Introduction
Cancer stage is defined according to the
International Union against Cancer tumor node metastasis (TNM)
classification system (1). Other
characteristics, including histological differentiation, tumor
infiltration (INF) pattern, stromal type, blood vessel invasion and
lymphatic invasion are also used to assess tumors (2–4).
These other characteristics are not used to determine pathological
stage; however, some studies have reported that they may help to
predict outcomes (2–10). Some patients with lung cancer only
undergo limited resection due to poor lung function (11,12).
Patients with lung squamous cell carcinoma (SqCC) occasionally
exhibit chronic obstructive pulmonary disease due to smoking
(13,14), and often require limited lung
resection without systematic lymph node dissection. In these cases,
the lymph nodes, which are the N factor in the TNM classification
system, cannot be pathologically evaluated, and thus the
pathological stage cannot be determined. Therefore, it is difficult
to evaluate the need for adjuvant chemotherapy and radiotherapy,
and to predict prognosis.
In our previous study, we examined the INF pattern
in lung SqCC specimens; the samples were divided into two groups:
The INFc(−) group, which exhibited clear borders between the tumor
and surrounding normal tissues, and the INFc(+) group, which did
not exhibit clear borders between the tumor and surrounding normal
tissues (6,15–17).
The results demonstrated that INFc(+) was significantly associated
with venous invasion, scirrhous stromal type and poorer
postoperative survival, thus suggesting that INFc(+) may be
considered a useful marker of local invasiveness. Determination of
various histological characteristics of primary lesions are
important for patients with recurrent lung SqCC, since there are
few therapeutic options available for these patients compared with
patients with adenocarcinoma (18–24).
Histological vascular invasion has been reported to predict
prognosis in non-small cell lung cancer (8–10).
Several studies regarding non-small cell lung cancer have
predominantly focused on patients with adenocarcinoma, whereas no
previous studies have focused specifically on patients with SqCC,
to the best of our knowledge (8–10).
The present study investigated the association between the degree
of lymphatic invasion and prognosis in patients with SqCC of the
lung. The aim of the present study was to investigate whether the
pattern of lymphatic invasion and other clinicopathological
characteristics may be used to predict prognosis in patients with
SqCC of the lung.
Materials and methods
Lung cancer specimens
Resected specimens were collected from patients
