Introduction
Clinical trauma patients are frequently diagnosed
with Staphylococcus aureus (S. aureus) and tetanus
infections, particularly those in developing countries (1,2).
These infections have contributed greatly to the morbidity and
mortality rates of neonates in developing countries such as Nigeria
(3). S. aureus colonizes in
approximately one-third of the human population (4), and is responsible for various
diseases ranging from skin and soft tissue infections to
life-threatening septicemia with metastatic complications (5). Tetanus is caused by tetanospasmin, a
neurotoxin produced by the obligate anaerobic bacterium
Clostridium tetani. Although the incidence of tetanus is low
in developed countries, the worldwide mortality rates range from
6–72% (6), depending on the
medical condition. In 2013, the World Health Organization estimated
that ~49,000 newborns died from neonatal tetanus (7).
Combination vaccines are a reliable strategy to
protect against two or more pathogens, and may reduce costs of mass
vaccination and disposables (8).
Those susceptible to trauma, including athletes, soldiers and the
police, as well as women of childbearing age in developing
countries, may benefit from the development of a combined vaccine
against S. aureus and tetanus infections. S. aureus
surface protein A (SasA), a cell wall-anchored protein of S.
aureus, consisting of 2,271 amino acid residues, is a potential
vaccine candidate for S. aureus infections (9). SasA has been reported to be a
virulence determinant in endovascular infections, as the SasA
mutant strain was observed to decrease the ability of catheterized
rabbits to form vegetative plaques on heart valves (10). In addition, SasA is prevalent in
S. aureus clinical isolates, and is expressed during in
vivo growth of the bacteria (11). Notably, immunization with SasA was
able to protect mice against a lethal S. aureus challenge
(9). The tetanus toxin is a 150
kDa protein that has three domains (12), including the N-terminal zinc
endopeptidase domain (13), the
internal heavy chain translocation domain and the C-terminal heavy
chain receptor-binding domain known as the C-fragment of tetanus
neurotoxin (TeNT-Hc) (14). The
genetic recombinant TeNT-Hc, which is nontoxic but has ganglioside
binding activities, has been proposed as a possible replacement for
the tetanus toxoid vaccine (15).
TeNT-Hc is expected to demonstrate clear advantages over the
existing tetanus toxoid vaccine with regard to ease of production,
homogeneity and characterization.
In the present study, mice were immunized with a
combined vaccine consisting of SasA (9) and TeNT-Hc (16) formulated by adsorption to
Alhydrogel. The antibody titers and protective efficacy were
measured for individual and combined vaccine administrations. The
results demonstrated that the combined vaccine was able to induce
protective immunity against S. aureus and tetanus
neurotoxin challenges.
Materials and methods
Bacterial strains and growth
conditions
The S. aureus strain USA300 (BAA-1556™;
American Type Culture Collection, Manassas, VA, USA) was cultivated
in tryptic soy broth (TSB; tryptone 15 g/l, soybean peptone 5 g/l,
NaCl 5 g/l, pH 7.2, used after 121°C, 20 min) at 37°C. The
Escherichia coli (E. coli) strain BL21 (DE3; Merck
Millipore, Darmstadt, Germany) were cultivated in Luria-Bertani
broth (tryptone 10 g/l, yeast extract 5 g/l, NaCl 10 g/l, pH 7.2,
used after 121°C, 20 min) at 37°C and 100 µg/ml ampicillin (10 g
ampicillin powder dissolved in 100 ml H2O, used after
filtration and diluted 1:1,000) was used for plasmid selection.
Subunit antigens
The antigens used in the current study were
expressed in E. coli BL21 (DE3) and purified to >90%. The
purification protocols were the same as described previously
(9,16). Briefly, the polyhistidine-tagged
recombinant SasA (rSasA) was purified by anion exchange, HisTrap
and gel filtered chromatography (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA). The non-tagged TeNT-Hc antigen was purified
by anion exchange, hydrophobic interactions and gel-filtered
chromatography (GE Healthcare Bio-Sciences).
