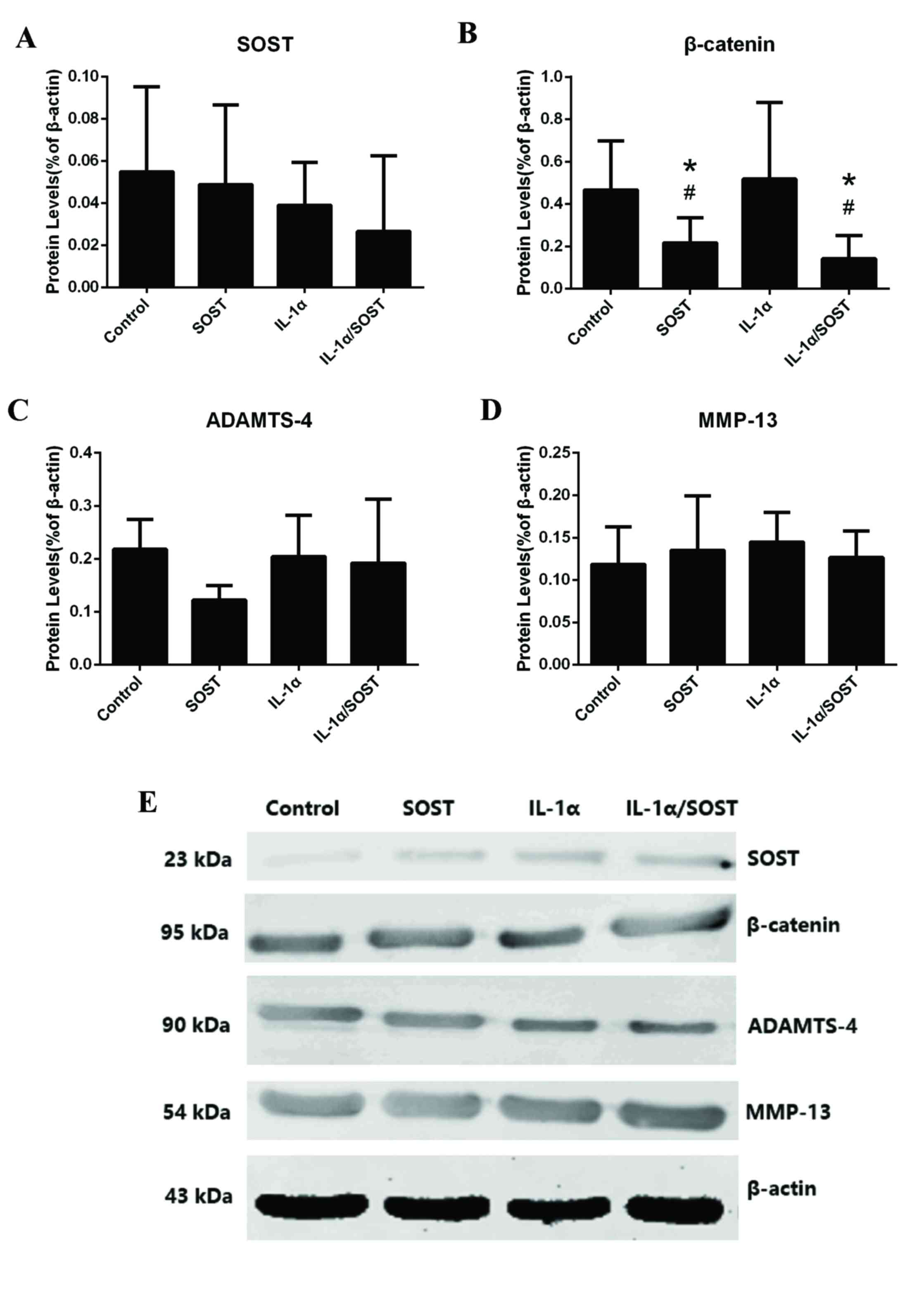
|
1
|
Bijlsma JW, Berenbaum F and Lafeber FP:
Osteoarthritis: An update with relevance for clinical practice.