treated for SqCC of the lung. The samples were examined after
receiving informed consent from the patients. The study protocol
was approved by the Institutional Review Board of Tokai University
Hospital (Isehara, Japan). The present study included 103 patients
with SqCC of the lung (97 males and 6 females; age range, 43–85
years; mean age, 67.2±9.1 years) who underwent radical surgery
(lobectomy and mediastinal lymphadenectomy) at Tokai University
Hospital. For each patient, tumor stage was defined according to
the TNM classification system (25) and the histological type was defined
according to the World Health Organization classification (26). The median postoperative follow-up
period was 1,528 days (range, 41-3,837 days).
Histological examination
The lung tissue specimens were fixed with 10%
buffered formalin for 24–48 h, embedded in paraffin according to
routine techniques, and 4-µm sections were sliced at 5–10 mm
intervals. Sections were examined using an optical microscope. INF
pattern and lymphatic invasion were examined on sections, which
were stained with hematoxylin and eosin. Vascular and pleural
invasion were examined using Verhoeff-van Gieson staining as
follows: Incubation with Verhoeff solution [5% alcohol hematoxylin,
10% ferric chloride and Weigert iodine solution (Muto Pure
Chemicals Co., Ltd., Tokyo, Japan)] for 60 min at room temperature;
and then van Gieson solution [1% aqueous acid fuchsin: (Muto Pure
Chemicals Co., Ltd.)] for 10 min at room temperature. The degree of
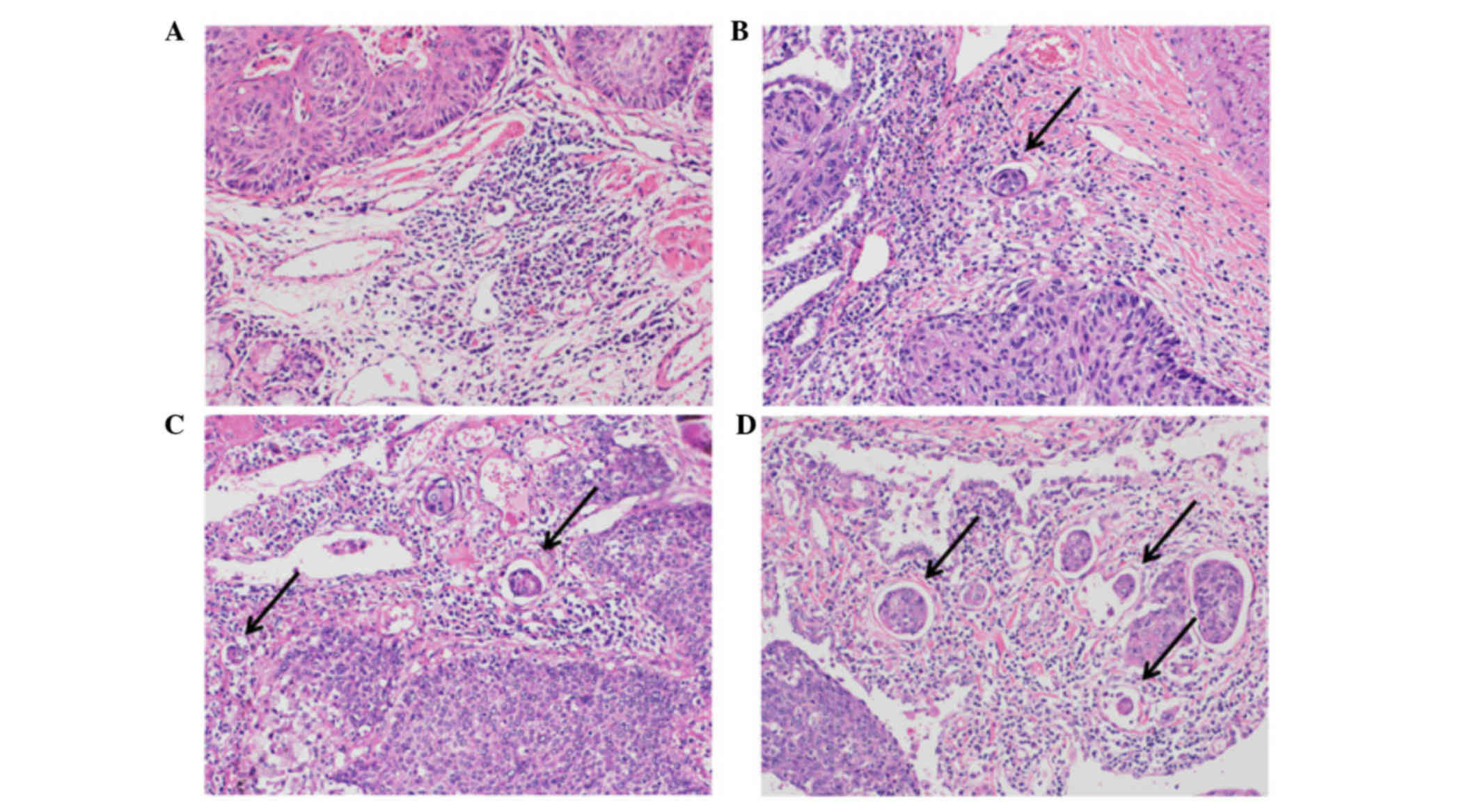
lymphatic invasion was classified as follows: Ly0, no lymphatic
invasion; ly1, mild lymphatic invasion; ly2, moderate lymphatic
invasion; or ly3, severe lymphatic invasion (Fig. 1). The degree of venous invasion was
classified as follows: V0, no venous invasion; v1, minimal venous
invasion (1 or 2 foci of venous invasion per histological section);
v2, moderate venous invasion (3 or 4 foci); or v3, severe venous
invasion (≥5 foci).
The INF pattern was described as previously reported
in gastric cancer studies (15–17):
INFa, cancer nests exhibited expanding growth and a distinct border
with the surrounding tissues; INFb, characteristics between those
of INFa and INFc; and INFc, cancer nests exhibited infiltrative
growth and an indistinct border with the surrounding tissues. Since
some specimens included two INF patterns, the patients were divided
into seven INF categories: INFa, INFa>b, INFa<b, INFb,
INFb>c, INFb<c, and INFc. These seven categories were divided
into an INFc(−) group, which consisted of INFa, INFa>b,
INFa<b and INFb; and an INFc(+) group, which consisted of
INFb>c, INFb<c and INFc (4,6).
