Introduction
Vascular remodeling is recognized as a primary
contributor to the damage of target organs in the development of
hypertension (1). Artery walls
that have undergone hypertension are identified by the increased
size of the vascular wall, particularly the tunica media, which is
accompanied by a narrowed lumen. Proliferation of vascular smooth
muscle cells (VSMC) occurs, along with alterations to the elastic
properties of the arterial wall (2). In the vascular wall extracellular
matrix (ECM), the abnormal deposition of collagen contributes to
vascular remodeling (3). Reactive
oxygen species (ROS) are believed to be involved in the development
of hypertension via the promotion of vascular remodeling (4), which induces collagen hyperplasia in
the vascular wall (5). GSPE is an
anti-free radical reagent (6),
which may be beneficial for the treatment of cardiovascular
diseases, including arteriosclerosis, hypertension and subsequent
organ damage (7). ROS are second
messengers that regulate cellular function via reversibly oxidizing
specific amino acid residues of key target proteins (8), adversely affecting various important
cell signaling pathways, including the transforming growth factor
(TGF)-β1 signaling cascade and the expression of ECM (9). The present study hypothesized that
GSPE may have a therapeutic effect on vascular remodeling
associated with collagen accumulation in the mesenteric small
arteries of spontaneous hypertensive rats (SHR). The understanding
of the association between the anti-oxidative effect of GSPE and
the underlying mechanisms involved in vascular remodeling may
support its use as a potential therapeutic agent for the treatment
of small vascular remodeling in cardiovascular disease.
Materials and methods
Animal model
All experimental procedures were approved by the
Animal Experiment Ethics Review Committee of Shandong University,
(Jinan, China). At total of 20 SHR and 10 Wistar-Kyoto (WKY) rats
(age, 20 weeks; weight, 369.25±12.11 g) were obtained from Vital
River Laboratory Animal Technology Co., Ltd. (Beijing, China). Rats
were maintained in individual cages at 22±2°C, 50–55% humidity,
with 12 h light/dark cycles. They were fed with standard rat chow
and had free access to tap water for 1 week. Rats were subsequently
randomly assigned to three groups (n=10): i) WKY control (WKY), ii)
spontaneous hypertension control (SHR-C), and iii) spontaneous
hypertension with GSPE treatment (SHR-T). Rats in the WKY and SHR-C
groups were orally administered 1.0 ml saline per day, where as
those in the SHR-T group were treated with 250 mg/kg/day GSPE (lot
no. 002-1312065-13; Natural Product R&D Co., Ltd., Tianjin,
China) dissolved in 1.0 ml saline. At the end of week 22, rats were
sacrificed following anesthesia with 10% chloral hydrate (300
mg/kg) by intraperitoneal injection provided by Qilu Hospital of
Shandong University (Jinan, China). Serum from peripheral blood
obtained from the abdominal aorta and the harvested mesenteric
small arteries were stored at −80°C.
Systolic blood pressure (SBP)
Tail SBPs were measured prior to experimentation,
and subsequently once a week until the end of the experiment using
a non-invasive sphygmomanometer (Softron tail-cuff; Softron Beijing
Incorporated, Beijing, China). All rats were conscious and free
when measurements were obtained.
ELISA
Malondialdehyde (MDA), H2O2,
ROS, superoxide dismutase (SOD), and catalase (CAT) levels in
mesenteric artery tissues were measured using the following
oxidative stress ELISA kits: H2O2 assay kit
(cat. no. 20131220; absorbance reading at 405 nm), ROS assay kit
(cat. no. 20131126; absorbance reading at 525 nm), SOD assay kit
(cat. no. 20131120; absorbance reading at 450 nm), CAT assay kit
(cat. no. 20131218; absorbance reading at 405 nm) and Microscale
MDA assay kit (cat. no. 20131112; absorbance reading at 523 nm),
which were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China) according to the manufacturer's
protocol.
Histopathology
Mesenteric small artery samples for protein and RNA
detection were washed in 4°C saline and frozen in liquid nitrogen
followed by storage at −80°C. Segments for electron microscopy were
immediately cut into 1×1×1 mm sections and fixed in 2.5%
glutaraldehyde solution. Fixed sections were processed with 10%
formalin and embedded in paraffin. Vascular tissue slices were
stained with Sirius red. Pathological alterations were observed and
analyzed in 5 randomly selected images per section using the
Olympus DP71 imaging system (Olympus Corporation, Tokyo, Japan) and
Image-Pro Plus software 5.0 (Media Cybernetics, Inc., Rockville,
MD, USA). The inner diameter (ID), vascular cross-sectional area
(VCSA), wall cross-sectional area (WCSA), luminal cross-sectional
area (LCSA) and wall thickness (WT) of the vascular tissue were
measured. The ratios of WT to ID (WT/ID) and WCSA to LCSA
(WCSA/LCSA) were subsequently calculated.
Type I and III collagen and elastin were stained
with Sirius red-victoria blue (10) and were observed using polarization
microscopy. The area and ratio of elastin and collagen were
calculated using Image Pro-Plus software in 5 different fields.
Western blotting
Total protein was extracted from the small
mesenteric arterial tissue. Tissues were washed twice with cold
phosphate buffered saline and were homogenized with ice-cold cell
radio immunoprecipitation assay lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology, Haimen, China) for 30 min on
ice and the supernatant was collected following centrifugation
(14,000 × g for 15 min) at 4°C. The concentration of total protein
was measured using a bicinchoninic acid protein assay kit (cat. no.
P0006; Beyotime Institute of Biotechnology). An equal quantity of
protein (60 µg) was loaded onto SDS-PAGE gels (12%
Mini-PROTEAN® Gel; cat. no. 456-1094; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The proteins were
transferred to polyvinylidene difluoride membranes which was
monitored by Ponceau S staining. Membranes were blocked with
blocking buffer [5% fat free milk in TBS plus 0.1% Tween-20 (TBST),
pH 7.5] at room temperature for 1 h and then incubated for 2 h with
the following primary antibodies: Mouse-anti-TGF-β1 (cat. no.
ab64715; dilution, 1:500; Abcam, Cambridge, MA, USA),
rabbit-anti-collagen I (cat. no. ab96723; dilution, 1:500; Abcam),
rabbit-anti-collagen III (cat. no. 13548-1; dilution, 1:400;
Protein Tech Group, Inc., Chicago, IL, USA) and mouse-anti-actin
(cat. no. ab3280; dilution, 1:2,000; Abcam) at 4°C overnight.
Following 3 washes with TBST (10 min each), membranes were
incubated with a species-specific horseradish peroxidase-conjugated
secondary antibodies: Goat anti-rabbit (cat. no. ZB-2301; dilution,
1:5,000; ZSGB-BIO, Beijing, China) and goat anti-mouse (cat. no.
Zf-0312; dilution, 1:5,000; ZSGB-BIO) at room temperature for 2 h.
Following 3 washes, membranes were soaked in Chemiluminescence
Detection reagent (EMD Millipore, Billerica, MA, USA) and protein
bands were visualized using an ImageQuant LAS-4000 imaging system
(GE Healthcare Life Sciences, Chalfont, UK). Quantitative analysis
was performed using AlphaEaseFC analysis software 4.1.0 (Alpha
Innotech, San Leandro, CA, USA) and normalized to densitometric
values of β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from small mesenteric
arterial tissue using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. First-strand cDNA was obtained using the
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) with oligo-d(T) as an initial primer. qPCR was
performed using a standard TaqMan® PCR kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the obtained cDNA
on an ABI PRISM® 7300 PCR instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in a 25 µl reaction
volume containing Maxima SYBR-Green qPCR Master Mix (2X) (Applied
Biosystems; Thermo Fisher Scientific, Inc.), cDNA and primers.
