Introduction
The kidneys are important in the regulation of salt
and water balance in the body. This function is finely regulated by
the tubular reabsorption of body fluid through renal water and
sodium channels, under the tight control of hormones, nerves and
intracellular signaling pathways (1,2).
Aquaporins (AQPs), expressed in the kidney, are divided based on
pore selectivity into water-specific, orthodox aquaporins and
solute-facilitating aquaglyceroporins, which conduct glycerol and
urea (3,4). AQP2 is expressed in epithelial cells
of the inner medullary collecting duct (IMCD) and are critical in
urine concentrating mechanisms. The water permeability of the IMCD
is rapidly regulated by the antidiuretic hormone, arginine
vasopressin (AVP) (5). AVP binds
to vasopressin V2 receptors, which are located
predominantly in the basolateral plasma membrane of principal cells
in collecting ducts. The activation of the V2 receptors
causes stimulation of adenylyl cyclase via the G protein, Gs,
leading to the elevation of cAMP. The subsequent activation of
protein kinase A (PKA) initiates the translocation of AQP2-bearing
vesicles from the cytosol to the plasma membrane, in which AQP2 is
inserted via an exocytosis-like process and AQP2 phosphorylation is
mediated by PKA (1,6). It has been suggested that
AVP-mediated signaling pathways are involved in hypertonic
stress-induced AQP trafficking in collecting ducts as the
expression and targeting of AQP2 are regulated by hypertonic stress
(7).
During antidiuresis, the IMCD adapts to the
hyperosmotic interstitial environment by the increased expression
of osmoprotective genes, which is driven by a common
transcriptional activator, tonicity-responsive enhancer binding
protein (TonEBP) (8). The
accumulation of compatible osmolytes, including sorbitol, inositol,
taurine, glycerophophorylcholine and betaine, is also essential for
the survival of medullary cells under hypertonic conditions
(9). Hyperosmotic stress
stimulates the activation of serum- and glucocorticoid-inducible
protein kinase 1 (Sgk1) via the p38 mitogen-activated protein
kinase-dependent pathway (10,11).
However, the complicated mechanisms induced by hypertonic stress
remain to be fully elucidated.
Wiryeongtang (Wei-Ling-Tang, Magnolia and Hoelen
combination; WRT), composed of pyeongwisan (Ping-Wei-San) and
oryeongsan (Wu-Ling-San) (12,13),
has been widely used in chronic edema and dysuresia of renal
homeostasis, and was originally recorded in an ancient Korean
medicinal book, ‘Donguibogam’ (14). WRT is composed of Alisma
orientalis, Cinnamomum cassia, Atractylodes lancea,
Magnolia officinalis, Paeonia lactiflora,
Polyporus umbellatus, Poria cocos, Glycyrrhiza
glabra and Citrus unshiu. However, the mechanism
responsible for the effects of WRT against hypertonicity remains to
be fully elucidated. Thus, the present study was performed to
determine the possible effects of WRT on water channel regulation
in response to hypertonic stress in mouse mIMCD-3 cells.
Materials and methods
Reagents
AQP1 (cat. no. sc-20810), AQP2 (cat. no. sc-28629),
AQP3 (cat. no. sc-20811), Na+/K+ ATPase α1 (cat. no. sc-21712),
p-SGK (cat. no. sc-16744), SGK (cat. no. sc-28338),
3-phosphoinositide-dependent kinase 1 (PDK1) (cat. no. sc-7140),
β-actin (cat. no. sc-4778) antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Anti-mouse (cat. no.
ADI-SAB-100-J), anti-rabbit (cat. no. ADI-SAB-300-J), anti-goat
(cat no. ADI-SAB-400-I) horseradish peroxidase (HRP)-conjugated
secondary antibodies were purchased from Enzo Life Sciences, Inc.
(Farmingdale, NY, USA). Forskolin (an adenylate cyclase activator)
was purchased from Sigma-Aldrich; Merck Millipore (Darmstadt,
Germany). KT5720 (a PKA inhibitor) was purchased from Alomone Labs,
Ltd. (Jerusalem, Israel). The other reagents used in the present
study were of the highest purity commercially available.
Preparation of WRT
The dried WRT was purchased from the Herbal Medicine
Co-operative Association of Chonbuk Province (Chonbuk, Korea) in
March 2010. A voucher specimen (no. HBI091) was deposited at the
Hanbang Body-Fluid Research Center, Wonkwang University (Iksan,
Korea). The ingredients of WRT comprised Alisma orientalis,
Cinnamomum cassia, Atractylodes lancea, Magnolia officinalis,
Paeonia lactiflora, Polyporus umbellatus, Poria cocos, Glycyrrhiza
glabra and Citrus unshiu. The dried WRT (300 g) was boiled in 2
liters of distilled water for 2 h at 100°C. The aqueous extract was
centrifuged at 890 × g for 20 min at 4°C and then concentrated
using a rotary evaporator. The supernatant extract was lyophilized
and the powder (yield rate, 1.77 g) was stored at 4°C until used in
experiments.
Cell cultures
The mIMCD-3 cell line was originally acquired from
American Type Culture Collection (Manassas, VA, USA) (2). The mIMCD-3 cells were routinely
cultured in Dulbecco's modified Eagle's medium/F-12 supplemented
with 10% FBS and antibiotics (Gibco and Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). mIMCD-3 cells in passages 9–11
were used and were maintained in mIMCD-3 medium in a humidified
chamber containing 5% CO2 at 37°C.