The degree of lymph node metastasis was classified
according to the TNM system as follows: N0, no lymph node
metastasis; N1, ipsilateral peribronchial and/or hilar lymph node
metastasis; or N2, ipsilateral mediastinal and/or subcarinal lymph
node metastasis (26). The stromal
type (i.e., the cancer-stroma relationship pattern) was classified
as follows: Medullary, with scanty stroma; intermediate, with a
quantity of stroma intermediate between the scirrhous and medullary
types; and scirrhous, with abundant stroma (15).
Statistical analysis
Univariate analyses were performed to identify
significant differences between the groups (χ2 test,
P<0.05). Cox univariate and multivariate proportional hazard
regression analyses were performed to determine the independent
effects of individual factors while controlling for the effects of
the other factors. Univariate and multivariate analyses were also
performed to investigate the association between the degree of
lymphatic invasion and patient prognosis. Multivariate analysis was
performed for all factors; five representative factors are
presented in the present study. Hazard ratios (HRs) with 95%
confidence intervals (CIs) were calculated to assess the impact of
individual factors on prognosis. P<0.05 was considered to
indicate a statistically significant difference.
Patient survival was measured from the date of
surgery until mortality from any cause. Survival curves were
constructed using the Kaplan-Meier method and were compared using
the log-rank test. All analyses were performed using the SPSS II
statistical software package (version 19.0; IBM SPSS, Tokyo,
Japan).
Results
Association between lymphatic invasion
and patient survival
The degree of lymphatic invasion was classified into
four groups: Ly0 in 43 cases (41.7%), ly1 in 41 cases (39.8%), ly2
in 12 cases (11.7%) and ly3 in 7 cases (6.8%). The association
between degree of lymphatic invasion and patient survival is
presented in Table I. Analysis
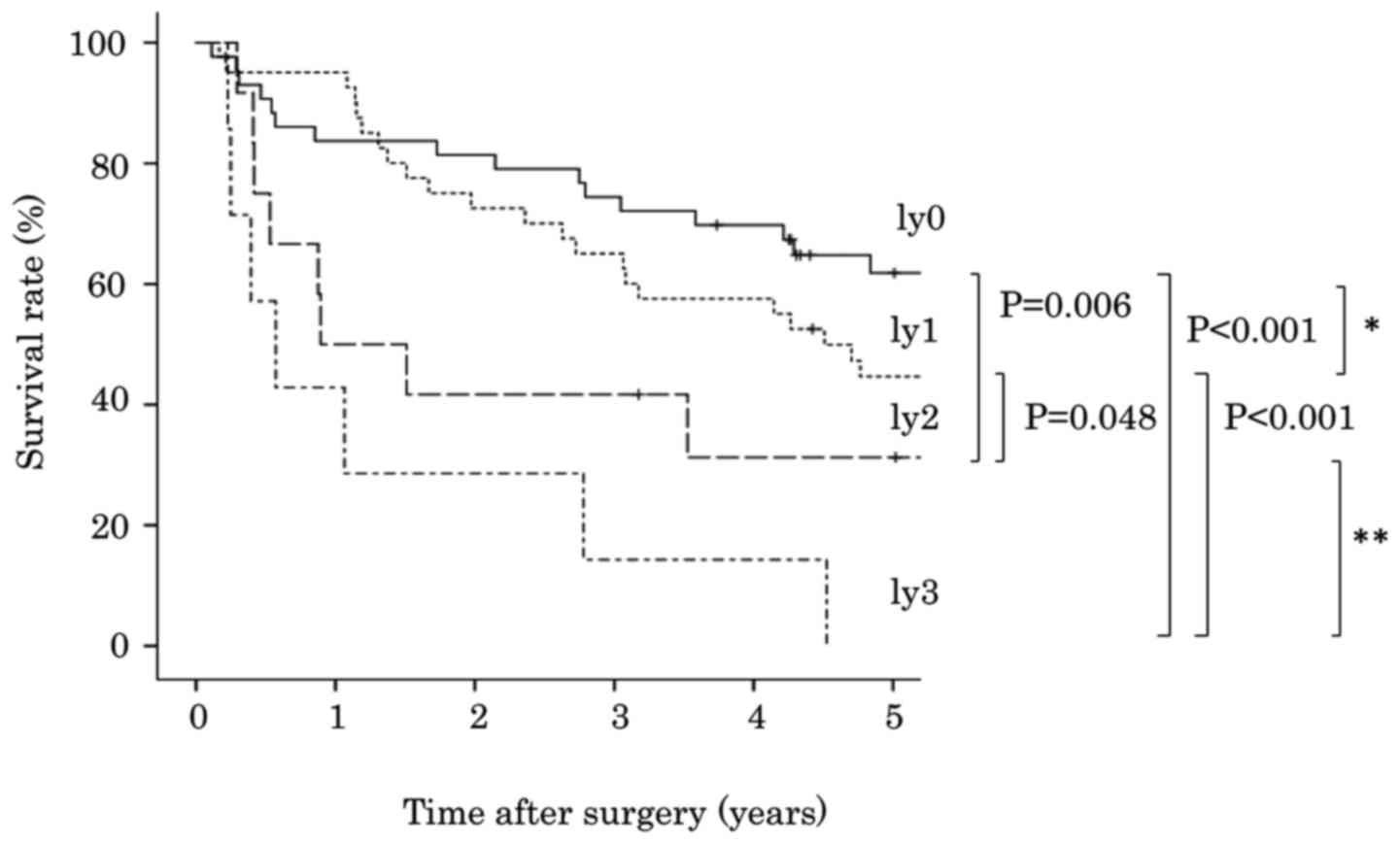
using the Kaplan-Meier method and the log-rank test indicated that
patients with ly2 exhibited poorer survival compared with patients
with ly1 (P=0.048; Fig. 2).
However, overall survival was not significantly different between
patients with ly0 and ly1 (P=0.237), or between patients with ly2
