Introduction
Osteoarthritis (OA) is a chronic inflammatory
disorder, its incidence increasing with obesity, poor lifestyle and
ageing. Hydrogen peroxide (H2O2)-induced
oxidative stress and loss of inflammatory homeostasis causes
cartilage degeneration leading to OA and H2O2
is released during acute inflammatory responses (1,2).
Oxidative damage and activation of cellular redox signaling
mechanisms by H2O2 in chondrocytes is
associated with the pathogenesis of OA (3). H2O2-induced
oxidative stress causes proteoglycan degradation with structural
and functional changes ultimately leading to apoptosis (2,4).
Since oxidative stress serves a critical role in the development of
OA, an effective preventative strategy may be stimulated through
antioxidants and thereby redress redox imbalance.
Plumbagin (5-hydroxy-2-methyl-1, 4-aphthoquinone;
C11H8O3) occurs in various plant
families including plumbaginaceae, Droseraceae and
Ebenceae. It exhibits antioxidant properties and is involved
in various biological activities against bacterial and fungal
infection, inflammation and various types of cancer (5–9). The
effect of plumbagin against osteosarcoma has been previously
demonstrated through reactive oxygen species (ROS)-mediated
activation of pro-apoptosis (10).
Increasing evidence suggests that plumbagin also prevents migration
of gastric and breast cancer by suppressing the chemokine receptor
type 4 (11). Plumbagin acts as
anti-inflammatory compound that suppresses
lipopolysaccharide-induced endotoxemia by regulating nuclear
factor-κB (NF-κB) and carrageenan-induced rat paw edema by
modulating inflammatory mediators (12,13).
The present study evaluated the potential role of
plumbagin against H2O2-induced oxidative
stress and inflammation in primary culture of rat chondrocytes. The
study evaluated various oxidative stress parameters, including ROS
levels, antioxidant status and redox regulation of nuclear factor
(erythroid-derived 2)-like 2 (Nrf-2), NF-κB and inflammatory
cytokine levels. Therefore, plumbagin may be able to provide
protection against osteoarthritis by modulating oxidative stress
and inflammatory pathways.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
calf serum, antibiotics, antimycolytic solution,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and 2′,7′-dichlorofluorescin diacetate (DCF-DA) were obtained from
Gibco BRL (Gaithersburg, MD, USA). Primary antibodies used at
1:1,000 dilutions [NF-κB (cat.no. 3031), cyclooxygenase-2 (COX-2)
(cat. no. 12282), Nrf-2 (cat. no. ab31163), inducible NO synthase
(iNOS) (cat. no. ab3523), NAD(P)H:quinone oxidoreductase 1 (cat.
no. ab2346), heme oxygenase 1 (HO-1) (cat. no. ab13243)] and
secondary antibodies used at 1:10,000 dilutions [(Anti-rabbit IgG,
horseradish peroxidase-conjugated (HRP)-linked antibody (cat. no.
7074) and anti-mouse IgG, HRP-conjugated antibody (cat. no. 7076)]
were used in the present study were purchased from CST Biological
Reagents Co., Ltd., Shanghai, China.
Cell culture and cell viability
Primary rat chondrocytes were used in the present
study. The isolation and cell culture were carried out as described
previously (14). Cell viability
was assessed by MTT assay. Cells (1×104) were seeded and allowed to
attach overnight. Subsequently, cells were treated with different
concentrations of H2O2 (0.2–1.0 µM) for 24 h.
The half maximal inhibitory concentration (IC50) was
identified to be 0.8 µM. In order to determine the protective
effect of plumbagin against H2O2-induced
oxidative stress, cells were pre-treated with plumbagin and then
with 0.8 µM H2O2 for 24 h at 37°C. Cells were
treated with MTT for 5 h at 37°C and quantified at 570 nm (15).
Oxidative stress markers
Intracellular ROS generation
Cells were pre-treated with plumbagin and then with
DCF-DA for 1 h at 37°C. To identify the protective effect of
plumbagin against H2O2-induced oxidative
stress, cells were washed and treated with
H2O2 for 24 h. Cells were treated with
plumbagin and H2O2 alone, and control cells
without any treatment were maintained. Following complete
treatment, cells were suspended in phosphate buffered saline and
ROS levels were measured fluorimetrically (16).
Lipid Peroxidation
Following treatment, cells were analyzed for lipid
peroxidation content using a Lipid Peroxidation (MDA) assay kit
(cat. no. ab118970; Abcam, Cambridge, UK) in accordance with the
manufacturer's protocol.
Antioxidant enzyme activities
Antioxidant assay kits were used for determining the
specific activities. Superoxide dismutase activity (cat. no.