Active immunization
A total of 40 female BALB/c mice (age, 6–8 weeks;
weight, 18–20 g) were purchased from The Beijing Laboratory Animal
Center (Beijing, China), and were divided into 4 groups of 10 mice
each. All mice had free access to food/water and were raised under
standard conditions (temperature 25±2°C, relative humidity 50±10%)
with a dark/light cycle (14/10 h). The mice were immunized
intraperitoneally with 10 µg rSasA, 10 µg TeNT-Hc, 10 µg rSasA + 10
µg TeNT-Hc adsorbed to 0.75 mg aluminum hydroxide adjuvant
(Brenntag Biosector A/S, Frederikssund, Denmark). The 0.75 mg
aluminum hydroxide adjuvant was used as a negative control. Mice
were injected at weeks 0, 2 and 4. Blood samples were drawn at
weeks 2, 4 and 6 and every 4 weeks thereafter until week 26 through
the tail vein and screened for reactivity to rSasA or TeNT-Hc
antigens. Subsequent to the experiments, all mice were sacrificed
by CO2 asphyxiation. All experiments were performed in
agreement with the institutional guidelines approved by the
Laboratory Animal Care and Use Committee of the Beijing Institute
of Biotechnology (IACUC of AMMS-08-2014-006).
Serological analysis of
antibodies
Serum antibody titers of the antigen-specific total
IgG, IgG1 and IgG2a were determined by enzyme-linked immunosorbent
assay (ELISA). Microplates (96-well) were coated overnight with
rSasA or TeNT-Hc (2 µg/ml) in coating buffer (50 mM carbonate
buffer, pH 9.6) at 4°C. The plates were blocked with 2% (w/v)
bovine serum albumin (2 g dissolved in 100 ml H2O;
Sigma-Aldrich; Merck Millipore) in PBS at 37°C for 1 h. Duplicate
two-fold serial dilutions of serum in an appropriate range
(1:100~1:409,600) were incubated in the plates at 37°C for 1 h
followed by washing with PBS with 3% Tween 20 (PBST). Then
horseradish peroxidase-labelled goat anti-mouse total IgG, IgG1
(ab97240; 1:10,000) or IgG2a (ab97245; 1:10,000) antibodies (Abcam,
Cambridge, MA, USA) were applied for 1 h at 37°C and then washed
with PBST. The plates were incubated with
3,3′,5,5′-tetramethylbenzidine dihydrochloride substrate
(Sigma-Aldrich; Merck Millipore) at room temperature for 10 min in
the dark. The colorimetric reaction was stopped with 2 M sulfuric
acid, and the optical density at 450 nm (OD450) was read
in a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Each step was followed by washing three times with PBST. The
antibody-positive cut-off values were set as two times greater than
the OD450 means of the pre-immunized sera. The ELISA
antibody titer was expressed as the highest serum dilution showing
a positive reaction.
S. aureus challenge model
Overnight cultures of S. aureus strain USA300
were diluted 1:100 in fresh TSB and cultivated at 37°C until
reaching the mid-late logarithmic phase. S. aureus challenge
was performed as described previously (17). Immunized mice were challenged by
intraperitoneal injection with a lethal dose of S. aureus
USA300 (3×109 colony-forming units in 100 µl/mouse), at 6 weeks
after the primary immunization. Infected animals were monitored for
survival for 5 days.
TeNT challenge model
At 6 weeks following primary immunization, the mice
were challenged with (2×103)xLD50s (the LD50 in mice was determined
by the improved Karber method (18). The LD50 of tetanus neurotoxin was
~15.8 ng/kg in mice) of tetanus neurotoxin [in 0.5 ml
borate-buffered saline (0.5 g borax, 4.5 g boric acid and 8.5 g
sodium chloride in 1 l distilled water)] by subcutaneous injection.
Survival was monitored for five days.
Statistical analysis
Unpaired Student's two-tailed t-test was used to
analyze the differences in ELISA titers between the groups. To
compare the survival rates in the challenge models, experiments
were analyzed using the Gehan-Breslow-Wilcoxon test with GraphPad
Prism 5.01 (GraphPad Software, Inc., La Jolla, CA, USA). P≤0.05 was
considered to indicate a statistically significant difference.