and ly3 (P=0.138). A more statistically significant difference was
detected between patients with ly0-1 and ly2-3 (P<0.001),
compared with between patients with ly0 and ly1-3 (P=0.018,
Fig. 3).
 | Table I.Lymphatic invasion and survival in
patients with lung squamous cell carcinoma. |
Table I.
Lymphatic invasion and survival in
patients with lung squamous cell carcinoma.
| Variable | No. of patients
(%) | P-value | Hazard ratio | 95% Confidence
interval |
|---|
| Lymphatic
invasion |
| <0.001 |
|
|
| ly0 | 43 (41.7) |
| 1.854 | 1.390–2.474 |
|
ly1-3 | 60 (58.3) |
|
|
|
| Lymphatic
invasion |
| <0.001 |
|
|
|
ly0-1 | 84 (91.6) |
| 3.298 | 1.827–5.952 |
|
ly2-3 | 19 (18.4) |
|
|
|
| Lymphatic
invasion |
| <0.001 |
|
ly0-2 | 96 (93.2) |
| 4.752 | 2.115–10.677 |
| ly3 | 7 (6.8) |
|
|
|
Association between lymphatic invasion
and clinicopathological features
The stromal type of SqCC was medullary in 39 cases
(37.9%), intermediate in 31 cases (30.1%), and scirrhous in 33
cases (32.0%). The INF patterns were classified as follows: INFa in
11 patients (10.7%), INFa>b in 10 patients (9.7%), INFb in 43
patients (41.7%), INFb>c in 31 patients (30.1%), INFb<c in 4
patients (3.9%), and INFc in 4 patients (3.9%); therefore, 64
patients (62.1%) were classified as INFc(+) and 39 patients (37.9%)
were classified as INFc(−). The associations between degree of
lymphatic invasion and clinicopathological features are presented
in Table II. Ly2-3 was
significantly associated with larger tumor size (P=0.028), lymph
node metastasis (P<0.001), venous invasion (P=0.001) and poor
differentiation (P=0.047), as compared with ly0-1. Univariate
analyses identified five factors that were significantly associated
with increased mortality (Table
III): Increased tumor size (HR, 1.897; 95% CI, 1.059–3.396);
lymph node metastasis (HR, 3.028; 95% CI, 1.785–5.136); lymphatic
invasion (HR, 3.298; 95% CI, 1.827–5.952); poor differentiation
(HR, 2.092; 95% CI, 1.050–4.168) and INFc(−) (HR, 2.209; 95% CI,
1.301–3.749). Scirrhous stromal type was not significantly
associated with survival (HR, 1.229; 95% CI, 0.706–2.139). In
addition, multivariate analysis identified ly2-3 as an independent
predictor of mortality (HR, 2.580; 95% CI, 1.376–4.839; Table IV).
 | Table II.Lymphatic invasion and
clinicopathological features of lung squamous cell carcinoma. |
Table II.