706002; Cayman Chemical Company, Ann Arbor, MI, USA), catalase
(CAT) activity (cat. no. 707002; Cayman Chemical Company),
Glutathione-S-Transferase (GST) activity (cat. no. 703302; Cayman
Chemical Company), Glutathione Peroxidase (GPx) activity: (cat. no.
703102; Cayman Chemical Compnay) and glutathione (GSH) levels (cat.
no. 703002; Cayman chemicals) were assessed.
Pro-inflammatory cytokine expression levels
Following treatment with plumbagin and
H2O2, cell supernatants were analyzed for
tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6
levels using ELISA kits (Abcam). The levels of interleukins were
expressed as pg/mg protein.
Immunoblot analysis
Cells were lysed using radioimmunoprecipitation
assay buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for the isolation of whole cell protein extract.
The nuclear extract was isolated using NE-PER Nuclear and
Cytoplasmic Extraction Reagents, Thermofisher Scientific, (cat. no.
78833) for the determination of the expression levels of NF-κB and
Nrf-2. The samples were run on 12% SDS-PAGE gels, and then
transferred onto nitrocellulose membranes. The membranes were
incubated with 5% non-fat milk for 2 h. Following washing, the blot
was incubated with primary (1:1,000) and secondary antibodies
(1:10,000). The bands were developed with enhanced
chemiluminescence and the images were analyzed using ImageJ
software (https://imagej.nih.gov/ij).
Statistical analysis
The results are expressed as mean ± standard
deviation. One-way analysis of variance followed by Tukey's
multiple comparison tests was performed for determining
significance. SPSS software (version, 16.0; SPSS, Inc., Chicago,
IL, USA) was used and statistical significance was set as
***P<0.001, when compared with the control, and ++P<0.01,
+++P<0.001, when compared to the H2O2
group.
Results
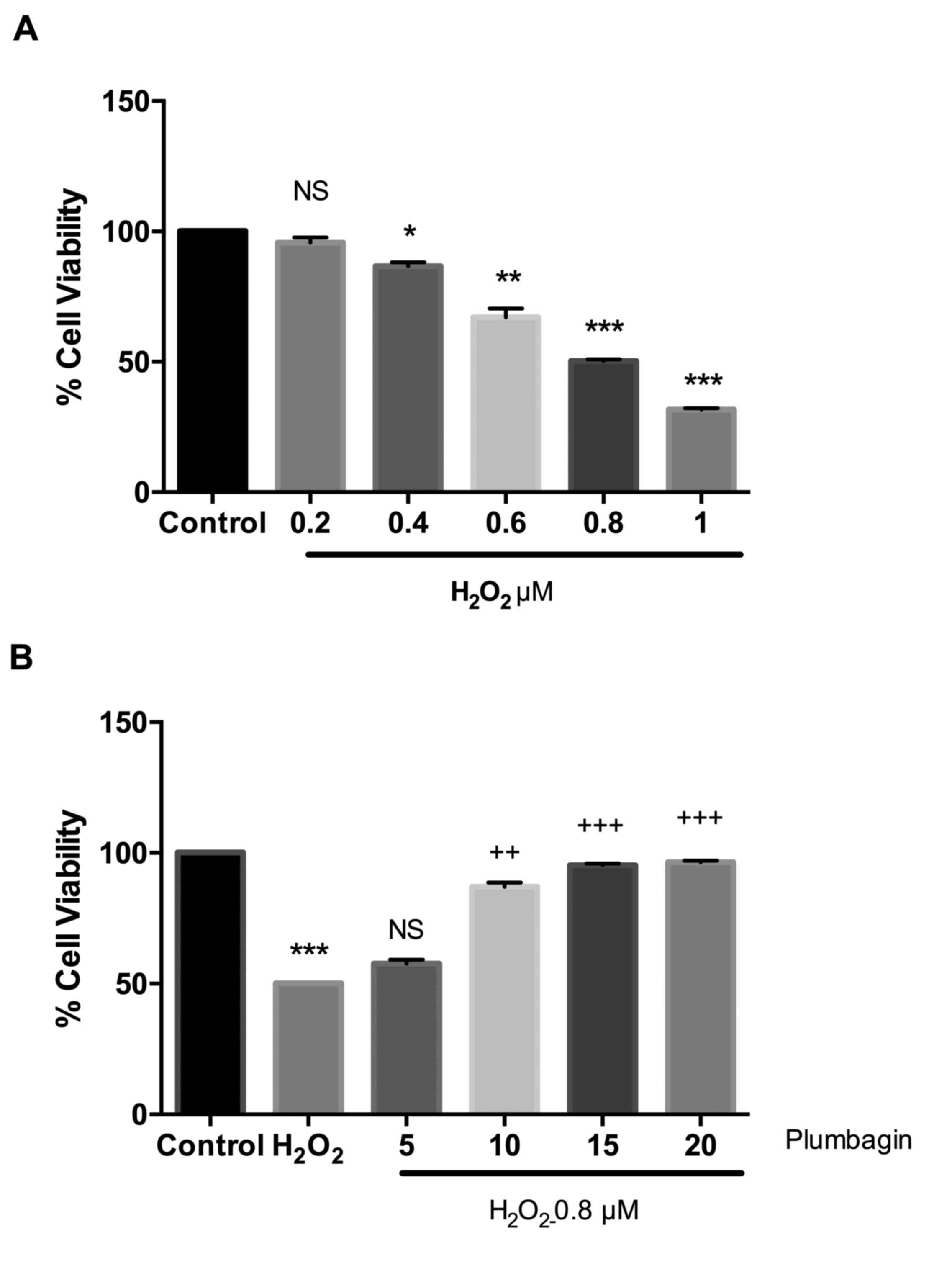
Cell viability was increased by plumbagin
treatment
In order to test H2O2-induced
cell death, an MTT viability assay was performed.