Primer sequences were as follows: Forward,
5′-CTGCTGACCCCCACTGATAC-3′ and reverse, 5′-GTGAGCACTGAAGCGAAAGC-3′
for TGF-β1; forward, 5′-GGCATAAAGGGTCATCGTG-3′ and reverse,
5′-GAACCTTCGCTTCCATACTC-3′ for collagen I; forward,
5′-AGATCATGTCTTCACTCAAGTC-3′ and reverse,
5′-TTTACATTGCCATTGGCCTAG-3′ for collagen III; forward,
5′-CGGGAAATCGTGCGTGAC-3′ and reverse, 5′-TCGCTCCAACCGACTGCT-3′ for
β-actin. Thermocycling conditions were set at 95°C for 20 sec,
followed by 60°C for 20 sec; 40 cycles were performed. The gene
expression was relatively quantified (11) and normalized by the expression of
β-actin which was performed according to the manufacturer's
protocol and the article (12).
Ultrastructure
The mesenteric arterial tissue was fixed with 3%
glutaraldehyde in cacodylate buffer at 4°C for 2 h and subsequently
post fixed in a 1% osmium-tetroxide phosphate buffer solution for 1
h. Dehydrated tissue sections were embedded in epoxy resin and were
sliced into 75-nm semi-thin sections, which were stained with
uranylacetate and lead citrate. Slides were observed under an H-800
transmission electron microscope (Hitachi, Ltd., Tokyo, Japan).
Statistical analysis
A one-way analysis of variance was performed using
SPSS software 17.0 (SPSS, Inc., Chicago, IL, USA), followed by a
Student-Newman-Keuls or least significant difference post-hoc test.
Results are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant difference
and the null hypothesis was that there were no differences between
the groups.
Results
Systolic blood pressure and body
weight
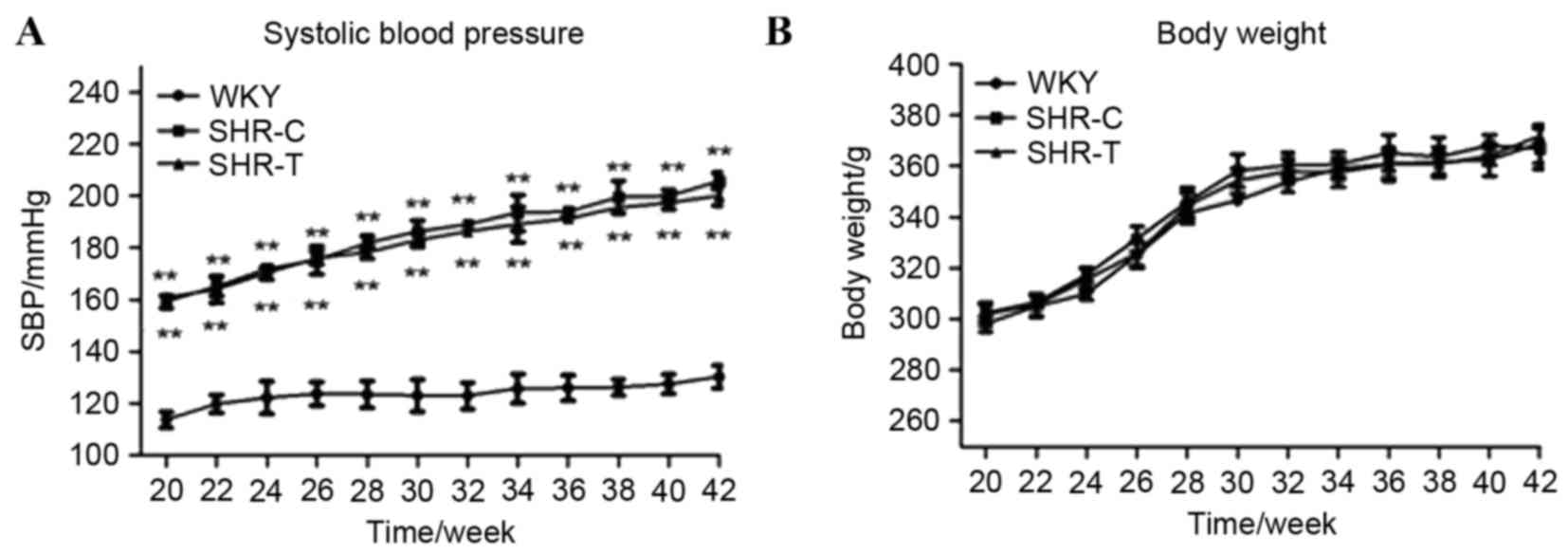
SBP increased with age in all groups, and was
significantly increased in the SHR-C compared with the WKY group
(P<0.01; Fig. 1A). Treatment
with GSPE did not significantly alter SBP compared with the SHR-C
group (P>0.5). No significant weight alterations were observed
between the groups at any time (P>0.5; Fig. 1B).
Anti-oxidative stress effect of GSPE
in spontaneous hypertension
Hypertensive rats exhibited significantly increased
levels of ROS (P<0.01; Fig.
2A), H2O2 (P<0.01; Fig. 2B) and MDA (P<0.01; Fig. 2C), compared with the WKY group.
However, this increase was partially reversed by treatment with
GSPE (P<0.05). The levels of the oxidative enzymes, SOD
(P<0.01; Fig. 2D) and CAT
(P<0.01; Fig. 2E) were
decreased in SHR-Compared with WKY controls; however, this decrease
was partially reversed by GSPE (P<0.05).
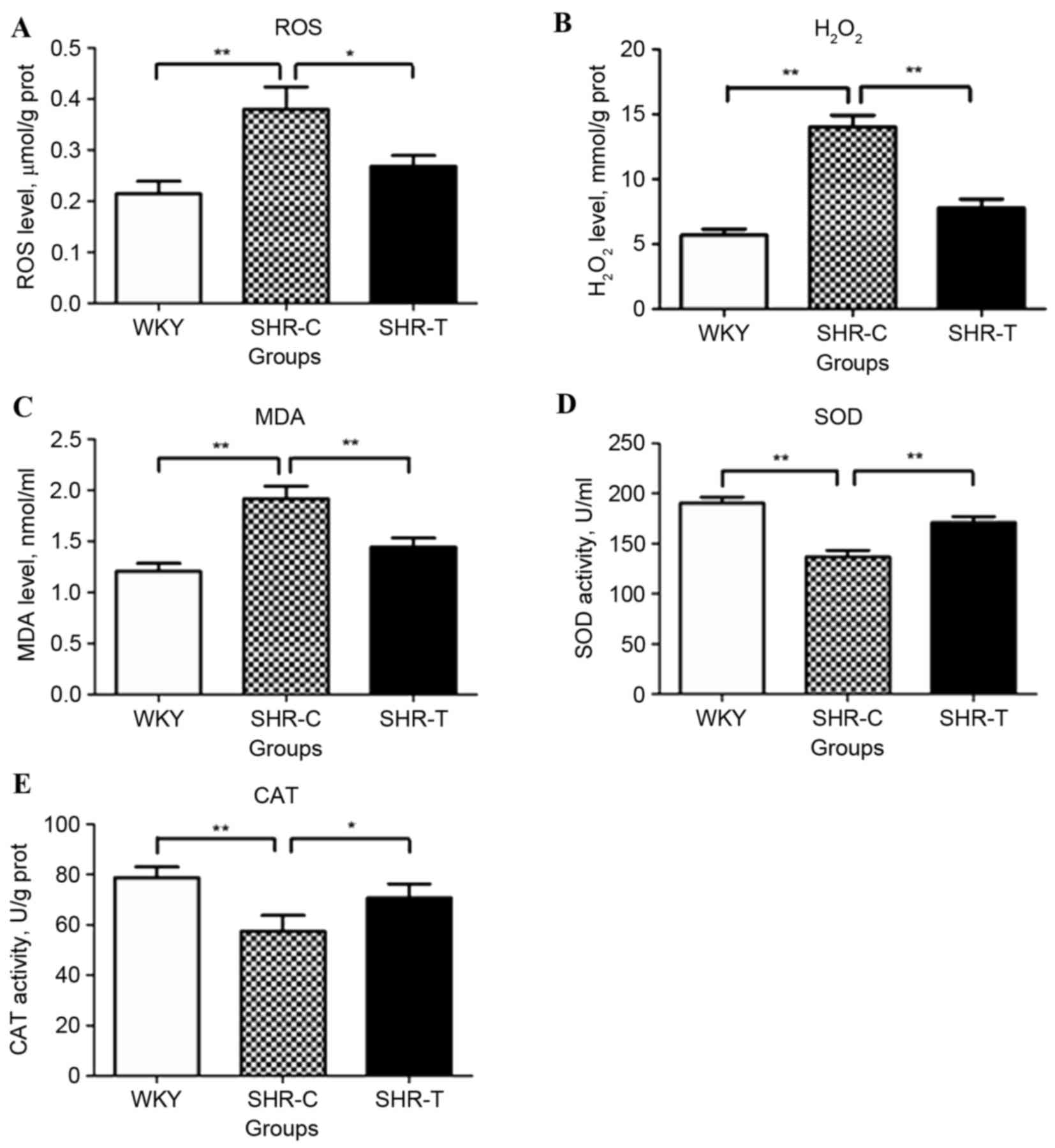 | Figure 2.Effect of GSPE on ROS,
H2O2, MDA, SOD, and CAT levels in mesenteric
arterial tissues. Hypertensive rats exhibited a significantly
increased level of (A) ROS, (B) H2O2 and (C)
MDA compared with the WKY control group. This was partially
reversed by treatment with GSPE. The levels of the oxidative
enzymes (D) SOD and (E) CAT were decreased in hypertensive
subjects; however, this was attenuated by administration of GSPE.