Lymphatic invasion and
clinicopathological features of lung squamous cell carcinoma.
| Variable | No. of patients
(%) | ly0-1 (%) | ly2-3 (%) | P-value |
|---|
| Age at surgery
(years) |
|
|
| 0.910 |
|
<68 | 53 (51.5) | 43 (81.1) | 10 (18.9) |
|
| ≥68 | 50 (48.5) | 41 (82.0) | 9 (18.0) |
|
| Gender |
|
|
| 0.230 |
| Male | 97 (94.2) | 78 (80.4) | 19 (19.6) |
|
|
Female | 6 (5.8) | 6 (100.0) | 0 (0.0) |
|
| Tumor size (mm) |
|
|
| 0.028 |
| ≤30 | 39 (37.9) | 36 (92.3) | 3 (7.7) |
|
|
>30 | 64 (62.1) | 48 (75.0) | 16 (25.0) |
|
| Lymph node
metastasis |
|
|
| <0.001 |
|
n(−) | 70 (68.0) | 66 (94.3) | 4 (5.7) |
|
|
n(+) | 33 (32.0) | 18 (54.5) | 15 (45.5) |
|
| Venous
invasion |
|
|
| 0.001 |
|
v(−) | 53 (51.5) | 50 (94.3) | 3 (5.7) |
|
|
v(+) | 50 (48.5) | 34 (68.0) | 16 (32.0) |
|
| Histological
differentiation |
|
|
| 0.047 |
| Well,
moderate | 90 (87.4) | 76 (84.4) | 14 (15.6) |
|
|
Poorly | 13 (12.6) | 8 (61.5) | 5 (38.5) |
|
| Stromal type |
|
|
| 0.298 |
|
Medullary, intermediate | 70 (68.0) | 59 (84.3) | 11 (15.7) |
|
|
Scirrhous | 33 (32.0) | 25 (75.8) | 8 (24.2) |
|
| Infiltrating
pattern |
|
|
| 0.142 |
|
INFc(−) | 64 (62.1) | 55 (85.9) | 9 (14.1) |
|
|
INFc(+) | 39 (37.9) | 29 (74.4) | 10 (25.6) |
|
 | Table III.Clinicopathological features and
survival in patients with lung squamous cell carcinoma. |
Table III.
Clinicopathological features and
survival in patients with lung squamous cell carcinoma.
| Variable | No. of patients
(%) | P-value | Hazard ratio | 95% Confidence
interval |
|---|
| Age at surgery
(years) |
| 0.131 |
|
|
|
<68 | 53 (51.5) |
| 1.502 | 0.885–2.548 |
|
≥68 | 50 (48.5) |
|
|
|
| Gender |
| 0.904 |
|
|
|
Male | 97 (94.2) |
| 0.939 | 0.339–2.602 |
|
Female | 6 (5.8) |
|
|
|
| Tumor size
(mm) |
| 0.031 |
|
|
|
≤30 | 39 (37.9) |
| 1.897 | 1.059–3.396 |
|
>30 | 64 (62.1) |
|
|
|
| Lymph node
metastasis |
| <0.001 |
|
|
|
n(−) | 70 (68.0) |
| 3.028 | 1.785–5.136 |
|
n(+) | 33 (32.0) |
|
|
|
| Lymphatic
invasion |
| <0.001 |
|
|
|
ly0 | 43 (41.7) |
| 1.854 | 1.390–2.474 |
|
ly1-3 | 60 (58.3) |
|
|
|
| Lymphatic
invasion |
| <0.001 |
|
|
|
ly0-1 | 84 (91.6) |
| 3.298 | 1.827–5.952 |
|
ly2-3 | 19 (18.4) |
|
|
|
| Venous
invasion |
| 0.145 |
|
|
|
v(−) | 53 (51.5) |
| 1.486 | 0.873–2.530 |
|
v(+) | 50 (48.5) |
|
|
|
| Histological
differentiation |
| 0.036 |
|
|
| Well,
mod | 90 (87.4) |
| 2.092 | 1.050–4.168 |
|
Poorly | 13 (12.6) |
|
|
|
| Stromal type |
| 0.465 |
|
|
|
Medullary, intermediate | 70 (68.0) |
| 1.229 | 0.706–2.139 |
|
Scirrhous | 33 (32.0) |
|
|
|
| Infiltrating
pattern |
| 0.003 |
|
|
|
INFc(−) | 64 (62.1) |
| 2.209 | 1.301–3.749 |
|
INFc(+) | 39 (37.9) |
|
|
|
 | Table IV.Multivariate analysis of
clinicopathological features and survival in patients with lung
squamous cell carcinoma. |
Table IV.