H2O2-induced dose-dependent cell viability
was compared with that of the control cells. The IC50
value was found to be 0.8 µM. Next, the cells were pre-treated with
different concentrations of plumbagin for 24 h and then treated
with H2O2. Plumbagin dose-dependently
improved the cell viability compared with
H2O2-treated cells. However, treatment with
plumbagin alone caused no cell death compared with that of the
control cells (data not shown). plumbagin at 15 and 20 µM showed a
protective effect and 15 µM was selected for further molecular
studies, (Fig. 1A and B).
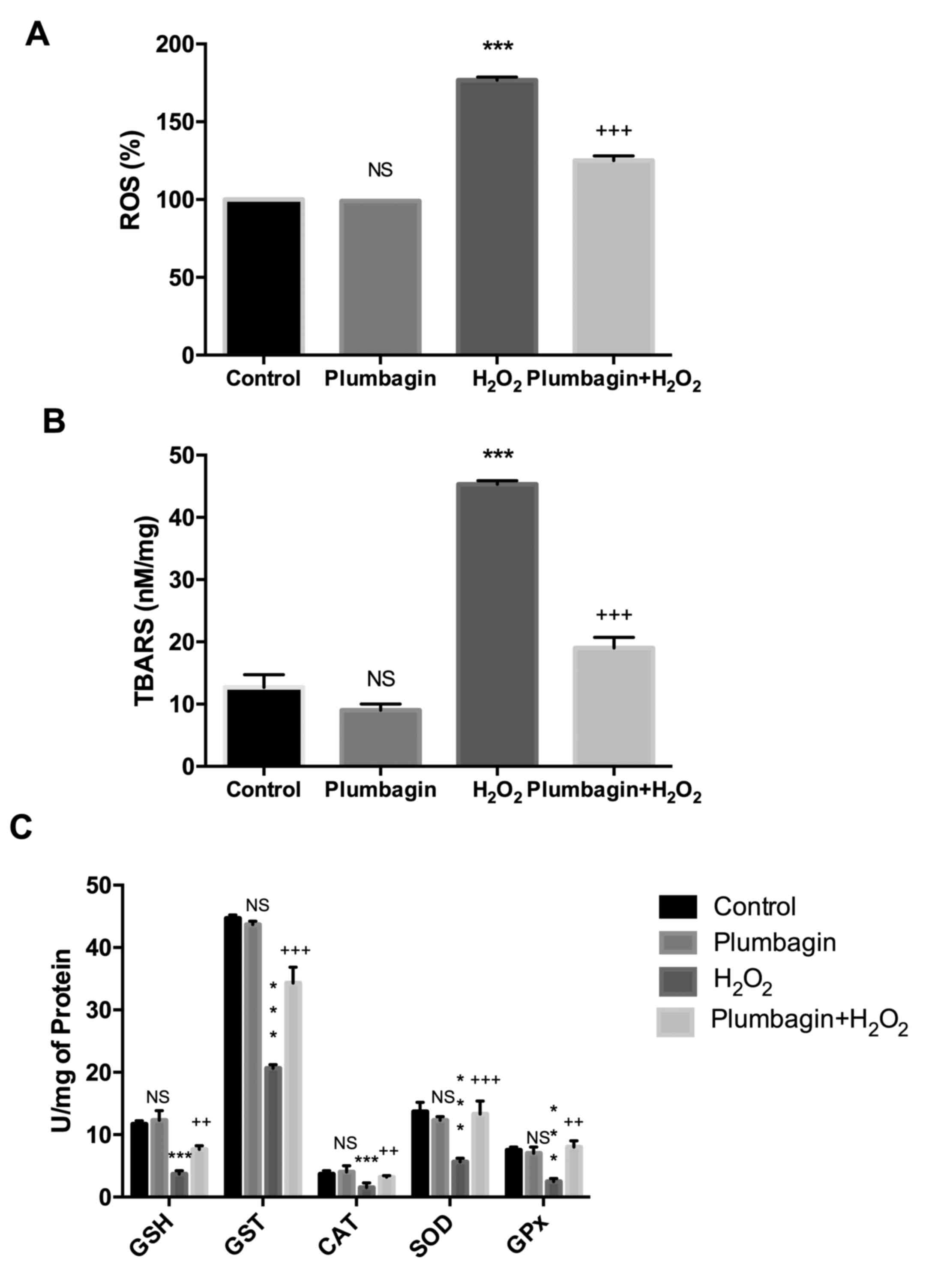
Plumbagin prevents
H2O2-induced oxidative stress
H2O2 treatment of chondrocytes
caused an increase in oxidative markers, including ROS and lipid
peroxides, compared with control cells. Pre-treatment with
plumbagin reduced these marker levels compared with those of
H2O2 treated cells. Plumbagin treatment alone
did not affect the oxidative stress marker levels (Fig. 2A and B). In addition, antioxidant
enzyme activities including GSH, SOD, GST, GPx and CAT activities,
were estimated for different groups. It was identified that
H2O2 treatment reduced the antioxidant
defense system by significantly reducing the levels compared with
those of control cells. However, treatment with plumbagin increased
the antioxidant enzyme activities to significant levels compared
with those of H2O2-treated cells (Fig. 2C).
Plumbagin modulates NF-κB and Nrf-2
signaling
Next the H2O2-induced
oxidative stress and inflammation in chondrocytes through NF-κB and
Nrf-2 signaling was evaluated. H2O2 treatment