*P<0.05 and **P<0.01. GSPE, grape seed proanthocyanidin
extract; SHR, spontaneous hypertension rat; T, treatment; C,
control; WKY, Wistar-Kyoto; ROS, reactive oxygen species,
H2O2, hydrogen peroxide; MDA,
malondialdehyde; SOD, superoxide dismutase; CAT, catalase. |
Effect of GSPE on morphological
alterations of small arteries
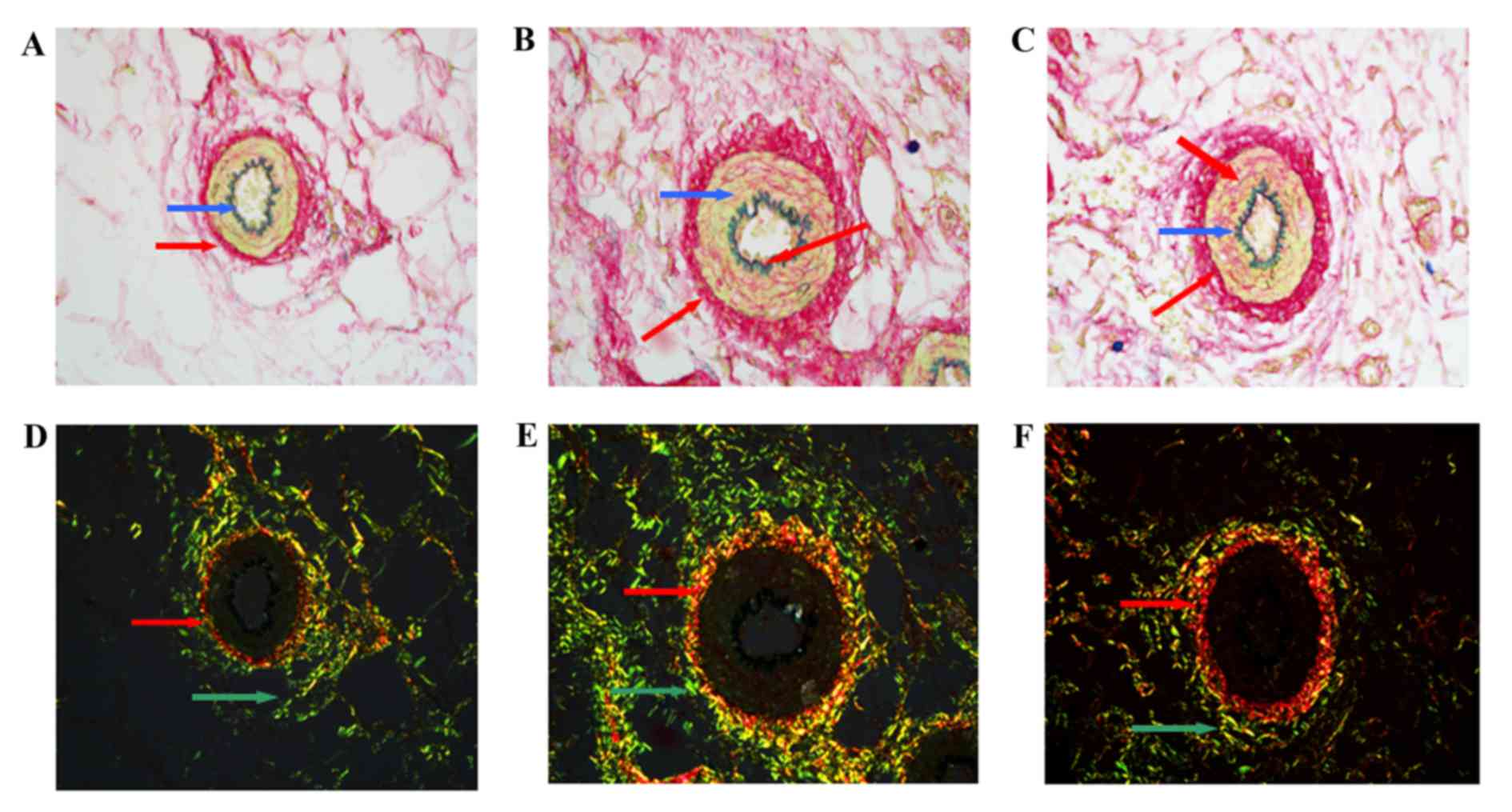
Vascular tissue slices were stained with Sirius
red-victoria blue and pathological changes were observed using
polarization microscopy primarily under a brightfield. In WKY, the
typical three-layer structure was observed. The inner layer was
thinner and the elastic lamina between the endangium and media was
dyed green-blue. VSMCs were arranged as a circular multilayer in
the media and collagens were observed as red, primarily resident in
the adventitia (Fig. 3A). In
SHR-C, broken endangium and collagen fibers in the lumen were
presented in images captured under a brightfield, and the small
mesenteric arteries had become narrowed and thickened. Disarranged
collagen was observed in the media and adventitia (Fig. 3B). Following GSPE treatment, wall
thickening and luminal stenosis of small mesenteric arteries were
reduced. In addition, collagen levels were reduced, particularly in
the media (Fig. 3C).
Effect of GSPE on collagen expression
and distribution in small mesenteric arteries
Type I/III collagen and elastin were stained with
Sirius red-victoria blue and were observed using polarization
microscopy. Collagen was observed as red and elastin was observed
as green-blue, under a brightfield. Under a darkfield, type I
collagen appeared as red/orange fibers with strong double
refraction, whereas type III collagen appeared as thin green fibers
with weak birefringence. In WKY, type I collagen was primarily
located in the media and type III collagen primarily appeared in
the adventitia, with reduced levels present in the media (Fig. 3D). There were no collagen fibers
present in the inner layer of the small artery. However, in the
SHR-C small mesenteric artery, an overload of disarranged collagen
was observed in the media and adventitia. In addition, collagen
fibers were observed in the inner layer (Fig. 3E). Following, GSPE treatment, no
collagen fibers were observed in the inner layer (Fig. 3F). Type I and III collagen were
reduced in the media compared with the SHR-C group.