Results
Antibody titer following
immunization
A total of 3 groups of 10 mice were
intraperitoneally immunized three times (at weeks 0, 2 and 4) with
10 µg rSasA, 10 µg TeNT-Hc or 10 µg rSasA + 10 µg TeNT-Hc. Blood
samples were collected periodically through the tail vein to assay
antigen-specific antibody responses by ELISA. The responses were
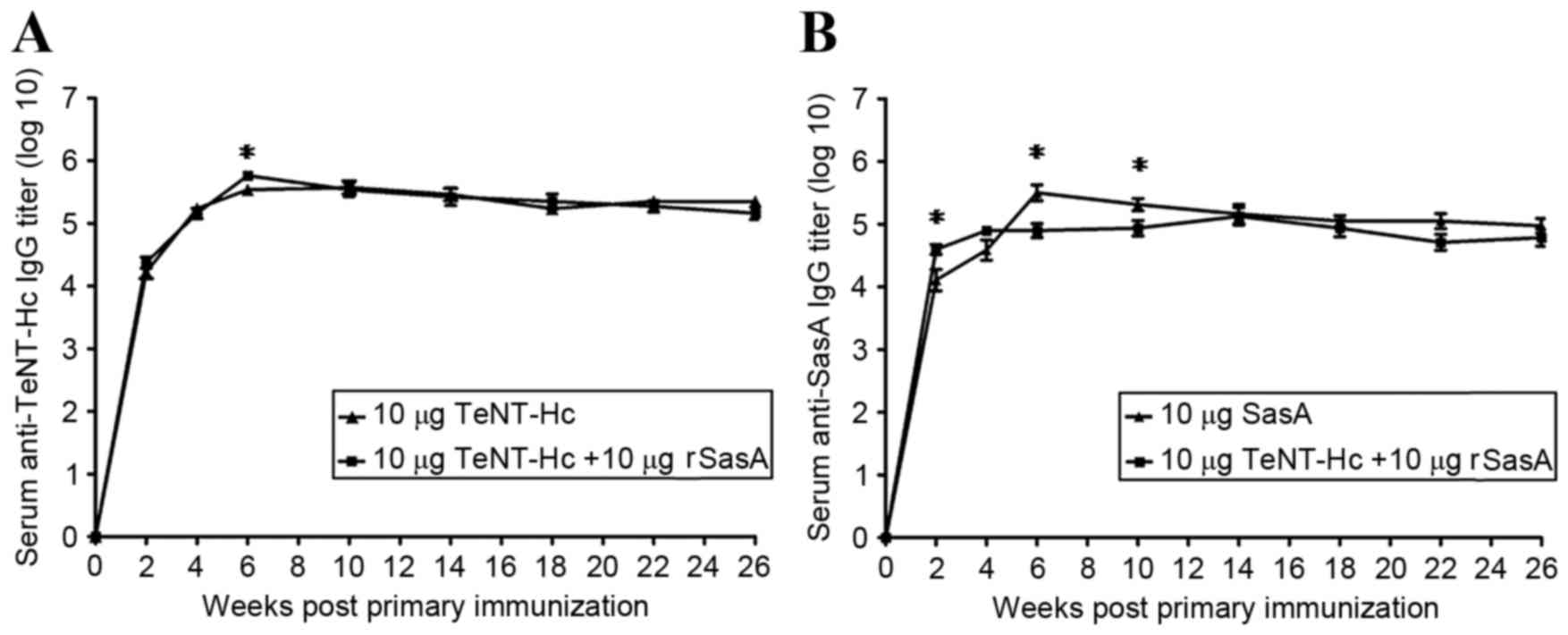
compared between the groups over 26 weeks (Fig. 1). Detectable levels of IgG against
rSasA and TeNT-Hc antigens were observed at 2 weeks following
primary immunization, and the titers were maintained over 6
months.
No significant differences in TeNT-Hc specific IgG
titers were observed between the TeNT-Hc immunized group and
TeNT-Hc + SasA immunized group over the 26 weeks (Fig. 1A), except for the week 6, when the
combined vaccine induced anti-TeNT-Hc IgG titers 1.683-fold higher
than TeNT-Hc (P=0.0313). Significant differences in rSasA specific
IgG titers were observed between the rSasA immunized group and the
TeNT-Hc + SasA immunized group in weeks 2, 6 and 10 (Fig. 1B). The combined vaccine induced
rSasA specific IgG titers 3.083-fold higher than rSasA alone at
week 2 (P=0.0210). However, at weeks 6 and 10, the combined vaccine
induced rSasA specific IgG titers 3.999 times (P=0.0032) and 2.377
times (P=0.0323) lower than rSasA alone, respectively.