Lancet. 377:2115–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felson DT: Developments in the clinical
understanding of osteoarthritis. Arthritis Res Ther. 11:2032009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Welgus HG: Stromelysin: Structure and
function. Agents Actions Suppl. 35:61–67. 1991.PubMed/NCBI
|
|
4
|
Karsdal MA, Leeming DJ, Dam EB, Henriksen
K, Alexandersen P, Pastoureau P, Altman RD and Christiansen C:
Should subchondral bone turnover be targeted when treating
osteoarthritis? Osteoarthritis Cartilage. 16:638–646. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoeppner LH, Secreto FJ and Westendorf JJ:
Wnt signaling as a therapeutic target for bone diseases. Expert
Opin Ther Targets. 13:485–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuasa T, Otani T, Koike T, Iwamoto M and
Enomoto-Iwamoto M: Wnt/beta-catenin signaling stimulates matrix
catabolic genes and activity in articular chondrocytes: Its
possible role in joint degeneration. Lab Invest. 88:264–274. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C,
Rosier RN, O'Keefe RJ, Zuscik M and Chen D: Activation of
beta-catenin signaling in articular chondrocytes leads to
osteoarthritis-like phenotype in adult beta-catenin conditional
activation mice. J Bone Miner Res. 24:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blom AB, Brockbank SM, van Lent PL, van
Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo
FA, Schreurs BW, Clements K, et al: Involvement of the Wnt
signaling pathway in experimental and human osteoarthritis:
Prominent role of Wnt-induced signaling protein 1. Arthritis Rheum.
60:501–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamamura Y, Otani T, Kanatani N, Koyama E,
Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici
M, et al: Developmental regulation of Wnt/beta-catenin signals is
required for growth plate assembly, cartilage integrity, and
endochondral ossification. J Biol Chem. 280:19185–19195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Bezooijen RL, Roelen BA, Visser A, van
der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE,
ten Dijke P and Löwik CW: Sclerostin is an osteocyte-expressed
negative regulator of bone formation, but not a classical BMP
antagonist. J Exp Med. 199:805–814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang
J, Harris SE and Wu D: Sclerostin binds to LRP5/6 and antagonizes
canonical Wnt signalin. J Biol Chem. 280:19883–19887. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karlsson C, Dehne T, Lindahl A, Brittberg
M, Pruss A, Sittinger M and Ringe J: Genome-wide expression
profiling reveals new candidate genes associated with
osteoarthritis. Osteoarthritis Cartilage. 18:581–592. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan BY, Fuller ES, Russell AK, Smith SM,
Smith MM, Jackson MT, Cake MA, Read RA, Bateman JF, Sambrook PN and
Little CB: Increased chondrocyte sclerostin may protect against
cartilage degradation in osteoarthritis. Osteoarthritis Cartilage.
19:874–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bouaziz W, Funck-Brentano T, Lin H, Marty
C, Ea HK, Hay E and Cohen-Solal M: Loss of sclerostin promotes
osteoarthritis in mice via β-catenin-dependent and -independent Wnt
pathways. Arthritis Res Ther. 17:242015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roudier M, Li X, Niu QT, Pacheco E,
Pretorius JK, Graham K, Yoon BR, Gong J, Warmington K, Ke HZ, et
al: Sclerostin is expressed in articular cartilage but loss or
inhibition does not affect cartilage remodeling during aging or
following mechanical injury. Arthritis Rheum. 65:721–731. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moody HR, Heard BJ, Frank CB, Shrive NG
and Oloyede AO: Investigating the potential value of individual
parameters of histological grading systems in a sheep model of
cartilage damage: The Modified Mankin method. J Anat. 221:47–54.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poole KE, van Bezooijen RL, Loveridge N,
Hamersma H, Papapoulos SE, Löwik CW and Reeve J: Sclerostin is a
delayed secreted product of osteocytes that inhibits bone
formation. FASEB J. 19:1842–1844. 2005.PubMed/NCBI
|
|
19
|
Irie K, Ejiri S, Sakakura Y, Shibui T and
Yajima T: Matrix mineralization as a trigger for osteocyte
maturation. J Histochem Cytochem. 56:561–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Bezooijen RL, Bronckers AL, Gortzak
RA, Hogendoorn PC, van der Wee-Pals L, Balemans W, Oostenbroek HJ,
Van Hul W, Hamersma H, Dikkers FG, et al: Sclerostin in mineralized
matrices and van Buchem disease. J Dent Res. 88:569–574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Winkler DG, Sutherland MK, Geoghegan JC,
Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR,
Staehling-Hampton K, et al: Osteocyte control of bone formation via
sclerostin, a novel BMP antagonist. EMBO J. 22:6267–6276. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ellies DL, Viviano B, McCarthy J, Rey JP,
Itasaki N, Saunders S and Krumlauf R: Bone density ligand,
Sclerostin, directly interacts with LRP5 but not LRP5G171 V to
modulate Wnt activity. J Bone Miner Res. 21:1738–1749. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang
J, Li Y, Feng G, Gao X and He L: Sclerostin mediates bone response
to mechanical unloading through antagonizing Wnt/beta-catenin
signaling. J Bone Miner Res. 24:1651–1661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang SG, Yu SS, Ryu JH, Jeon HB, Yoo YJ,
Eom SH and Chun JS: Regulation of beta-catenin signaling and
maintenance of chondrocyte differentiation by ubiquitin-independent
proteosomal degradation of alpha-catenin. J Biol Chem.