Quantification of pathological
alterations
Image-ProPlus software was used to analyze
microscope images and to calculate the ID, WT, WT/ID, WCSA, LCSA
and WCSA/LCSA of small mesenteric arteries. Hypertension increased
WT (P<0.01; Fig. 4A) and
decreased ID (P<0.05; Fig. 4B),
and these effects were significantly abrogated by treatment with
GSPE (P<0.05). Notably, the ratio of WT/ID was significantly
greater in SHR-C compared with WKY and SHR-T groups (P<0.01;
Fig. 4C). Similarly, significantly
increased levels of WCSA (P<0.01; Fig. 4D) and decreased levels of LCSA
(P<0.05; Fig. 4E) were observed
in the SHR-C group compared with the WKY group, and these effects
were attenuated by treatment with GSPE (P<0.05). The ratio of
WCSA/LCSA was increased in the SHR-C group, compared with the WKY
control and SHR-T groups (P<0.05; Fig. 4F).
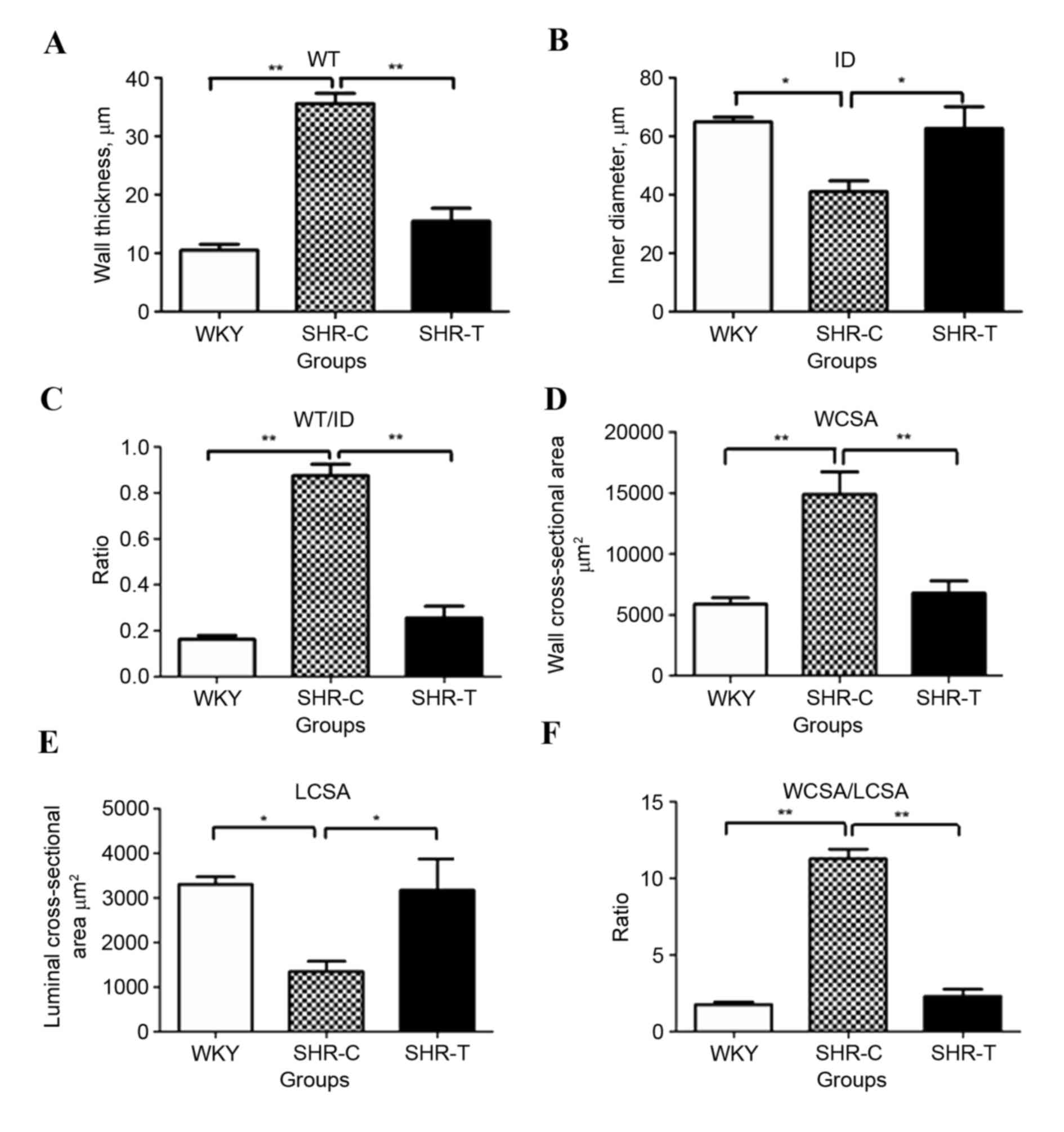 | Figure 4.Effect of GSPE on vascular
geometrical measurement of small mesenteric arteries. The diameter
of vascular wall and lumen and the thickness of vascular wall was
measured in Sirius red-victoria blue stained sections. (A) WT was
increased in the SHR-C group compared with the WKY group, and this
was significantly reversed by treatment with GSPE. (B) ID was
decreased in the SHR-C group compared with the WKY group, and this
was significantly reversed by treatment with GSPE. (C) The ratio of
WT/ID was significantly greater in the SHR-C group compared with
the WKY and SHR-T groups. (D) WCSA levels were significantly
increased in the SHR-C group compared with the control group,
whereas (E) LCSA levels were significantly decreased in the SHR-C
group compared with the control group. (F) The ratio of WCSA/LCSA
was increased in the SHR-C group; however, this increase was
significantly abrogated by treatment with GSPE. *P<0.05 and
**P<0.01. GSPE, grape seed proanthocyanidin extract; SHR,
spontaneous hypertension rat; T, treatment; C, control; WKY,
Wistar-Kyoto; ID, inner diameter; VCSA, vascular cross-sectional
area; WCSA, wall cross-sectional area; LCSA, luminal
cross-sectional area; WT, wall thickness. |
Quantification of collagen/elastin
expression and ratio
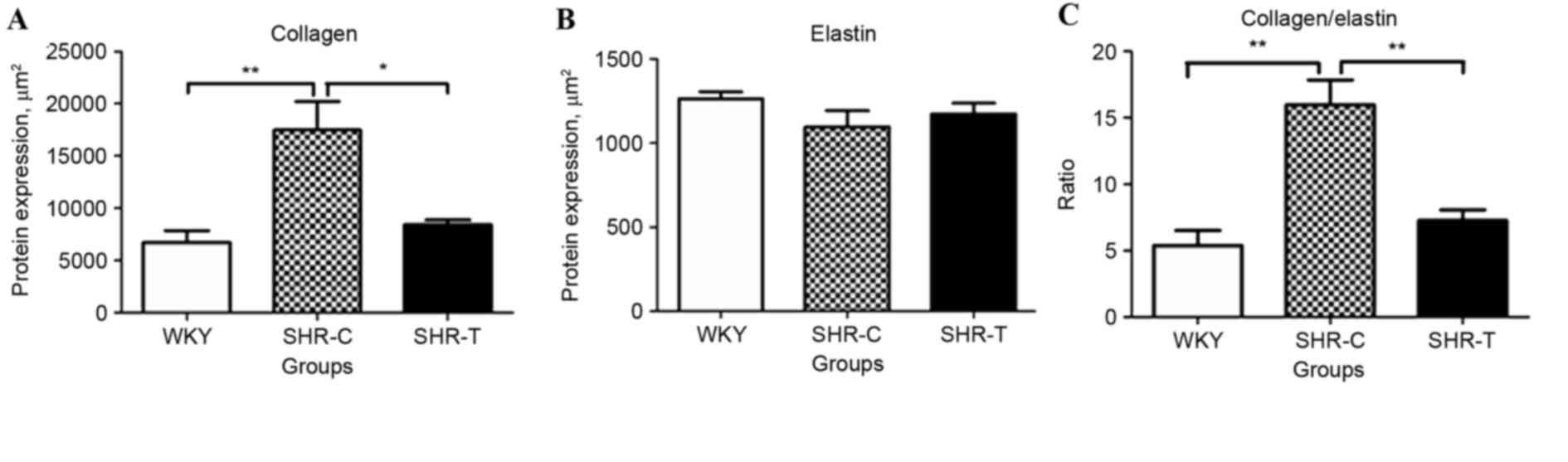
Image Pro-Plus software was used to investigate
collagen and elastin expression in 5 different fields of view.