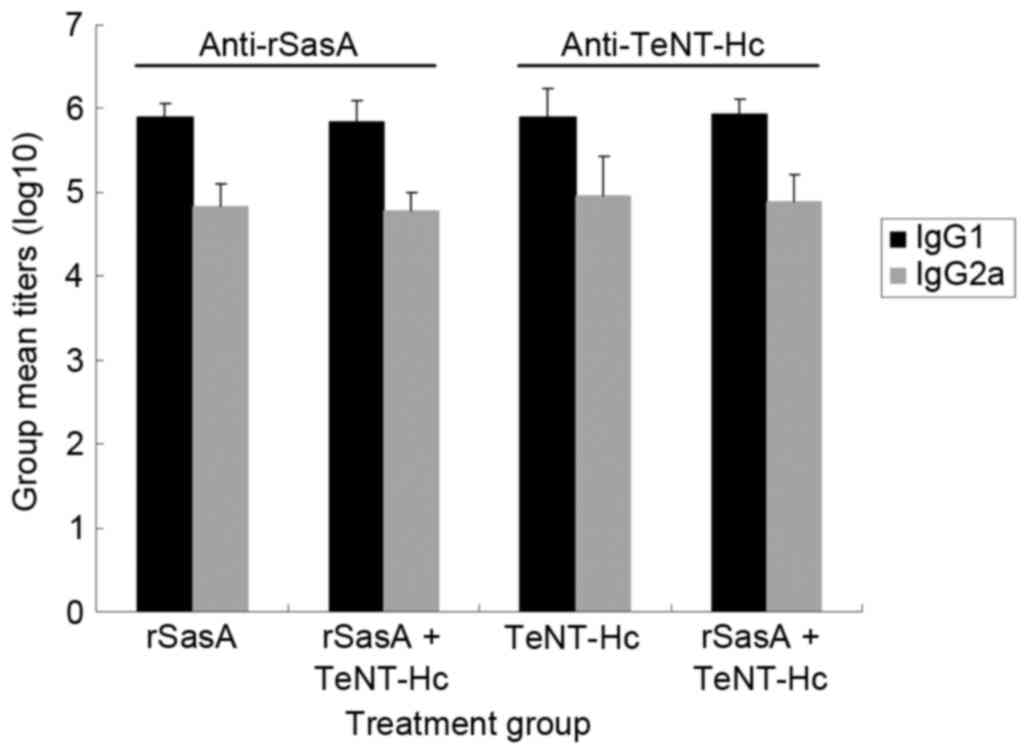
IgG isotyping
Serum samples from immunized mice at week 6 were
assayed for the presence of antigen-specific IgG1 and IgG2a
antibodies by ELISA. rSasA and the combined vaccine induced the
robust production of IgG1 and IgG2a antibodies specific to rSasA
(Fig. 2). No significant
differences in rSasA IgG1 and IgG2a titers were observed between
the rSasA and rSasA + TeNT-Hc groups. Similar results were observed
from TeNT-Hc-immunized mice and the combined vaccine-immunized mice
(Fig. 2). The combined vaccine
induced specific IgG1 and IgG2a titers comparable to rSasA or
TeNT-Hc vaccines. Additionally, the antigen-specific IgG1/IgG2a
ratio was not influenced by co-administration of the two antigens
(data not shown).