induced inflammatory signaling through increased NF-κB expression
levels and the downstream targets (COX-2 and iNOS). Furthermore,
Nrf-2 levels were significantly downregulated in chondrocytes
treated with H2O2 compared with those of
control cells. Nrf-2-induced expression levels of HO-1 and NQO-1
were significantly reduced. Plumbagin treatment followed by
H2O2 treatment downregulated NF-κB and
upregulated Nrf-2 levels (Fig. 3A and
B).
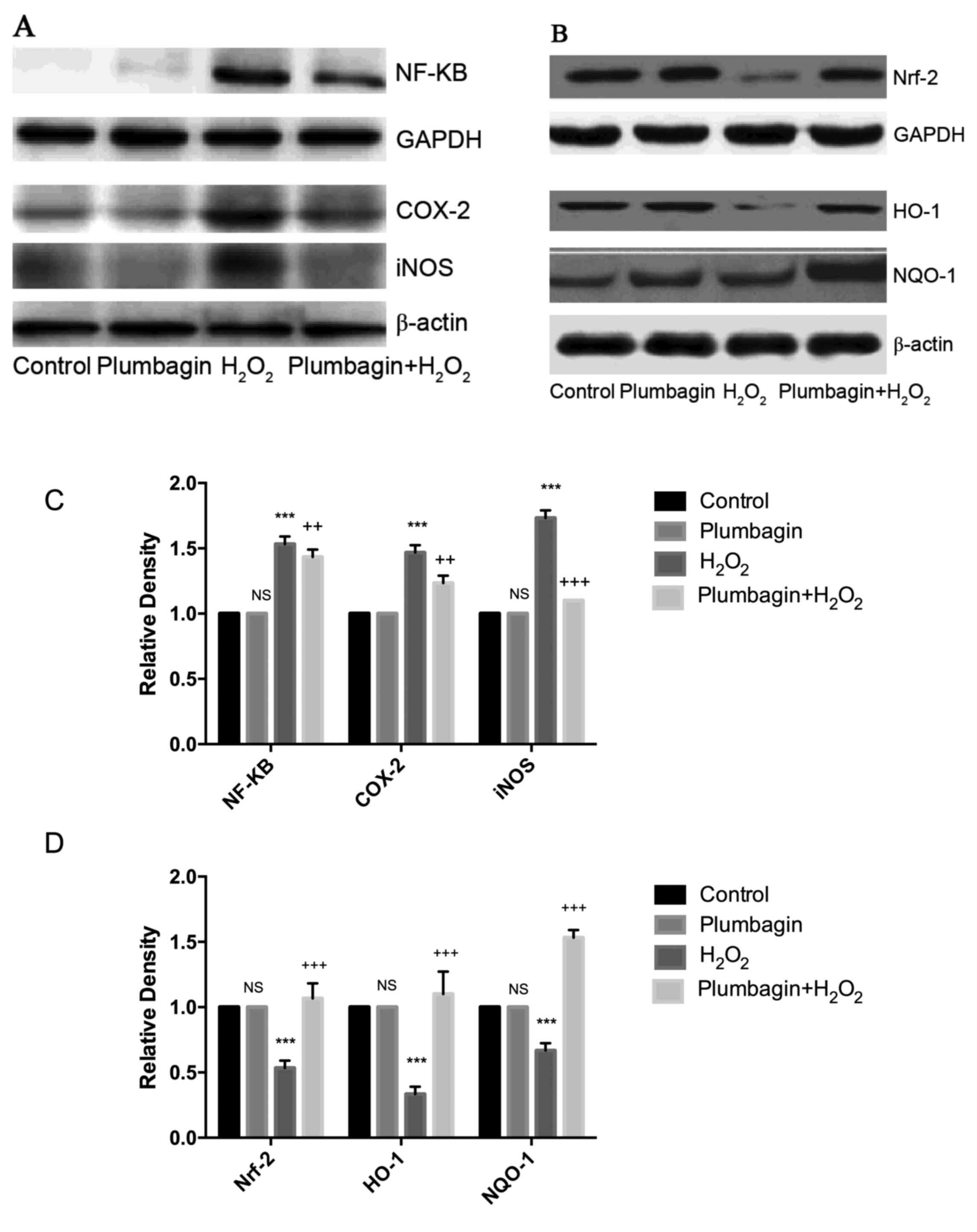 | Figure 3.(A) Plumbagin downregulates
H2O2-induced NF-κB, COX-2 and iNOS
expressions in rat chondrocytes. (B) Plumbagin upregulates Nrf-2,
HO-1 and NQO1 expressions in rat chondrocytes. (C and D)
Densitometric analysis on the effect of plumbagin and
H2O2 against NF-κB and Nrf-2 signaling
proteins. ***P<0.001, when compared with the control.
++P<0.01, +++P<0.001, when compared to
H2O2 group. Plumbagin and
H2O2 group vs. control; plumbagin +
H2O2 vs. H2O2 group
(One-way analysis of variance followed by Tukey's multiple
comparison). NF-κB, nuclear factor-κB; Nrf-2, nuclear factor
(erythroid-derived 2)-like 2; H2O2, hydrogen
peroxide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; COX-2,
cyclooxygenase-2; iNOS, inducible NO synthase; NS, non significance
vs. control group; HO-1, heme oxygenase 1; NQO-1, NAD(P)H:quinone
oxidoreductase 1. |
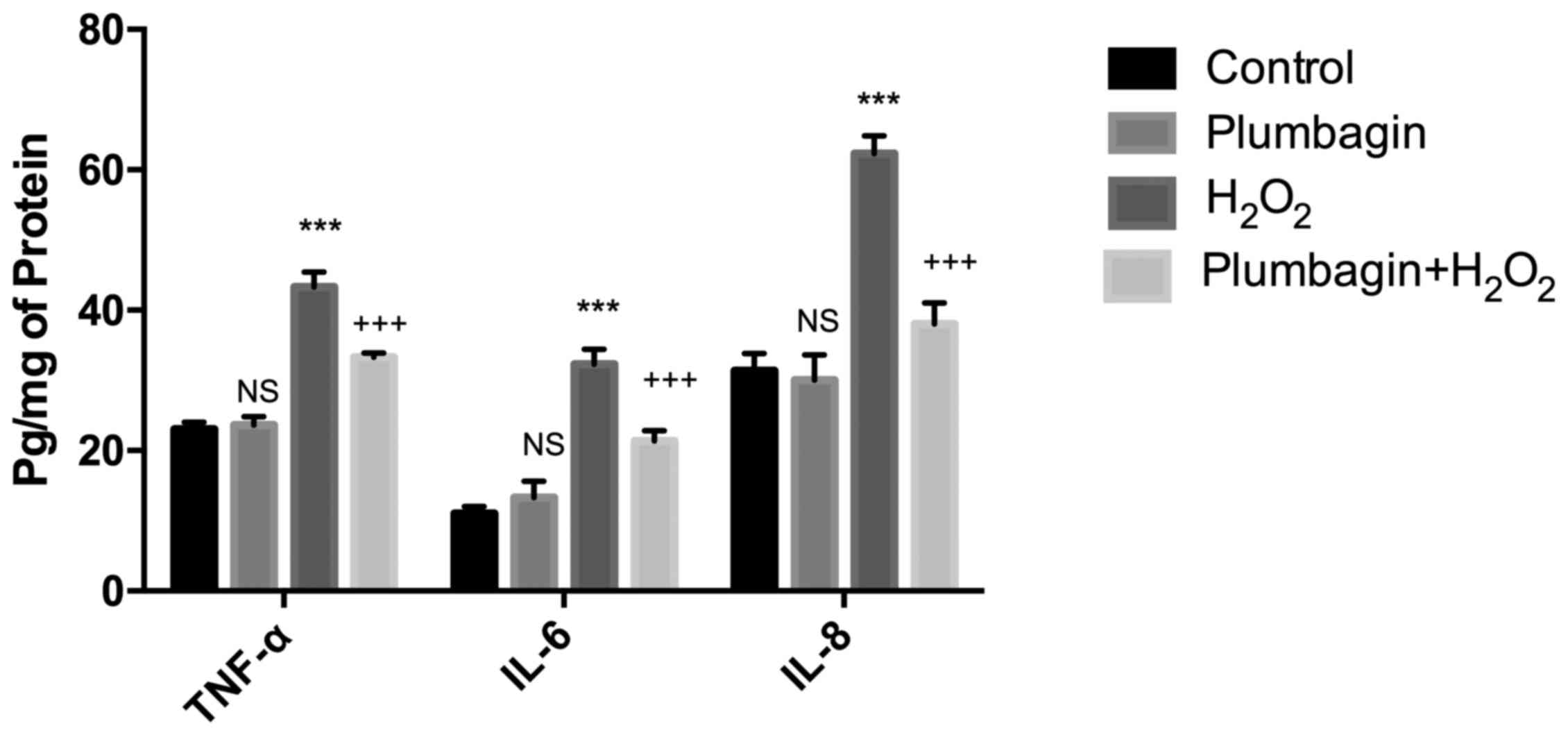
Plumbagin reduces inflammatory cytokine
expression
Pro-inflammatory cytokine expression, including
TNF-α, IL-6 and IL-8, were evaluated by ELISA.
H2O2 treatment increased the pro-inflammatory
