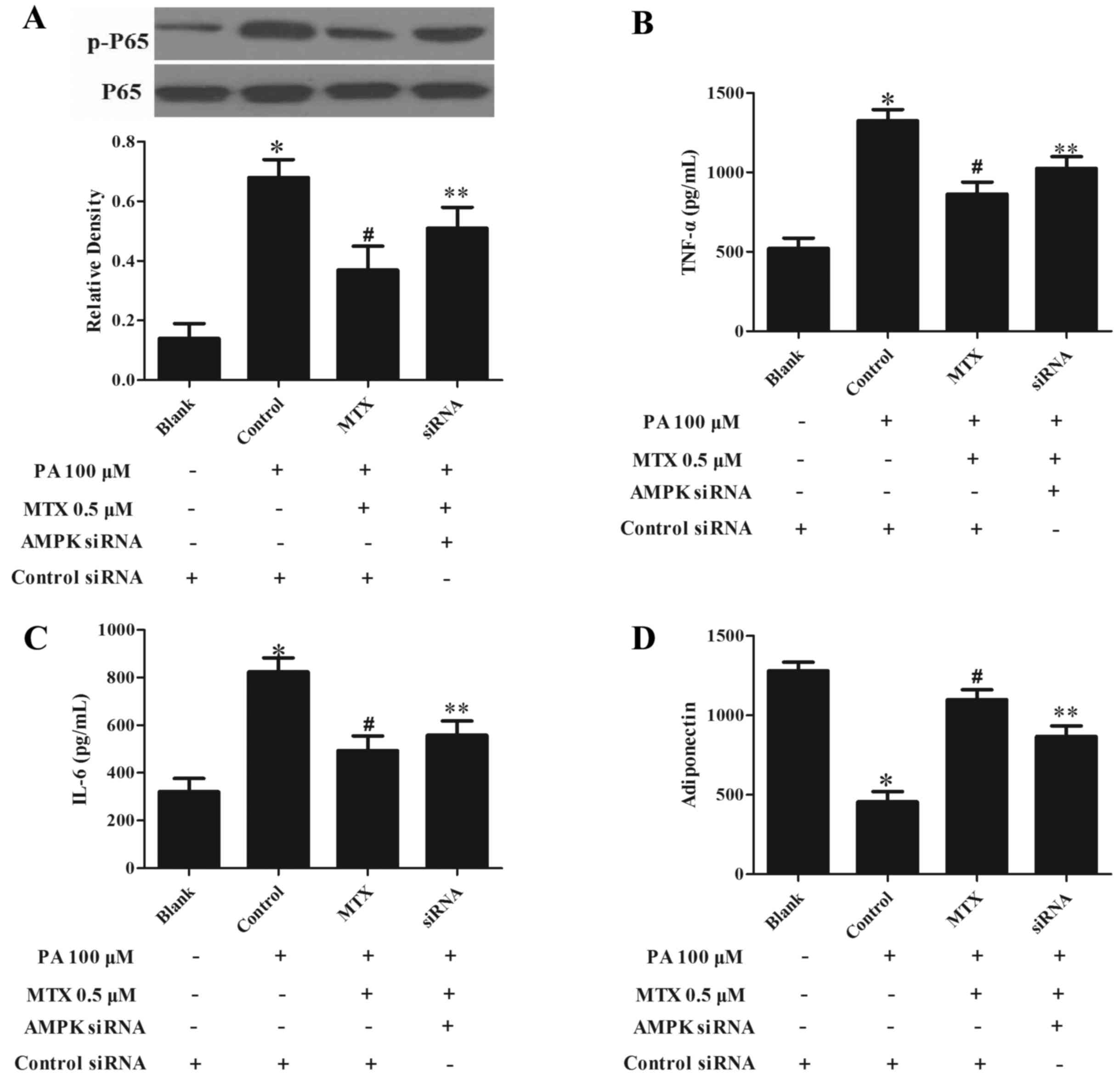
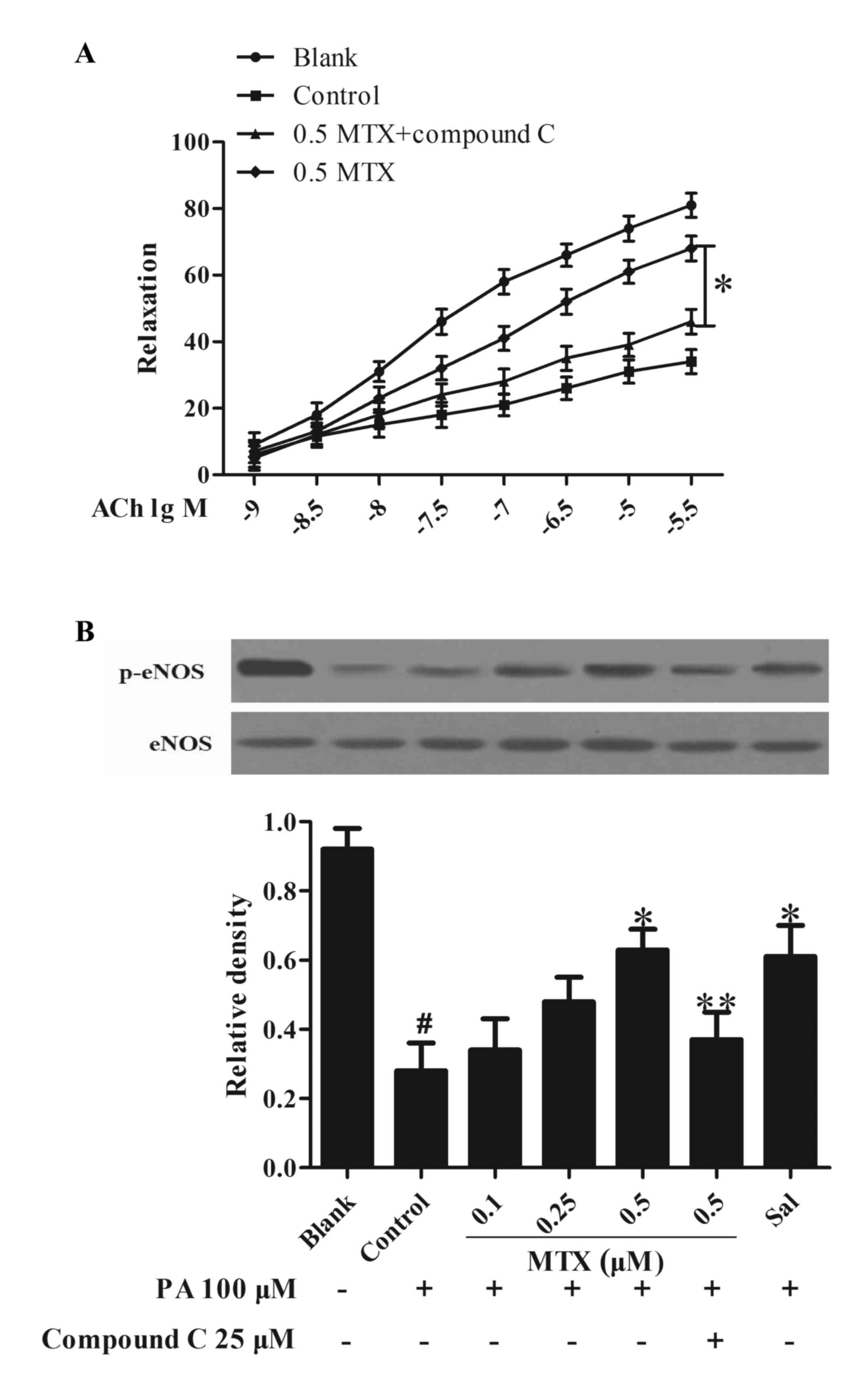
|
1
|
World Health Statistics, . 2012, World
Health Organization. Geneva: 2012
|
|
2
|
Vita JA and Keaney JF: Endothelial
function: A barometer for cardiovascular risk? Circulation.