280:12758–12765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldring MB and Goldring SR: Articular
cartilage and subchondral bone in the pathogenesis of
osteoarthritis. Ann N Y Acad Sci. 1192:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewiecki EM: Role of sclerostin in bone
and cartilage and its potential as a therapeutic target in bone
diseases. Ther Adv Musculoskelet Dis. 6:48–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Findlay DM and Atkins GJ:
Osteoblast-chondrocyte interactions in osteoarthritis. Curr
Osteoporos Rep. 12:127–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bove SE, Calcaterra SL, Brooker RM, Huber
CM, Guzman RE, Juneau PL, Schrier DJ and Kilgore KS: Weight bearing
as a measure of disease progression and efficacy of
anti-inflammatory compounds in a model of monosodium
iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage.
11:821–830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teichtahl AJ, Davies-Tuck ML, Wluka AE,
Jones G and Cicuttini FM: Change in knee angle influences the rate
of medial tibial cartilage volume loss in knee osteoarthritis.
Osteoarthritis Cartilage. 17:8–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baron R and Rawadi G: Targeting the
Wnt/beta-catenin pathway to regulate bone formation in the adult
skeleton. Endocrinology. 148:2635–2643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mohan G, Perilli E, Kuliwaba JS, Humphries
JM, Parkinson IH and Fazzalari NL: Application of in vivo
micro-computed tomography in the temporal characterisation of
subchondral bone architecture in a rat model of low-dose monosodium
iodoacetate-induced osteoarthritis. Arthritis Res Ther.
13:R2102011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Findlay DM and Atkins GJ:
Osteoblast-chondrocyte interactions in osteoarthritis. Current
osteoporosis reports. 12:127–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amin AK, Huntley JS, Simpson AH and Hall
AC: Chondrocyte survival in articular cartilage: The influence of
subchondral bone in a bovine model. J Bone Joint Surg Br.
91:691–699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sanchez C, Deberg MA, Piccardi N, Msika P,
Reginster JY and Henrotin YE: Osteoblasts from the sclerotic
subchondral bone downregulate aggrecan but upregulate
metalloproteinases expression by chondrocytes. This effect is
mimicked by interleukin-6, −1beta and oncostatin M pre-treated
non-sclerotic osteoblasts. Osteoarthritis Cartilage. 13:979–987.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang B, Zhou X, Price C, Li W, Pan J and
Wang L: Quantifying load-induced solute transport and solute-matrix
interaction within the osteocyte lacunar-canalicular system. J Bone
Miner Res. 28:1075–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imhof H, Sulzbacher I, Grampp S, Czerny C,
Youssefzadeh S and Kainberger F: Subchondral bone and cartilage
disease: A rediscovered functional unit. Invest Radiol. 35:581–588.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Botter SM, van Osch GJ, Clockaerts S,
Waarsing JH, Weinans H and van Leeuwen JP: Osteoarthritis induction
leads to early and temporal subchondral plate porosity in the
tibial plateau of mice: An in vivo microfocal computed tomography
study. Arthritis Rheum. 63:2690–2699. 2011. View Article : Google Scholar : PubMed/NCBI
|