Multivariate analysis of
clinicopathological features and survival in patients with lung
squamous cell carcinoma.
| Variable | No. of patients
(%) | P-value | Hazard ratio | 95% Confidence
interval |
|---|
| Age at surgery
(years) |
| 0.179 |
|
|
|
<68 | 53 (51.5) |
| 1.461 | 0.840–2.540 |
|
≥68 | 50 (48.5) |
|
|
|
| Gender |
| 0.784 |
|
|
|
Male | 97 (94.2) |
| 1.157 | 0.408–3.281 |
|
Female | 6 (5.8) |
|
|
|
| Tumor size
(mm) |
| 0.083 |
|
|
|
≤30 | 39 (37.9) |
| 1.700 | 0.933–3.098 |
|
>30 | 64 (62.1) |
|
|
|
| Infiltrating
pattern |
| 0.058 |
|
|
|
INFc(−) | 64 (62.1) |
| 1.723 | 0.981–3.027 |
|
INFc(+) | 39 (37.9) |
|
|
|
| Lymphatic
invasion |
| 0.003 |
|
|
|
ly0-1 | 84 (81.6) |
| 2.580 | 1.376–4.839 |
|
ly2-3 | 19 (18.4) |
|
|
|
Discussion
The present study investigated the degree of
lymphatic invasion and other clinicopathological features of lung
SqCC, and demonstrated that ly2-3 was associated with higher
malignant potential compared with ly0-1. A total of 18% of patients
with lung SqCC had ly2-3, and exhibited higher rates of lymph node
metastasis and poorer overall survival compared with those with
ly0-1. A previous study reported that vessel invasion was a
predictor of poor prognosis in patients with non-small cell lung
cancer (8–10). However, the majority of patients in
that previous study had adenocarcinoma, and lymphatic invasion has
not previously been reported as a potential prognostic factor in
patients with non-small cell lung cancer. To the best of our
knowledge, this is the first study to report an association between
the degree of lymphatic invasion and prognosis in patients with
lung SqCC.
Several patients with lung SqCC have undergone
surgical resection due to advances in imaging, other diagnostic
techniques, and operative procedures (27,28).
In these patients, the most important prognostic factor is thought
to be pathological stage according to the TNM classification
system. Although lobectomy and lymph node dissection are standard
surgical procedures, some patients with poor pulmonary function
only undergo limited resection without lymph node dissection. In
these patients, lymph node metastasis (N factor) is histologically
unclear, and the pathological stage cannot be determined. The main
histological information is obtained from the primary tumor (T
factor, which predominantly accounts for tumor size), which is
insufficient to determine prognosis. Lung cancer is evaluated
according to morphological features, including histological type,
histological differentiation, pleural invasion, blood vessel
invasion and lymphatic invasion (8–10).
Evaluations of cancer in other organs include histological factors,
such as the INF pattern and stromal type (4–7,29,30).
Therefore, the present study considered it important to evaluate
these factors in lung cancer, in combination with the conventional
factors. Various treatments are currently available for patients
who develop postoperative recurrence of lung adenocarcinoma;
however, there are not any effective treatments available for
patients who develop postoperative recurrence of lung SqCC
(20–24). Therefore, the identification of
factors that predict patient prognosis is important for SqCC, in
order to enable early detection and treatment of recurrence, and to
determine the need for postoperative adjuvant therapy. In addition,
adenocarcinoma, SqCC and large cell carcinoma are all categorized
as non-small cell carcinoma. However, ~35% of non-small cell
carcinoma cases are SqCC, and SqCC must be studied separately from
adenocarcinoma.