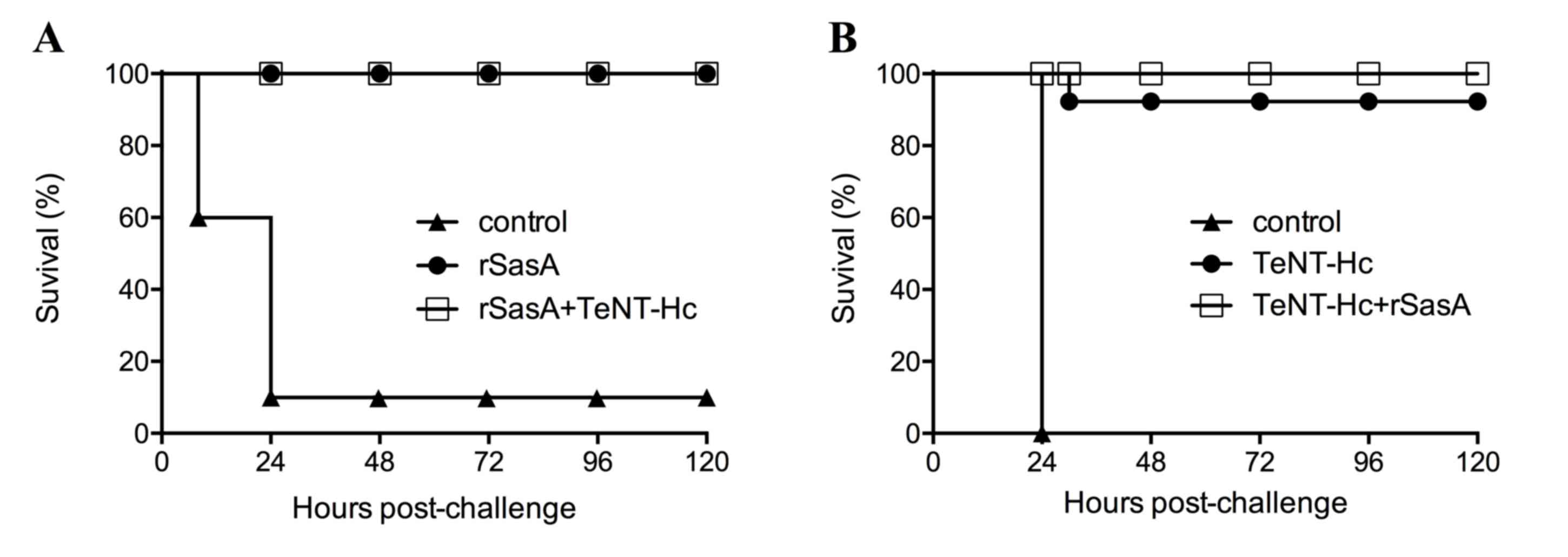
In vivo protection against S. aureus
challenge
Immunized mice were challenged by intraperitoneal
inoculation with 3×109 colony-forming units of S. aureus
USA300, 6 weeks following primary immunization. Approximately 90%
of mice in the control group died within 24 h (Fig. 3A). By contrast, immunized mice
survived for 120 h post challenge. All mice immunized with 10 µg
rSasA or 10 µg rSasA+10 µg TeNT-HC survived the challenge with
symptoms of infection such as temporary leg paralysis.
In vivo protection against tetanus
neurotoxin
The mice vaccinated three times with 10 µg TeNT-Hc
or 10 µg rSasA + 10 µg TeNT-Hc were challenged with tetanus
neurotoxin, and its protective abilities were evaluated. When
challenged with 2×103 LD50s of tetanus neurotoxin, TeNT-Hc and the
combined vaccine provided excellent protection; 9 mice vaccinated
by TeNT-Hc survived and 1 mouse died from tetanus poisoning. All
mice vaccinated by 10 µg rSasA + 10 µg TeNT-Hc survived without any
observed symptoms (Fig. 3B).
However, all mice immunized with aluminum hydroxide adjuvant alone
did not survive at 24 h post-challenge.
Discussion
A combined vaccine may be a reliable and
cost-effective strategy to prevent infections caused by two or more
pathogens or a single pathogen with various serotypes. Several
successful combined vaccines for children have been used for a
number of years including, heterologous combinations such as
diphtheria-tetanus-pertussis vaccine (19), the combined Hemophilus
influenzae type B (HIB), the HIB/hepatitis B (HepB) vaccine
(20), the combined HepA-HepB
(21), in addition to homologous
combinations such as multivalent pneumococcal vaccines (22). The results of the present study
suggested that SasA and TeNT-Hc are promising candidates for a
combined vaccine against S. aureus and tetanus
infections.
Due to antibiotic resistance and a general lack of
novel classes of antimicrobial agents against S. aureus,
vaccines against this pathogen have been extensively investigated.