Collagen expression levels were increased in the SHR-C group
compared with the WKY control (P<0.01); this increase was
abrogated by GSPE treatment (P<0.05; Fig. 5A). Although there was no
significant difference in the expression of elastin among the
different groups (P>0.05; Fig.
5B), a significantly greater ratio of collagen/elastin was
observed in the SHR-C group compared with the WKY control, which
was partially reversed by treatment with GSPE (P<0.01; Fig. 5C).
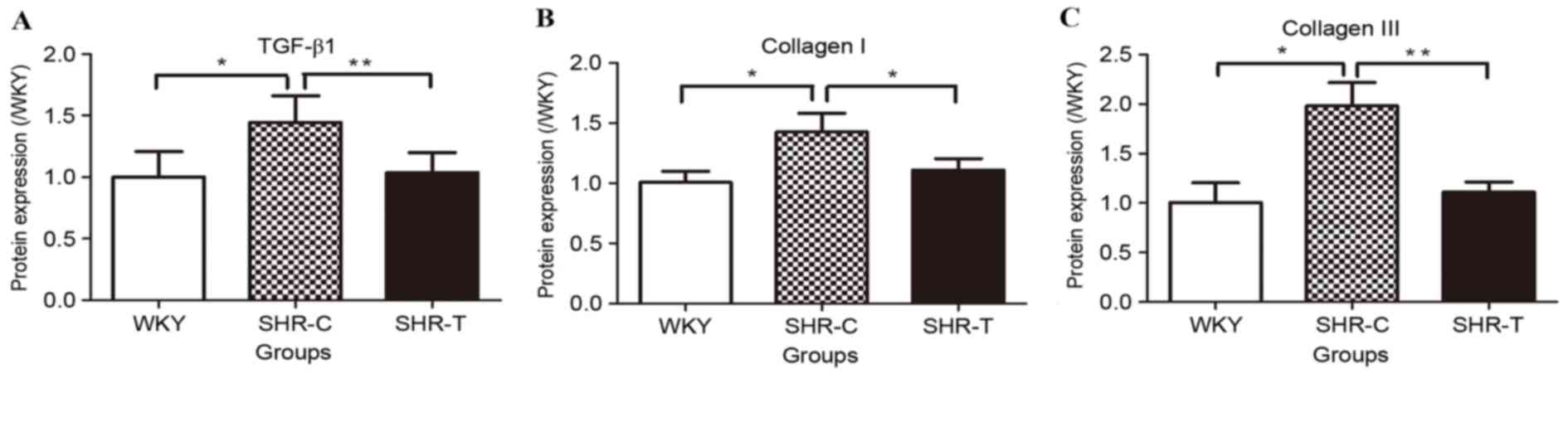
Effect of GSPE on the expression of
TGF-β1 and collagen
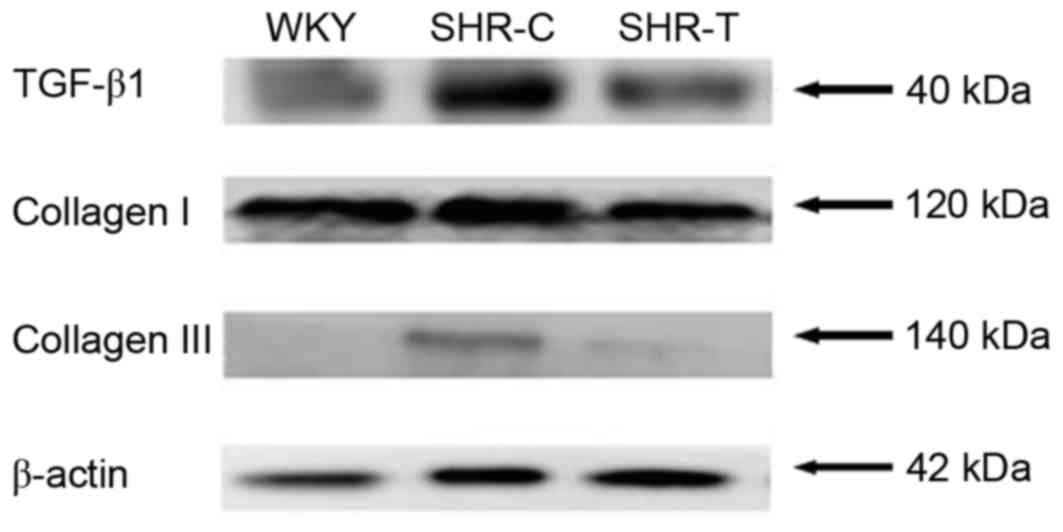
The protein expression levels of collagen and TGF-β1
were detected by western blotting, as presented in Fig. 6. The expression of collagen I and
III was increased by hypertension and this increase was attenuated
by GSPE treatment. In addition, TGF-β1 expression was increased in
the SHR-C group compared with the WKY group, which was abrogated by
GSPE treatment.
TGF-β1 and collagen I/III protein expression levels
were normalized to β-actin levels using densitometry, as presented
in Fig. 7. The expression of
TGF-β1 was significantly increased in the SHR-C group compared with
the WKY group (P<0.05); this increase was attenuated by
treatment with GSPE (P<0.01; Fig.
7A). Significantly increased levels of collagen I (P<0.05;
Fig. 7B) and III (P<0.05;
Fig. 7C) were observed in the
SHR-C group compared with the WKY group; GSPE treatment
significantly reduced this hypertension-induced collagen
hyperplasia (P<0.05).
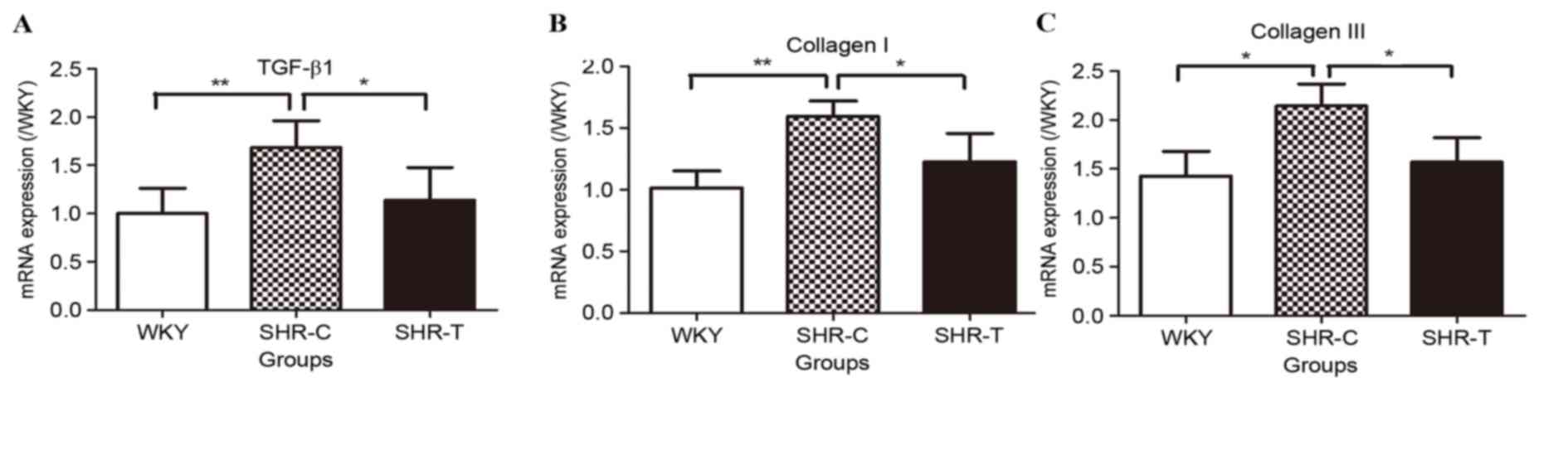
The mRNA expression levels of TGF-β1 and collagen
I/III were detected using RT-qPCR (Fig. 8). mRNA expression levels of TGF-β1
were significantly increased by hypertension (P<0.01); this
increase was abrogated by GSPE treatment (P<0.05; Fig. 8A). Collagen I (Fig. 8B) and III (Fig. 8C) mRNA expression levels were
upregulated in the SHR-C group compared with the WKY group
(P<0.05); this increase was partially reversed by treatment with
GSPE (P<0.05).