Our previous study reported an association between
INF pattern and survival in patients undergoing treatment for SqCC
of the lung (6). The present study
evaluated the local aggressiveness of SqCC by the degree of
lymphatic invasion. When patients were classified into two groups,
namely, those with positive (ly1-3) or negative (ly0) lymphatic
invasion, a significant difference in survival was observed between
them. However, there was a more statistically significant
difference in survival between patients with ly0-1 and ly2-3.
Univariate analysis indicated a significant difference in survival
between patients with ly1-3 and ly0; however, the HR was 1.854,
which was lower than the HR of 3.298 for the comparison of survival
between patients with ly0-1 and ly2-3. Lymph node metastasis was
excluded from the multivariate analysis since there was a moderate
linear relationship between lymphatic invasion and lymph node
metastasis. This finding may help to predict the prognosis in
patients undergoing limited resection. In the present study,
patients with ly2-3 exhibited a significantly higher rate of lymph
node metastasis compared with those with ly0-1 (P<0.001). Since
the prediction of lymph node metastasis is important in patients
with lung cancer who may undergo limited resection, the authors of
the present study aim to conduct further studies to clarify the
relationship between lymph node metastasis and the morphological
features of SqCC using immunohistochemical/molecular analyses. The
present study also analyzed the relationship between venous
invasion and prognosis, but found no significant differences in
prognosis among the categories of blood vessel invasion (data not
shown).
In conclusion, the degree of lymphatic invasion in
lung SqCC is associated with local tumor aggressiveness, and may be
a useful indicator of prognosis.
References
|
1
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung CK, Zaino R, Stryker JA, O'Neill M
Jr and DeMuth WE Jr: Carcinoma of the lung: Evaluation of
histological grade and factors influencing prognosis. Ann Thorac
Surg. 33:599–604. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gajra A, Newman N, Gamble GP, Abraham NZ,
Kohman LJ and Graziano SL: Impact of tumor size on survival in
stage IA non-small cell lung cancer: A case for subdividing stage
IA disease. Lung Cancer. 42:51–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito E, Ozawa S, Kijima H, Kazuno A, Nishi
T, Chino O, Shimada H, Tanaka M, Inoue S, Inokuchi S and Makuuchi
H: New invasive patterns as a prognostic factor for superficial
esophageal cancer. J Gastroenterol. 47:1279–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okada K, Kijima H, Imaizumi T, Hirabayashi
K, Matsuyama M, Yazawa N, Dowaki S, Tobita K, Ohtani Y, Tanaka M,
et al: Clinical significance of wall invasion pattern of
subserosa-invasive gallbladder carcinoma. Oncol Rep. 28:1531–1536.
2012.PubMed/NCBI
|
|
6
|
Masuda R, Kijima H, Imamura N, Aruga N,
Nakamura Y, Masuda D, Takeichi H, Kato N, Nakagawa T, Tanaka M, et
al: Tumor budding is a significant indicator of a poor prognosis in
lung squamous cell carcinoma patients. Mol Med Rep. 6:937–943.
2012.PubMed/NCBI
|
|
7
|
Yokota T, Kunii Y, Teshima S, Yamada Y,
Saito T, Takahashi M, Kikuchi S and Yamauchi H: Significant
prognostic factors in patients with early gastric cancer. Int Surg.
85:286–290. 2000.PubMed/NCBI
|
|
8
|
Harpole DH Jr, JE II Herndon, Young WG Jr,
Wolfe WG and Sabiston DC Jr: Stage I nonsmall cell lung cancer. A
multivariate analysis of treatment methods and patterns of
recurrence. Cancer. 76:787–796. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichinose Y, Yano T, Asoh H, Yokoyama H,
Yoshino I and Katsuda Y: Prognostic factors obtained by a
pathologic examination in completely resected non-small-cell lung
cancer. An analysis in each pathologic stage. J Thorac Cardiovasc
Surg. 110:601–605. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duarte IG, Bufkin BL, Pennington MF, Gal
AA, Cohen C, Kosinski AS, Mansour KA and Miller JI: Angiogenesis as
a predictor of survival after surgical resection for stage I
non-small-cell lung cancer. J Thorac Cardiovasc Surg. 115:652–659.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crabbe MM, Patrissi GA and Fontenelle LJ:
Minimal resection for bronchogenic carcinoma. An update. Chest.