A number of S. aureus antigens, including capsular
polysaccharide (23),
poly-N-acetylglucosamine (24),
iron-responsive surface determinant B (25), clumping factor A (26), clumping factor B (27), protein A (28), fibronectin-binding protein
(29), collagen adhesion (30), coagulase (31), α-hemolysin (32), Panton-Valentine leukocidin
(33), staphylococcal enterotoxins
(34) and toxic shock syndrome
toxin 1 (35) have been studied in
animal models. However, no vaccine developed thus far, has
demonstrated efficacy in humans (36) for unknown reasons. In the present
study, SasA induced a robust specific-antibody reaction and
complete protection against the USA300 strain, which is a common
source of methicillin-resistant S. aureus infections in the
USA (37). In addition, SasA is
prevalent in S. aureus clinical isolates and is immunogenic
in humans, as titers of SasA-specific antibodies in the sera of
convalescent patients infected by S. aureus were higher than
that in the sera of healthy people (11). Taken together, SasA is a promising
component for a combined vaccine against S. aureus.
Recombinant TeNT-Hc has exhibited considerable promise as a
next-generation subunit vaccine against tetanus (15,16),
particularly in formulations that can be administered orally
(38) or by intranasal routes
(39). Notably, the results of the
present study demonstrated no mutual interference between TeNT-Hc
and SasA in the antibody response, which is consistent with the
fact that recombinant TeNT-Hc has been used as a vaccine carrier to
enhance the immunogenicity of conjugate or fusion vaccines
(40).
As S. aureus consists of a number of
virulence factors, the development of a vaccine against this
pathogen has proven challenging (41). Therefore, a multi-component vaccine
has been suggested to achieve greater protective immunity against
S. aureus (42). In future
studies, more antigens of S. aureus may be added the
combined vaccine, to determine whether it may confer protection
against more clinical S. aureus strains and in different
animal models.
In conclusion, the present study demonstrated that
combined immunization with SasA and TeNT-Hc was as effective as
individual immunizations for induction of a serological antibody
response. The protective efficacy was not impaired when the
antigens were administered in combination. The results provided
preliminary evidence for the development of a combined vaccine
against S. aureus and tetanus infections.
Glossary
Abbreviations
Abbreviations:
|
S. aureus
|
Staphylococcus aureus
|
|
MRSA
|
Methicillin-resistant S.
aureus
|
|
IsdB
|
iron-responsive surface determinant
B
|
|
TeNT-Hc
|
C fragment of tetanus neurotoxin
|
|
SasA
|
Staphylococcus aureus surface
protein A
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
TMB
|
3,3′,5,5′-tetramethylbenzidine
dihydro-chloride
|
References
|
1
|
Zhang B, Liu Z, Lin Z, Zhang X and Fu W:
Microbiologic characteristics of pathogenic bacteria from
hospitalized trauma patients who survived Wenchuan earthquake. Eur
J Clin Microbiol Infect Dis. 31:2529–2535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Afshar M, Raju M, Ansell D and Bleck TP:
Narrative review: Tetanus-a health threat after natural disasters
in developing countries. Ann Intern Med. 154:329–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Udo JJ, Anah MU, Ochigbo SO, Etuk IS and
Ekanem AD: Neonatal morbidity and mortality in Calabar, Nigeria: A
hospital-based study. Niger J Clin Pract. 11:285–289.
2008.PubMed/NCBI
|
|
4
|
Peacock SJ, de Silva I and Lowy FD: What
determines nasal carriage of Staphylococcus aureus? Trends
Microbiol. 9:605–610. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lowy FD: Staphylococcus aureus infections.
N Engl J Med. 339:520–532. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chalya PL, Mabula JB, Dass RM, Mbelenge N,
Mshana SE and Gilyoma JM: Ten-year experiences with Tetanus at a
Tertiary hospital in Northwestern Tanzania: A retrospective review
of 102 cases. World J Emerg Surg. 6:202011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
World Health Organization, . Immunization,
vaccines and biologicals: Tetanus. http://www.who.int/immunization/diseases/teta-nus/enNovember
3–2016.
|
|
8
|
Skibinski DA, Baudner BC, Singh M and
O'Hagan DT: Combination vaccines. J Glob Infect Dis. 3:63–72. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi SQ, Zhang XY, Yang YL, Yang Y, Liu SL,
Fu L, Yu CM and Chen W: Immunity induced by Staphylococcus aureus
surface protein A was protective against lethal challenge of
Staphylococcus aureus in BALB/c mice. Microbiol Immunol.
56:406–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siboo IR, Chambers HF and Sullam PM: Role
of SraP, a Serine-Rich Surface Protein of Staphylococcus aureus, in
binding to human platelets. Infect Immun. 73:2273–2280. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roche FM, Massey R, Peacock SJ, Day NP,
Visai L, Speziale P, Lam A, Pallen M and Foster TJ:
Characterization of novel LPXTG-containing proteins of
Staphylococcus aureus identified from genome sequences.