106:640–642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bijland S, Mancini SJ and Salt IP: Role of
AMP-activated protein kinase in adipose tissue metabolism and
inflammation. Clin Sci (Lond). 124:491–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gauthier MS, O'Brien EL, Bigornia S, Mott
M, Cacicedo JM, Xu XJ, Gokce N, Apovian C and Ruderman N: Decreased
AMP-activated protein kinase activity is associated with increased
inflammation in visceral adipose tissue and with whole-body insulin
resistance in morbidly obese humans. Biochem Bioph Res Commun.
404:382–387. 2011. View Article : Google Scholar
|
|
6
|
Tsai KL, Chen LH, Chiou SH, Chiou GY, Chen
YC, Chou HY, Chen LK, Chen HY, Chiu TH, Tsai CS, et al: Coenzyme
Q10 suppresses oxLDL-induced endothelial oxidative injuries by the
modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH
oxidase signaling pathway. Mol Nutr Food Res. 55 Suppl 2:S227–S240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Li J, Xiao N, Wang M, Kou J, Qi L,
Huang F, Liu B and Liu K: Pharmacological activation of AMPK
ameliorates perivascular adipose/endothelial dysfunction in a
manner interdependent on AMPK and SIRT1. Pharmacol Res. 89:19–28.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saag KG, Teng GG, Patkar NM, Anuntiyo J,
Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M,
et al: American College of Rheumatology 2008 recommendations for
the use of nonbiologic and biologic disease-modifying antirheumatic
drugs in rheumatoid arthritis. Arthritis Rheum. 59:762–784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ridker PM: Testing the inflammatory
hypothesis of atherothrombosis: Scientific rationale for the
cardiovascular inflammation reduction trial (CIRT). J Thromb
Haemost. 7 Suppl 1:S332–S339. 2009. View Article : Google Scholar
|
|
10
|
Bulgarelli A, Dias AA Martins, Caramelli B
and Maranhão RC: Treatment with methotrexate inhibits atherogenesis
in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 59:308–314.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mugabo Y, Mukaneza Y and Renier G:
Palmitate induces C-reactive protein expression in human aortic
endothelial cells. Relevance to fatty acid-induced endothelial
dysfunction. Metabolism. 60:640–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calles-Escandon J and Cipolla M: Diabetes
and endothelial dysfunction: A clinical perspective. Endocr Rev.
22:36–52. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gollasch M: Vasodilator signals from
perivascular adipose tissue. Br J Pharmacol. 165:633–642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steinberg GR, Michell BJ, van Denderen BJ,
Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Görgün CZ,
Carling D, et al: Tumor necrosis factor alpha-induced skeletal
muscle insulin resistance involves suppression of AMP-kinase
signaling. Cell Metab. 4:465–474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng HB, Libby P and Liao JK: Induction
and stabilization of I kappa B alpha by nitric oxide mediates
inhibition of NF-kappa B. J Biol Chem. 270:14214–14219. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maloney E, Sweet IR, Hockenbery DM, Pham
M, Rizzo NO, Tateya S, Handa P, Schwartz MW and Kim F: Activation
of NF-kappaB by palmitate in endothelial cells: A key role for
NADPH oxidase-derived superoxide in response to TLR4 activation.
Arterioscl Throm Vas Biol. 29:1370–1375. 2009. View Article : Google Scholar
|
|
18
|
Félétou M and Vanhoutte PM: Endothelial
dysfunction: A multifaceted disorder (The Wiggers Award Lecture).
Am J Physiol Heart Circ Physiol. 291:H985–H1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cersosimo E and DeFronzo RA: Insulin
resistance and endothelial dysfunction: The road map to
cardiovascular diseases. Diabetes Metab Res Rev. 22:423–436. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moncada S: Nitric oxide in the
vasculature: Physiology and pathophysiology. Ann NY Acad Sci.
811:60–69. 1997. View Article : Google Scholar : PubMed/NCBI
|