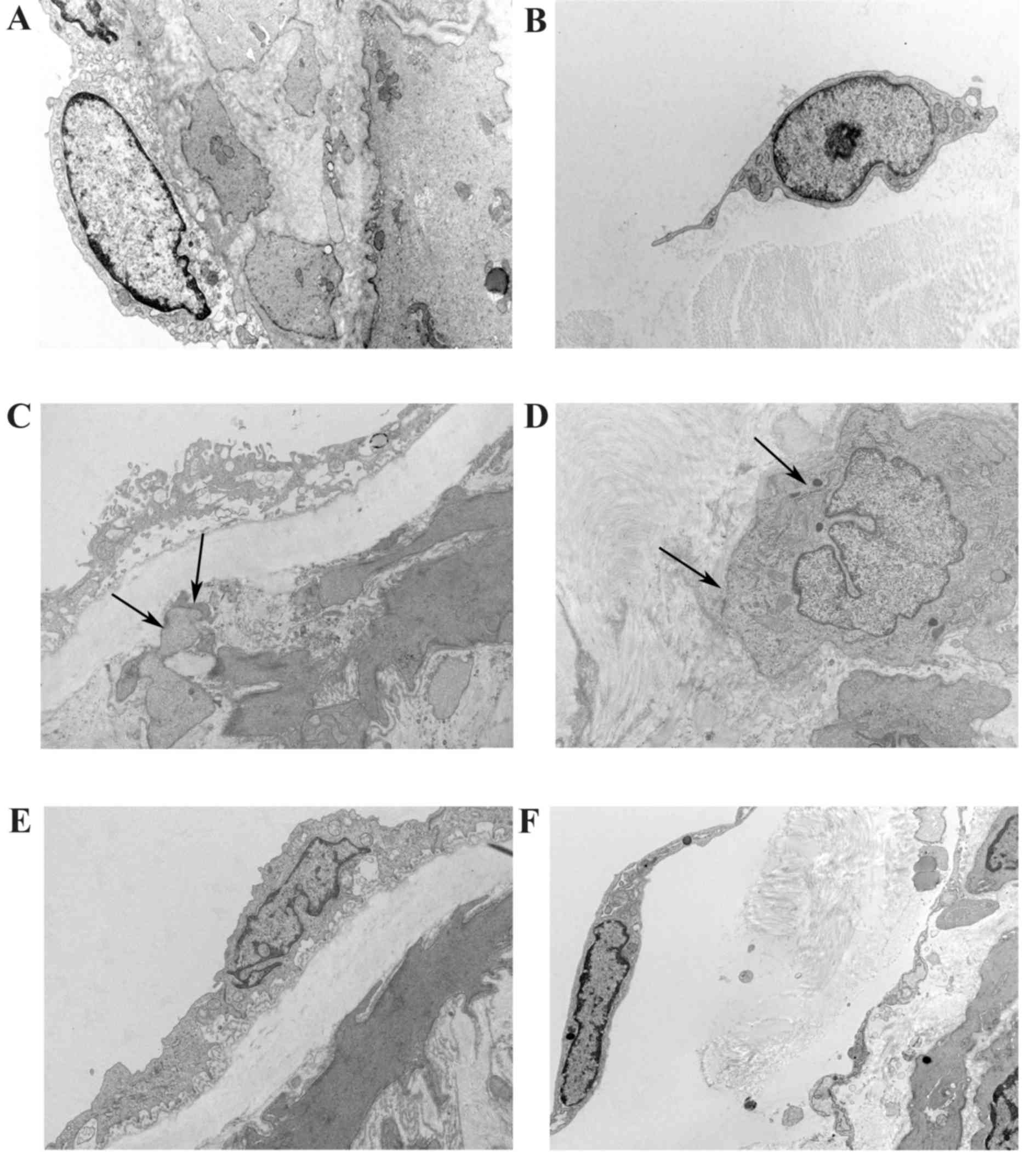
Role of GSPE in the alteration of the
ultrastructure of fibroblast cells in the mesenteric small
arteries
The ultrastructure of the mesenteric small artery
was observed using a transmission electron microscope. In
photomicrographs taken from WKY, the intima was smooth with a clear
edge and collagen was homogenously distributed in the media and in
adventitia (Fig. 9A). Fibroblast
cells exhibited a normal morphology in the adventitia (Fig. 9B). In the SHR-C group, the inner
membrane appeared to be damaged, a large quantity of irregular
collagen was located in the media and invading adventitia
fibroblasts (AF) appeared (Fig.
9C). In AF, the endoplasmic reticulum became dilated and the
formation of a dense patch under the membrane, in addition to the
development of collagen hyperplasia, were observed in the
adventitia (Fig. 9D).
Hypertension-induced endangium damage to the inner membrane and
accumulation of collagen in the vascular wall were attenuated by
GSPE (Fig. 9E). In addition, the
translocation of AF into the media of the small artery, as a result
of hypertension, was abrogated by GSPE. Furthermore, no dense patch
was observed under the membrane, however, the endoplasmic reticulum
was dilated in AFs (Fig. 9F).
Discussion
Hypertension is an important risk factor and
contributes to cardiovascular diseases. Vascular remodeling,
particularly in small arteries, is considered critical in the
development of hypertension (13).
The increased thickness of the vascular wall and the increased
narrowing of the lumen are primary pathological developments in
hypertension, along with the proliferation of VSMC and the
accumulation of collagen in the ECM (14).
In the present study, hypertrophic vascular
remodeling was observed in the small mesenteric arteries of rats
with spontaneous hypertension, which presented as a reduction in
the size of the lumen. This pathological reconstruction of the
artery in hypertensive rats includes an increase in wall thickness,
collagen overexpression and AF relocates to the media of the
artery. A model of hypertension similarly demonstrated that the ID
was reduced and the wall/lumen ratio was increased in small
arteries as a consequence of increased wall thickness (15). The present study further confirmed
that the accumulation of collagen was a primary feature that
resulted in a narrowed lumen in SHR-C animals, indicated as an
increase in ratio of WT/ID and WCSA/LCSA. A previous study
similarly reported findings indicating that concentric remodeling
occurred in humans from autopsy readings of cardiac and renal
arterioles in 25 essential hypertension patients (16). An increased wall-to-lumen ratio and
WCSA of retinal arterioles was observed in cerebrovascular patients
compared with subjects with mild arterial hypertension (17). Thus, the critical role of vascular
remodeling in the development of hypertension and the association
between the increase in vascular wall thickness and the development
and progression of hypertension was investigated. CSA and
intima-media thickness have been considered as independently
diagnostic morphological parameters, which were significantly
increased in patients with coronary artery disease compared with
those without (18). The results
of the present study suggested that hypertension resulting from
hypertrophic vascular remodeling was significantly decreased by
orally-administered GSPE. GSPE treatment significantly reduced wall
thickness and enlarged the vascular lumen of SHR mesenteric small
arteries. However, the SBP of hypertensive rats was not affected by
GSPE administration. This indicated that the vascular remodeling
was improved independent of decreasing blood pressure. The
therapeutic benefits of GSPE on cardiovascular remodeling have been
investigated through its inhibitory effect on the
ROS/mitogen-activated protein kinase pathway (19). In our previous study, GSPE was
examined as an antagonist of chemical-induced vascular remodeling
(19).