Microbiology. 149:643–654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montecucco C and Schiavo G: Tetanus and
botulism neurotoxins: A new group of zinc proteases. Trends Biochem
Sci. 18:324–327. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schiavo G, Benfenati F, Poulain B,
Rossetto O, De Laureto P Polverino, DasGupta BR and Montecucco C:
Tetanus and botulinum-B neurotoxins block neurotransmitter release
by proteolytic cleavage of synaptobrevin. Nature. 359:832–835.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sinha K, Box M, Lalli G, Schiavo G,
Schneider H, Groves M, Siligardi G and Fairweather N: Analysis of
mutants of tetanus toxin Hc fragment: Ganglioside binding, cell
binding and retrograde axonal transport properties. Mol Microbiol.
37:1041–1051. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu R, Yi S, Yu C, Fang T, Liu S, Yu T,
Song X, Fu L, Hou L and Chen W: A conformational change of C
fragment of tetanus neurotoxin reduces its ganglioside-binding
activity but does not destroy its immunogenicity. Clin Vaccine
Immunol. 18:1668–1672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu R, Hou L, Yu C, Liu S, Ren J, Fang T,
Zhang X and Chen W: Enhanced expression of soluble recombinant
tetanus neurotoxin Hc in Escherichia coli for use as a tetanus
vaccine candidate. Immunobiology. 216:485–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rauch S, DeDent AC, Kim HK, Wardenburg J
Bubeck, Missiakas DM and Schneewind O: Abscess formation and
alpha-hemolysin induced toxicity in a mouse model of Staphylococcus
aureus peritoneal infection. Infect Immun. 80:3721–3732. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ulrich R and Miller J: Threshold
estimation in two-alternative forced-choice (2AFC) tasks: The
Spearman-Kärber method. Percept Psychophys. 66:517–533. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Booy R, Aitken SJ, Taylor S,
Tudor-Williams G, Macfarlane JA, Moxon ER, Ashworth LA, Mayon-White
RT, Griffiths H and Chapel HM: Immunogenicity of combined
diphtheria, tetanus, and pertussis vaccine given at 2, 3 and 4
months versus 3, 5 and 9 months of age. Lancet. 339:507–510. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
West DJ, Rabalais GP, Watson B, Keyserling
HL, Matthews H and Hesley TM: Antibody responses of healthy infants
to concurrent administration of a bivalent haemophilus influenzae
type b-hepatitis B vaccine with diphtheria-tetanus-pertussis, polio
and measles-mumps-rubella vaccines. BioDrugs. 15:413–418. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joines RW, Blatter M, Abraham B, Xie F, De
Clercq N, Baine Y, Reisinger KS, Kuhnen A and Parenti DL: A
prospective, randomized, comparative US trial of a combination
hepatitis A and B vaccine (Twinrix) with corresponding monovalent
vaccines (Havrix and Engerix-B) in adults. Vaccine. 19:4710–4719.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ortqvist A, Hedlund J, Burman LA, Elbel E,
Höfer M, Leinonen M, Lindblad I, Sundelöf B and Kalin M: Randomised
trial of 23-valent pneumococcal capsular polysaccharide vaccine in
prevention of pneumonia in middle-aged and elderly people. Swedish
Pneumococcal Vaccination Study Group. Lancet. 351:399–403. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robbins JB, Schneerson R, Horwith G, Naso
R and Fattom A: Staphylococcus aureus types 5 and 8 capsular
polysaccharide-protein conjugate vaccines. Am Heart J. 147:593–598.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maira-Litran T, Kropec A, Goldmann D and
Pier GB: Biologic properties and vaccine potential of the
staphylococcal poly-N-acetyl glucosamine surface polysaccharide.