cytokine expression compared with that of control cells, while
plumbagin pre-treatment significantly downregulated the cytokine
expression levels (Fig. 4).
Discussion
The present study demonstrated certain important
findings on plumbagin-mediated cytoprotection against OA in
chrondrocytes by the regulation of redox signaling and
inflammation. H2O2 treatment dose-dependently
decreased chondrocyte viability, whereas plumbagin pre-treatment
improved the cell viability of chondrocytes by up to 97%.
Furthermore, H2O2 caused severe oxidative
stress in chondrocytes by increasing the ROS and lipid peroxide
levels. Previous studies (17,18)
have demonstrated the potential role of H2O2
in inducing oxidative stress with functional loss of chondrocytes
and the development of OA. Plumbagin treatment reduced the
oxidative stress markers perhaps due to its antioxidant potential.
Plumbagin has been previously reported (19,20)
as having strong antioxidant properties and thereby associated
anti-mutagenic and anti-cancer effects.
The present study analyzed the detailed molecular
mechanism of plumbagin's protection against
H2O2-induced effects on chondrocytes. It
identified that H2O2 treatment increased the
oxidative stress by downregulating Nrf-2 signaling. Nrf-2 is the
nuclear transcription factor activated under oxidative stress and
translocates into the nucleus. Nrf-2 induces expression of
antioxidant responsive element (ARE)-responsive genes including,
NQO-1, GST, HO-1 at the transcriptional level (21). In the current study,
H2O2-induced redox signaling is mediated