99:1421–1424. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martini N, Rusch VW, Bains MS, Kris MG,
Downey RJ, Flehinger BJ and Ginsberg RJ: Factors influencing
ten-year survival in resected stages I to IIIa non-small cell lung
cancer. J Thorac Cardiovasc Surg. 117:32–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hylkema MN, Sterk PJ, deBoer WI and Postma
DS: Tobacco use in relation to COPD and asthma. Eur Respir J.
29:438–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gratziou Ch, Florou A, Ischaki E,
Eleftheriou K, Sachlas A, Bersimis S and Zakynthinos S: Smoking
cessation effectiveness in smokers with COPD and asthma under real
life conditions. Respir Med. 108:577–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maehara Y, Oshiro T, Adachi Y, Ohno S,
Akazawa K and Sugimachi K: Growth pattern and prognosis of gastric
cancer invading the subserosa. J Surg Oncol. 55:203–208. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haraguchi M, Yamamoto M, Saito A, Kakeji
Y, Orita H, Korenaga D and Sugimachi K: Prognostic value of depth
and pattern of stomach wall invasion in patients with an advanced
gastric carcinoma. Semin Surg Oncol. 10:125–129. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dembitzer FR, Flores RM, Parides MK and
Beasley MB: Impact of histological subtyping on outcome in lobar vs
sublobar resections for lung cancer: A pilot study. Chest.
146:175–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koike T, Koike T, Yoshiya K, Tsuchida M
and Toyabe S: Risk factor analysis of locoregional recurrence after
sublobar resection in patients with clinical stage IA non-small
cell lung cancer. J Thorac Cardiovasc Surg. 146:372–378. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rossi A, Ricciardi S, Maione P, de Marinis
F and Gridelli C: Pemetrexed in the treatment of advanced
non-squamous lung cancer. Lung Cancer. 66:141–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosell R, Perez-Roca L, Sanchez JJ, Cobo
M, Moran T, Chaib I, Provencio M, Domine M, Sala MA, Jimenez U, et
al: Customized treatment in non-small-cell lung cancer based on
EGFR mutations and BRCA1 mRNA expression. PLoS One. 4:e51332009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong J, Kyung SY, Lee SP, Park JW, Jung
SH, Lee JI, Park SH, Sym SJ, Park J, Cho EK, et al: Pemetrexed
versus gefitinib versus erlotinib in previously treated patients
with non-small cell lung cancer. Korean J Intern Med. 25:294–300.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumors. 7th edition.
Wiley; Oxford: 2009
|
|
26
|
Travis WD, Brambilla E, Müller-Hermelin HK
and Harris CC: World Health Organization Classification of Tumors.
Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and
Heart. IARC Press; Lyon: 2004
|
|
27
|
Beattie G, Bannon F and McGuigan J: Lung
cancer resection rates have increased significantly in females
during a 15-year period. Eur J Cardiothorac Surg. 38:484–490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin-Ucar AE, Waller DA, Atkins JL,
Swinson D, O'Byrne KJ and Peake MD: The beneficial effects of
specialist thoracic surgery on the resection rate for
non-small-cell lung cancer. Lung Cancer. 46:227–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masuda R, Kijima H, Imamura N, Aruga N,
Nakazato K, Oiwa K, Nakano T, Watanabe H, Ikoma Y, Tanaka M, et al:
Laminin-5γ2 chain expression is associated with tumor cell
invasiveness and prognosis of lung squamous cell carcinoma. Biomed
Res. 33:309–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okada K, Kijima H, Imaizumi T, Hirabayashi
K, Matsuyama M, Yazawa N, Oida Y, Dowaki S, Tobita K, Ohtani Y, et
al: Stromal laminin-5gamma2 chain expression is associated with the
wall-invasion pattern of gallbladder adenocarcinoma. Biomed Res.
30:53–62. 2009. View Article : Google Scholar : PubMed/NCBI
|