Significant accumulation of type I and III collagen
and the invasion of AF in the media were identified as contributors
to the hypertrophic vascular remodeling in the present study.
Morphological staining revealed that collagen had accumulated in
the walls of small mesenteric arteries of hypertensive rats
compared with healthy controls. However, this deregulated collagen
deposition and the resulting angiostenosis appeared to be
antagonized by oral administration of GSPE. The translocation of AF
into the media and the proliferation of VSMC (data not shown) were
observed in arterial tissues with hypertension. In addition,
invading fibroblasts exhibited an altered morphology, to
myofibroblasts, due to the appearance of dense paths and enlarged
endoplasmic reticulum. These alterations indicated that the
movement of AF, along with an associated increase in collagen, was
an important factor in vascular remodeling in the development of
hypertension. Previous studies have investigated the function of AF
in the development of hypertension and the regulation of
biopathological vascular remodeling (20). Collagen was observed to be
synthesized by AF and was a predominant compound of the structural
skeleton of tissues and organs (21). Notably, collagen and fibronectin
were increased but elastin was decreased in the vessel wall during
hypertension (13).
The results of the present study revealed that in
SHR, AF metastasized into vascular media and subsequently presented
a morphological transition associated with the overexpression of
collagen, whereas the expression of elastin was constant. The
underlying mechanism may be that oxidative stress and the
subsequent generation of cytokines, such as TGF-β1, induced AF
thereby altering macrophage phenotype and invasion into the
vascular wall, resulting in vascular remodeling (22,23).
Notably, the dysfunction resulting in the translocation of AF was
reduced by GSPE treatment, which was exhibited as the endoplasmic
reticulum decreasing in size and a reduction in the dense path.
Oxidative stress activates a cascade of intercellular and
intracellular signals, which result in functional and morphological
cell alterations, which are considered to be critical in the
initiation and development of hypertension (24,25).
Therefore, the therapeutic benefit of GSPE in vascular remodeling
partly depends on its anti-oxidative ability to inhibit the
pathological alteration of AF.
Oxidative stress has been widely accepted as a
primary factor during the development of hypertension (26). A large body of evidence suggests
that when an imbalance occurs between oxidant species and the
antioxidant defense system, ROS is generated in the vascular system
and serves as a key trigger of hypertension (27). In the present study, greater levels
of ROS, MDA and H2O2 were detected in
vascular tissue lysates, whereas reduced levels of the oxidant
enzymes SOD and CAT were observed. This imbalance triggered AF
translocation into the media of the mesenteric artery and
subsequently resulted in an overexpression of collagen. A previous
study demonstrated that a high level of ROS was observed in a
sterone-induced hypertensive rat model, which was closely linked to
the increased collagen deposition in the thoracic aorta (28). Behbahani et al (29) demonstrated that resveratrol from
grapes rescued the dysfunction of the oxidation-reduction system in
rat mesenteric small arterial tissue, via its antioxidant
properties. In a controlled study involving 119 healthy, pre- and
mildly hypertensive subjects, GSPE significantly decreased blood
pressure and reduced the oxidation of membrane lipids (30). The data obtained from the present
study suggested that the anti-oxidative effects of GSPE may result
in the inhibition of morphological and functional alterations of
AF; the primary contributor to vascular remodeling, and
subsequently hypertension and cardiac disease.
Oxidative production activates the redox-sensitive
regulation of multiple signaling pathways and second messengers,
such as TGF-β1, which responds to fibrogenic events and cell
differentiation (31,32). Vascular remodeling follows a set
pathway of events; ROS induces expression of angiotensin II,
followed by the release of TGF-β1, which modifies ECM composition
and structure (33). TGF-β1
subsequently enhances collagen expression in AF (34,35).
In addition, TGF-β1 induces the proliferation and phenotype
transformation of AF, as well as their migration, and increases the
expression of ECM compounds (36).
The present study investigated the potential association between
oxidation and TGF-β1 elevation, the consequent ECM upregulation,
and vascular wall remodeling in the small mesenteric arteries of
SHR. In hypertension groups, expression levels of TGF-β1 mRNA and
protein were increased; however, this increase was abrogated by
treatment with GSPE.
TGF-β1 promoted the differentiation of cardiac
fibroblasts into myofibroblasts and furthermore triggered collagen
gel contraction by cardiac fibroblasts (37). Similar findings from the present
study suggested that AF translocated into the media and this was
associated with the overexpression of collagen, which may explain
vascular remodeling resulting from spontaneous hypertension in
rats. The present study linked the reversible effect of GSPE on
vascular remodeling with the downregulation of the TGF-β1 signaling
pathway, and the subsequent prevention of AF metastasis,
proliferation, phenotype alteration and the reduction of
overexpression and deposition of collagen in vessels. TGF-β1 was
observed to be important in the pathogenesis of pulmonary arterial
hypertension and its blockade resulted in a decrease in pulmonary
vascular remodeling (38). The
expression of collagen in the media of carotid arteries in SHR was
stimulated by TGF-β1 via the Smad pathway, which was blocked by the
deregulation of TGF-β1 expression (39).
In conclusion, the present study confirmed that
hypertension resulted in hypertrophic vascular remodeling of the
small mesenteric arteries, which was partially reversed by
treatment with GSPE. The benefit of GSPE to vascular remodeling was
not due to a reduction in the SBP. The novel evidence presented in
the current study suggested that the anti-remodeling effect of GSPE
was achieved through inhibition of ROS generation, overexpression
of TGF-β1, AF differentiation and translocation, and collagen
hyperplasia in small mesenteric arteries. The anti-oxidative
properties of GSPE suggested it may be a potential therapeutic
agent; however, further studies are required to investigate this
potential.
Acknowledgements
The present study was supported by grants from the
Science and Technology Development Plan of Shandong Province (grant
no. ZR2014HM071), the National Natural Science Foundation of China
(grant no. 30700884), the Shandong Science and Technology Research
Plan (grant no. 2010GGC10294), the Shandong Science and Technology
Project Plan (grant no. 2012GB021817) and the National Science and
Technology Major Project: Technology Platform Construction for
Clinical Evaluation of Cardiovascular New Drug (grant no.
2012ZX09303016-003).
Glossary
Abbreviations
Abbreviations:
|
GSPE
|
grape seed proanthocyanidin
extract
|
|
TGF
|
transforming growth factor
|
|
SHR
|
spontaneous hypertensive rat
|
|
AF
|
adventitia fibroblasts
|
|
VSMC
|
vascular smooth muscle cell
|
|
ECM
|
extracellular matrix
|
|
WKY
|
Wistar-Kyoto rats
|
|
SBP
|
systolic blood pressure
|
|
MDA
|
malondialdehyde
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
|
ID
|
inner diameter
|
|
VCSA
|
vascular cross-sectional area
|
|
WCSA
|
wall cross-sectional area
|
|
LCSA
|
luminal cross-sectional area
|
|
WT
|
wall thickness
|
|
CSA
|
cross-sectional area
|
References
|
1
|
Wang M, Kim SH, Monticone RE and Lakatta
EG: Matrix metalloproteinases promote arterial remodeling in aging,
hypertension, and atherosclerosis. Hypertension. 6:698–703. 2015.