through increased ROS levels and a decline in the expression levels
of Nrf-2 and downstream proteins. This imbalance may be due to
exacerbated oxidative stress, which is not sufficiently ameliorated
by the endogenous antioxidant defence system inside the cells.
However, treatment with plumbagin improved the antioxidant defence
mechanism by inducing nuclear Nrf-2, HO-1 and NQO-1 expression
levels. HO-1 is activated by oxidative stress and inflammatory
cytokines, and produces biliverdin and bilirubin, which exert a
cytoprotective effect by ameliorating ROS (22–24).
Another important antioxidant enzyme is NQO-1 (also called
DT-diaphorase). This serves a major role in preventing oxidative
stress by reducing quinones to hydroquinones (25). A previous study by Son et al
(26), reported the protective
role of plumbagin by activating Nrf-2/ARE signaling against
cerebral ischemia.
NF-κB is a redox sensitive transcription factor that
is central to inflammation. NF-κB is held in the cytoplasm by
inhibitor of κB (IκB) proteins, which thereby regulates its
expression levels. Under oxidative stress, IκB undergoes
proteosomal degradation resulting in nuclear localization of NF-κB,
which induces the expression levels of various inflammatory
proteins, including COX-2, iNOS and inflammatory cytokines
(27). In the present study, it
was identified that H2O2 induces NF-κB
activation and expression and further upregulated its downstream
genes COX-2 and iNOS. The results of the present study are
consistent with previous reports on
H2O2-induced NF-κB activation, regulated
through phosphorylation of IκB (28,29).