Vaccine. 22:872–879. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joshi A, Pancari G, Cope L, Bowman EP, Cua
D, Proctor RA and McNeely T: Immunization with Staphylococcus
aureus iron regulated surface determinant B (IsdB) confers
protection via Th17/IL17 pathway in a murine sepsis model. Hum
Vaccin Immunother. 8:336–346. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Josefsson E, Hartford O, O'Brien L, Patti
JM and Foster T: Protection against experimental Staphylococcus
aureus arthritis by vaccination with clumping factor A, a novel
virulence determinant. J Infect Dis. 184:1572–1580. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schaffer AC, Solinga RM, Cocchiaro J,
Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale
P, Walsh E, et al: Immunization with Staphylococcus aureus clumping
factor B, a major determinant in nasal carriage, reduces nasal
colonization in a murine model. Infect Immun. 74:2145–2153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HK, Cheng AG, Kim HY, Missiakas DM and
Schneewind O: Nontoxigenic protein A vaccine for
methicillin-resistant Staphylococcus aureus infections in mice. J
Exp Med. 207:1863–1870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arrecubieta C, Matsunaga I, Asai T, Naka
Y, Deng MC and Lowy FD: Vaccination with clumping factor A and
fibronectin binding protein A to prevent Staphylococcus aureus
infection of an aortic patch in mice. J Infect Dis. 198:571–575.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nilsson IM, Patti JM, Bremell T, Höök M
and Tarkowski A: Vaccination with a recombinant fragment of
collagen adhesin provides protection against Staphylococcus
aureus-mediated septic death. J Clin Invest. 101:2640–2649. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng AG, McAdow M, Kim HK, Bae T,
Missiakas DM and Schneewind O: Contribution of coagulases towards
Staphylococcus aureus disease and protective immunity. PLoS Pathog.
6:e10010362010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wardenburg J Bubeck and Schneewind O:
Vaccine protection against Staphylococcus aureus pneumonia. J Exp
Med. 205:287–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown EL, Dumitrescu O, Thomas D, Badiou
C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch
F and Bowden MG: The Panton-Valentine leukocidin vaccine protects
mice against lung and skin infections caused by Staphylococcus
aureus USA300. Clin Microbiol Infect. 15:156–164. 2009. View Article : Google Scholar :
|
|
34
|
Hu DL, Omoe K, Narita K, Cui JC, Shinagawa
K and Nakane A: Intranasal vaccination with a double mutant of
staphylococcal enterotoxin C provides protection against
Staphylococcus aureus infection. Microbes Infect. 8:2841–2848.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu DL, Omoe K, Sasaki S, Sashinami H,
Sakuraba H, Yokomizo Y, Shinagawa K and Nakane A: Vaccination with
nontoxic mutant toxic shock syndrome toxin 1 protects against
Staphylococcus aureus infection. J Infect Dis. 188:743–752. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bagnoli F, Bertholet S and Grandi G:
Inferring reasons for the failure of Staphylococcus aureus vaccines
in clinical trials. Front Cell Infect Microbiol. 2:162012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Diep BA, Gill SR, Chang RF, Phan TH, Chen
JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, et al:
Complete genome sequence of USA300, an epidemic clone of
community-acquired meticillin-resistant Staphylococcus aureus.
Lancet. 367:731–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tacket CO, Galen J, Sztein MB, Losonsky G,
Wyant TL, Nataro J, Wasserman SS, Edelman R, Chatfield S, Dougan G
and Levine MM: Safety and immune responses to attenuated Salmonella
enterica serovar typhi oral live vector vaccines expressing tetanus
toxin fragment C. Clin Immunol. 97:146–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee S, Belitsky BR, Brown DW, Brinker JP,
Kerstein KO, Herrmann JE, Keusch GT, Sonenshein AL and Tzipori S:
Efficacy, heat stability and safety of intranasally administered
Bacillus subtilis spore or vegetative cell vaccines expressing
tetanus toxin fragment C. Vaccine. 28:6658–6665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bongat AF, Saksena R, Adamo R, Fujimoto Y,
Shiokawa Z, Peterson DC, Fukase K, Vann WF and Kovác P: Multimeric
bivalent immunogens from recombinant tetanus toxin HC fragment,
synthetic hexasaccharides, and a glycopeptide adjuvant. Glycoconj
J. 27:69–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salgado-Pabón W and Schlievert PM: Models
matter: The search for an effective Staphylococcus aureus vaccine.
Nat Rev Microbiol. 12:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stranger-Jones YK, Bae T and Schneewind O:
Vaccine assembly from surface proteins of Staphylococcus aureus.
Proc Natl Acad Sci USA. 103:16942–16947. 2006. View Article : Google Scholar : PubMed/NCBI
|