View Article : Google Scholar
|
|
2
|
van Guldener C and Stehouwer CD:
Hyperhomocysteinemia, vascular pathology, and endothelial
dysfunction. Semin Thromb Hemost. 26:281–289. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ponticos M and Smith BD: Extracellular
matrix synthesis in vascular disease: Hypertension and
atherosclerosis. J Biomed Res. 28:25–39. 2014.PubMed/NCBI
|
|
4
|
Rodrigo R, Passalacqua W, Araya J,
Orellana M and Rivera G: Implications of oxidative stress and
homocysteine in the pathophysiology of essential hypertension. J
Cardiovasc Pharmacol. 42:453–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lijnen P, Papparella I, Petrov V,
Semplicini A and Fagard R: Angiotensin II-stimulated collagen
production in cardiac fibroblasts is mediated by reactive oxygen
species. J Hypertens. 24:757–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Pieniek M, Fard A, O'Brien J,
Mannion JD and Zalewski A: Adventitial remodeling after coronary
arterial injury. Circulation. 93:340–348. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen WL, Gao PJ, Che ZQ, Ji KD, Yin M, Yan
C, Berk BC and Zhu DL: NAD(P)H oxidase-derived reactive oxygen
species regulate angiotensin-II induced adventitial fibroblast
phenotypic differentiation. Biochem Biophys Res Commun.
339:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holmström KM and Finkel T: Cellular
mechanisms and physiological consequences of redox-dependent
signalling. Nat Rev Mol Cell Biol. 15:411–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharifi AM, Li JS, Endemann D and
Schiffrin EL: Effects of enalapril and amlodipine on small-artery
structure and composition, and on endothelial dysfunction in
spontaneously hypertensive rats. J Hypertens. 16:457–466. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Junqueira LC, Cossermelli W and Brentani
R: Differential staining of collagens type I, II and III by Sirius
Red and polarization microscopy. Arch Histol Jpn. 41:267–274. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jun L, Yuanshu W, Yanying X, Zhongfa X,
Jian Y, Fengling W, Xianjun Q, Kokudo N, Wei T, Weixia Z and
Shuxiang C: Altered mRNA expressions of sialyltransferases in human
gastric cancers. Med Oncol. 29:84–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rattazzi M, Bertacco E, Puato M, Faggin E
and Pauletto P: Hypertension and vascular calcification: A vicious
cycle? J Hypertens. 30:1885–1893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gündüz F, Baskurt OK and Meiselman HJ:
Vascular dilation responses of rat small mesenteric arteries at
high intravascular pressure in spontaneously hypertensive rats.
Circ J. 73:2091–2097. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang E, Maruyama J, Yokochi A, Mitani Y,
Sawada H, Nishikawa M, Ma N and Maruyama K: Sarpogrelate
hydrochloride, a serotonin 5HT2A receptor antagonist, ameliorates
the development of chronic hypoxic pulmonary hypertension in rats.
J Anesth. 29:715–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pei F, Li XY, Fang Y, Shi HY and Diao HJ:
Cardiac and renal arteriolar pathological changes in the autopsied
elderly hypertensive patients with left ventricular hypertrophy.
Zhonghua Xin Xue Guan Bing Za Zhi. 36:872–877. 2008.(In Chinese).
PubMed/NCBI
|
|
17
|
Baleanu D, Ritt M, Harazny J, Heckmann J,
Schmieder RE and Michelson G: Wall-to-lumen ratio of retinal
arterioles and arteriole-to-venule ratio of retinal vessels in
patients with cerebrovascular damage. Invest Ophthalmol Vis Sci.
50:4351–4359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frick M, Schwarzacher SP, Alber HF, Rinner
A, Ulmer H, Pachinger O and Weidinger F: Morphologic rather than
functional or mechanical sonographic parameters of the brachial
artery are related to angiographically evident coronary
atherosclerosis. J Am Coll Cardiol. 40:1825–1830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang LL, Pan C, Wang L, Ding L, Guo K,
Wang HZ, Xu AM and Gao S: Protective effects of grape seed
proanthocyanidins on cardiovascular remodeling in DOCA-salt
hypertension rats. J Nutr Biochem. 26:841–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Qiu J, Zhao S, You B, Ji X, Wang Y,
Cui X, Wang Q and Gao H: Grape seed proanthocyanidin extract
alleviates ouabain-induced vascular remodeling through regulation
of endothelial function. Mol Med Rep. 6:949–954. 2012.PubMed/NCBI
|
|
21
|
Arteaga-Solis E, Gayraud B and Ramirez F:
Elastic and collagenous networks in vascular diseases. Cell Struct
Funct. 25:69–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siow RC, Mallawaarachchi CM and Weissberg
PL: Migration of adventitial myofibroblasts following vascular
balloon injury: Insights from in vivo gene transfer to rat carotid
arteries. Cardiovasc Res. 59:212–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stenmark KR, Yeager ME, El Kasmi KC,
Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR and Frid MG: The
adventitia: Essential regulator of vascular wall structure and
function. Annu Rev Physiol. 75:23–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siow RC and Churchman AT: Adventitial
growth factor signalling and vascular remodelling: Potential of
perivascular gene transfer from the outside-in. Cardiovasc Res.
75:659–668. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barnes JL and Gorin Y: Myofibroblast
differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney
Int. 79:944–956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montezano AC, Dulak-Lis M, Tsiropoulou S,
Harvey A, Briones AM and Touyz RM: Oxidative stress and human
hypertension: Vascular mechanisms, biomarkers, and novel therapies.
Can J Cardiol. 31:631–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brito R, Castillo G, González J, Valls N
and Rodrigo R: Oxidative stress in hypertension: Mechanisms and
therapeutic opportunities. Exp Clin Endocrinol Diabetes.
123:325–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen QZ, Han WQ, Chen J, Zhu DL Chen-Yan
and Gao PJ: Anti-stiffness effect of apocynin in
deoxycorticosterone acetate-salt hypertensive rats via inhibition
of oxidative stress. Hypertens Res. 36:306–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Behbahani J, Thandapilly SJ, Louis XL,
Huang Y, Shao Z, Kopilas MA, Wojciechowski P, Netticadan T and
Anderson HD: Resveratrol and small artery compliance and remodeling
in the spontaneously hypertensive rat. Am J Hypertens.
23:1273–1278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belcaro G, Ledda A, Hu S, Cesarone MR,
Feragalli B and Dugall M: Grape Seed Procyanidins in Pre- and Mild
Hypertension: A registry study. Evid Based Complement Alternat Med.
2013:3131422013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Purnomo Y, Piccart Y, Coenen T, Prihadi JS
and Lijnen PJ: Oxidative stress and transforming growth
factor-β1-induced cardiac fibrosis. Cardiovasc Hematol Disord Drug
Targets. 13:165–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Z, Liu L, Xi Z, Huang J and Lin B:
Single-walled carbon nanotubes promote rat vascular adventitial
fibroblasts to transform into myofibroblasts by SM22-α expression.
Int J Nanomedicine. 7:4199–4206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshioka J, Schreiter ER and Lee RT: Role
of thioredoxin in cell growth through interactions with signaling
molecules. Antioxid Redox Signal. 8:2143–2151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding WJ and Hong L: Progress research on
relationship between oxidative stress and collagen metabolism
diseases. Journal of Jilin University (Medicine Edition).
39:1302–1306. 2013.
|
|
35
|
Silacci P and Hayoz D: Oxidative stress as
the triggering event for vascular remodelling. Nephrol Dial
Transplant. 13:1343–1346. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takimoto E and Kass DA: Role of oxidative
stress in cardiac hypertrophy and remodeling. Hypertension.
49:241–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lijnen P, Petrov V, Rumilla K and Fagard
R: Transforming growth factor-beta 1 promotes contraction of
collagen gel by cardiac fibroblasts through their differentiation
into myofibroblasts. Methods Find Exp Clin Pharmacol. 25:79–86.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Graham BB, Chabon J, Gebreab L, Poole J,
Debella E, Davis L, Tanaka T, Sanders L, Dropcho N, Bandeira A, et
al: Transforming growth factor-β signaling promotes pulmonary
hypertension caused by Schistosoma mansoni. Circulation.
128:1354–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen JL, Shang QH, Hu W, Liu C, Mao WH and
Liu HQ: Role of TGF-β1/Smads pathway in carotid artery remodeling
in renovascular hypertensive rats and prevention by Enalapril and
Amlodipine. J Geriatr Cardiol. 9:185–191. 2012. View Article : Google Scholar : PubMed/NCBI
|