H2O2 induces COX-2 expression through
tyrosine kinase phosphorylation and is involved in wound repair in
human umbilical vein endothelial cells (30). The
H2O2-induced NF-κB levels and increased
expression levels of inflammatory cytokines observed in the present
study are consistent with the previous study of Lim et al
(31), where the authors
demonstrated the protective role of melatonin against
H2O2-induced inflammatory effects on the
CHON-001 human chondrocyte cell line and a rabbit OA model. These
H2O2-induced inflammatory effects were
significantly downregulated by plumbagin preventing NF-κB
expression levels and their downstream targets (COX-2, iNOS, TNF-α,
IL-18 and IL-6). plumbagin suppressed osteoclastogenesis and
osteolytic metastasis by downregulating receptor activator of NF-κB
ligand-induced NF-κB activation (32). The immunomodulatory activity of
plumbagin has been previously demonstrated by Checker et al
(33) by suppressing Concanavalin
A-induced NF-κB expression levels and various cytokine (IL-2, IL-4,
IL-6 and interferon-γ) levels. Plumbagin also suppresses
carrageenan induced edema by downregulating pro-inflammatory
cytokines (13). Thus,
H2O2-induced inflammation may be regulated by
plumbagin modulating the NF-κB transcription factor and thereby
regulating its downstream targets.
The findings of the present study are that plumbagin
is a cytoprotective compound against
H2O2-induced oxidative stress and
inflammatory responses in chondrocyte cells. Additionally,
plumbagin prevents oxidative stress by regulating Nrf-2 levels,
induces the expression of antioxidant genes and improves the
overall cellular antioxidant defense mechanism. In addition,
plumbagin modulates NF-κB levels and suppresses inflammation by
downregulating COX-2, iNOS and inflammatory cytokine expression
levels. As a result, the present study provide evidence that
plumbagin has the potential to protect against osteoarthritis by
regulating redox signaling and inflammation.
References
|
1
|
Martin JA and Buckwalter JA: Roles of
articular cartilage aging and chondrocyte senescence in the
pathogenesis of osteoarthritis. Iowa Orthop J. 21:1–7.
2001.PubMed/NCBI
|
|
2
|
Shah R, Raska K Jr and Tiku ML: The
presence of molecular markers of in vivo lipid peroxidation in
osteoarthritic cartilage: A pathogenic role in osteoarthritis.
Arthritis Rheum. 52:2799–2807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maneesh M, Jayalekshmi H, Suma T,
Chatterjee S, Chakrabarti A and Singh TA: Evidence for oxidative
stress in osteoarthritis. Indian J Clin Biochem. 20:129–130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ziskoven C, Jäger M, Zilkens C, Bloch W,
Brixius K and Krauspe R: Oxidative stress in secondary
osteoarthritis: From cartilage destruction to clinical
presentation? Orthop Rev (Pavia). 2:e232010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Durga R, Sridhar P and Polasa H: Effects
of plumbagin on antibiotic resistance in bacteria. Indian J Med
Res. 91:18–20. 1990.PubMed/NCBI
|
|
6
|
Dzoyem JP, Tangmou JG, Lontsi D, Etoa FX
and Lohoue PJ: In vitro antifungal activity of extract and
plumbagin from the stem bark of Diospyros crassiflora hiern
(Ebenaceae). Phytotherapy Res. 21:671–674. 2007. View Article : Google Scholar
|
|
7
|
Thasni KA, Rakesh S, Rojini G,
Ratheeshkumar T, Srinivas G and Priya S: Estrogen-dependent cell
signaling and apoptosis in BRCA1-blocked BG1 ovarian cancer cells
in response to plumbagin and other chemotherapeutic agents. Ann
Oncol. 19:696–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuo PL, Hsu YL and Cho CY: Plumbagin
induces G2-M arrest and autophagy by inhibiting the AKT/mammalian
target of rapamycin pathway in breast cancer cells. Mol Cancer
Ther. 5:3209–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu YL, Cho CY, Kuo PL, Huang YT and Lin
CC: Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) induces
apoptosis and cell cycle arrest in A549 cells through p53
accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation
at serine 15 in vitro and in vivo. J Pharmacol Exp Ther.
318:484–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian LQ, Yin DL, Ren Y, Gong C, Chen AM
and Guo FJ: Plumbagin induces apoptosis via the p53 pathway and
generation of reactive oxygen species in human osteosarcoma cells.
Mol Med Rep. 5:126–132. 2012.PubMed/NCBI
|
|
11
|
Manu KA, Shanmugam MK, Rajendran P, Li F,
Ramachandran L, Hay HS, Kannaiyan R, Swamy SN, Vali S, Kapoor S, et
al: Plumbagin inhibits invasion and migration of breast and gastric
cancer cells by downregulating the expression of chemokine receptor
CXCR4. Mol Cancer. 10:1072011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo P, Wong YF, Ge L, Zhang ZF, Liu Y, Liu
L and Zhou H: Anti-inflammatory and analgesic effect of plumbagin
through inhibition of nuclear factor-κB activation. J Pharmacol Exp
Ther. 335:735–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Checker R, Patwardhan R, Sharma D, Menon
J, Thoh M, Sandur SK, Sainis KB and Poduval TB: Plumbagin, a
vitamin K3 Analogue, abrogates lipopolysaccharide-induced oxidative
stress, inflammation and endotoxic shock via NF-κB suppression.
Inflammation. 37:542–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Li L, Geng C, Gong D, Jiang L,
Ishikawa N, Kajima K and Zhong L: Monosodium iodoacetate induces
apoptosis via the mitochondrial pathway involving ROS production
and caspase activation in rat chondrocytes in vitro. J Orthop Res.
31:364–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Royall JA and Ischiropoulos H: Evaluation
of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent
probes for intracellular H2O2 in cultured endothelial cells. Arch
Biochem Biophys. 302:348–355. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan IM, Gilbert SJ, Caterson B, Sandell
LJ and Archer CW: Oxidative stress induces expression of
osteoarthritis markers procollagen IIA and 3B3(−) in adult bovine
articular cartilage. Osteoarthritis Cartilage. 16:698–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yudoh K, Nguyen vT, Nakamura H,
Hongo-Masuko K, Kato T and Nishioka K: Potential involvement of
oxidative stress in cartilage senescence and development of
osteoarthritis: Oxidative stress induces chondrocyte telomere
instability and downregulation of chondrocyte function. Arthritis
Res Ther. 7:R380–R391. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar S, Gautam S and Sharma A:
Antimutagenic and antioxidant properties of plumbagin and other
naphthoquinones. Mutat Res. 755:30–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan M, Liu Y, Luo X, Chen Z and Liang H:
Antioxidant activities of plumbagin and its Cu (II) complex.
Bioinorg Chem Appl. 2011:8987262011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiological Rev. 86:583–650. 2006. View Article : Google Scholar
|
|
23
|
Tenhunen R, Marver HS and Schmid R: The
enzymatic conversion of heme to bilirubin by microsomal heme
oxygenase. Proc Natl Acad Sci USA. 61:748–755. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terry CM, Clikeman JA, Hoidal JR and
Callahan KS: Effect of tumor necrosis factor-alpha and
interleukin-1 alpha on heme oxygenase-1 expression in human
endothelial cells. Am J Physiol. 274:H883–H891. 1998.PubMed/NCBI
|
|
25
|
Gutierrez PL: The role of NAD(P)H
oxidoreductase (DT-diaphorase) in the bioactivation of
quinone-containing antitumor agents: A review. Free Radic Biol Med.
29:263–275. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Son TG, Camandola S, Arumugam TV, Cutler
RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA,
et al: Plumbagin, a novel Nrf2/ARE activator, protects against
cerebral ischemia. J Neurochem. 112:1316–1326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kutuk O and Basaga H: Aspirin prevents
apoptosis and NF-kappaB activation induced by H2O2 in hela cells.
Free Radic Res. 37:1267–1276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takada Y, Mukhopadhyay A, Kundu GC,
Mahabeleshwar GH, Singh S and Aggarwal BB: Hydrogen peroxide
activates NF-kappa B through tyrosine phosphorylation of I kappa B
alpha and serine phosphorylation of p65: Evidence for the
involvement of I kappa B alpha kinase and Syk protein-tyrosine
kinase. J Biol Chem. 278:24233–24241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eligini S, Arenaz I, Barbieri SS, Faleri
ML, Crisci M, Tremoli E and Colli S: Cyclooxygenase-2 mediates
hydrogen peroxide-induced wound repair in human endothelial cells.
Free Radic Biol Med. 46:1428–1436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG,
Chun YH, Choi BJ and Kim EC: Cytoprotective and anti-inflammatory
effects of melatonin in hydrogen peroxide-stimulated CHON-001 human
chondrocyte cell line and rabbit model of osteoarthritis via the
SIRT1 pathway. J Pineal Res. 53:225–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung B, Oyajobi B and Aggarwal BB:
Plumbagin Inhibits osteoclastogenesis and reduces human breast
cancer-induced osteolytic bone metastasis in mice through
suppression of RANKL signaling. Mol Cancer Ther. 11:350–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Checker R, Sharma D, Sandur SK, Khanam S
and Poduval TB: Anti-inflammatory effects of plumbagin are mediated
by inhibition of NF-kappaB activation in lymphocytes. Int
Immunopharmacol. 9:949–958. 2009. View Article : Google Scholar : PubMed/NCBI
|