Cell viability assay
Cell viability was determined using
3-(4,5-dimethylthinazol-2-yl)-2,3-diphenyl tetrazolium bromide
(MTT) assay. mIMCD-3 was seeded into 96-well culture plates at a
density of 1×104 cells/well. Following incubation with
various concentrations of WRT (1–500 mg/ml) for 24 h, then 20 ml of
MTT solution (0.5 mg/ml) was added to each culture well and further
incubated at 37°C for 4 h. Following incubation for 4 h at 37°C,
the MTT solution was removed and 200 ml of dimethyl sulfoxide
(DMSO; Amresco, LLC, Solon, OH, USA) was added and mixtures were
shaken, the crystals were fully dissolved for about 10 min. The
absorbance of the dissolved formazan was read at 590 nm using a
microplate reader (Multiskan, Thermo Fisher Scientific, Inc.). The
absorbance was used as a measurement of cell viability, normalized
to cells incubated in control medium, which were considered 100 %
viable.
Measurements of osmolality
The mIMCD-3 cells were pretreated with WRT (1, 5, 10
and 30 g/ml) for 1 h, and stimulated with 175 mM NaCl for 1 h at
37°C. The supernatant, conditioned medium was collected by 210 × g
at 4°C for 5 min for measurement of osmolality. Osmolality was
measured using an Advanced osmometer (Model 3900; Advanced
Instruments, Norwood, MA, USA).
Western blot analysis
Harvested cell pellet was lysed with RIPA lysis
buffer. Following extraction, protein was quantified with Bradford
method. Homogenates (40 mg total protein) was loaded on the gel for
10% SDS-polyacrylamide gel and transferred onto a nitrocellulose
membrane. The blots were blocked with 5% non-fat milk and incubated
with the appropriate primary antibody at dilutions (1:1,000)
recommended by the manufacturer and incubated overnight at 4°C. The
membrane was washed with TBST (0.1% Tween-20 in 1X TBS), and
primary antibodies were detected with goat anti-rabbit-IgG or
anti-mouse-IgG antibody conjugated to HRP, following which the
bands were visualized with enhanced chemiluminescence (GE
Healthcare Life Sciences, Chalfont, UK). Protein expression levels
were determined by analyzing the signals captured on the
nitrocellulose membranes using the ChemiDoc image analyzer version
4.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA isolation and RT-PCR analysis were
performed using Maxime PCR PreMix (i-StarMAX II, Intron
Biotechnology, Inc., Seongnam, South Korea) (2). cDNA was prepared from 500 ng RNA by
reverse transcription in a final volume of 20 ml in an Opticon MJ
Research instrument. The samples were incubated at 37°C for 60 min
and 94°C for 5 min. The following sets of primers were used for PCR
amplification: AQP1, sense 5′-GTCCCACATGGTCTAGCCTTGTCTG-3′ and
antisense 5′-GGGAAGGGTCCTGGAGGTAAGTCA-3′; AQP2, sense
5′-TGCATCTTTGCCTCCACCGACGAG-3′ and antisense
5′-CATGGAGCAACCGGTGAAATAG-3′; AQP3, sense
5′-GGCTAAAAACGCTCCCTGTATCCA-3′ and antisense
5′-GGAGTTTCCCACCCCTATTCCTAAA-3′; SMIT, sense
5′-CACTGTGAGTGGATACTTCC-3′ and antisense
5′-TCTCTTAACTTCCTCAAACC-3′; Sgk1, sense
5′-GGAACACAGCCGAGATGTATGACAA-3′ and antisense
5′-AACTGCTTCCGCGGCTTCTTTCACAC-3′; TonEBP, sense
5′-ATGCAATTTCAGAATCAGCC-3′ and antisense 5′-GCATTTGCGAGAAAGAAG-3′;
GAPDH, sense 5′-TCACCATCTTCCAGGAGCGAG-3′ and antisense
5′-AAGGTGCAGAGATGATGACCCTC-3′. Synthesized cDNA 1 µl, 50 nM each
primer, butters were placed up to 20 µl in the PCR Pre-mix
according to the manufacturer's protocol (Intron Biotechnology,
Inc.). The amplification profile was as follows: Initial cycling at
94°C for 15 min, followed by 45 cycles of 94°C for 20 sec, 60°C for
20 sec, 72°C for 30 sec, and a final extension step at 72°C for 5
min. The PCR products were subjected to 1.2% agarose gel
electrophoresis.
Immunofluorescence
The mIMCD-3 cells were seeded at a density of
1×106 cells/well on sterile slide coverslips in a 60 mm
culture dish and treated with 175 mM NaCl, with or without WRT (5,
10 and 30 µg/ml), for 1 h at 37°C. Following incubation, the medium
was removed and the cells were fixed with 4% PFA in PBS at room
temperature for 5 min, and permeabilized with 0.1% Triton X-100 in
PBS for 15 min, as described previously (15). The cells were overlaid with 1% BSA
(Santa Cruz Biotechnology, Inc.) in PBS, and then incubated with
AQP2 antibody (1:100, Santa Cruz Biotechnology, Inc.) incubated at
4°C overnight. The cells were then incubated with FITC
green-conjugated rabbit anti-goat IgG secondary antibody (1:1,000,
cat. no. A11078, Invitrogen; Thermo Fisher Scientific, Inc.). The
slides were incubated at room temperature for 1 h, and nucleus
staining was performed with 4′,6-diamino-2-phenylindole (Molecular
Probes; Thermo Fisher Scientific, Inc.) diluted 1:500 in PBS.
Coverslips were mounted with mounting medium,
ProLong®Gold antifade reagents (Molecular Probes; Thermo
Fisher Scientific, Inc.) onto glass slides and examined using
fluorescence microscopy (ECLIPSETi; Nikon Corporation; Tokyo,
Japan).
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. The statistical significance of differences
between group means was determined using one-way analysis of
variance and Student's t-test using Sigma Plot version 10.0 (Systat
Software, Inc., San Jose, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of WRT on hypertonic
stress-induced regulation of AQP2
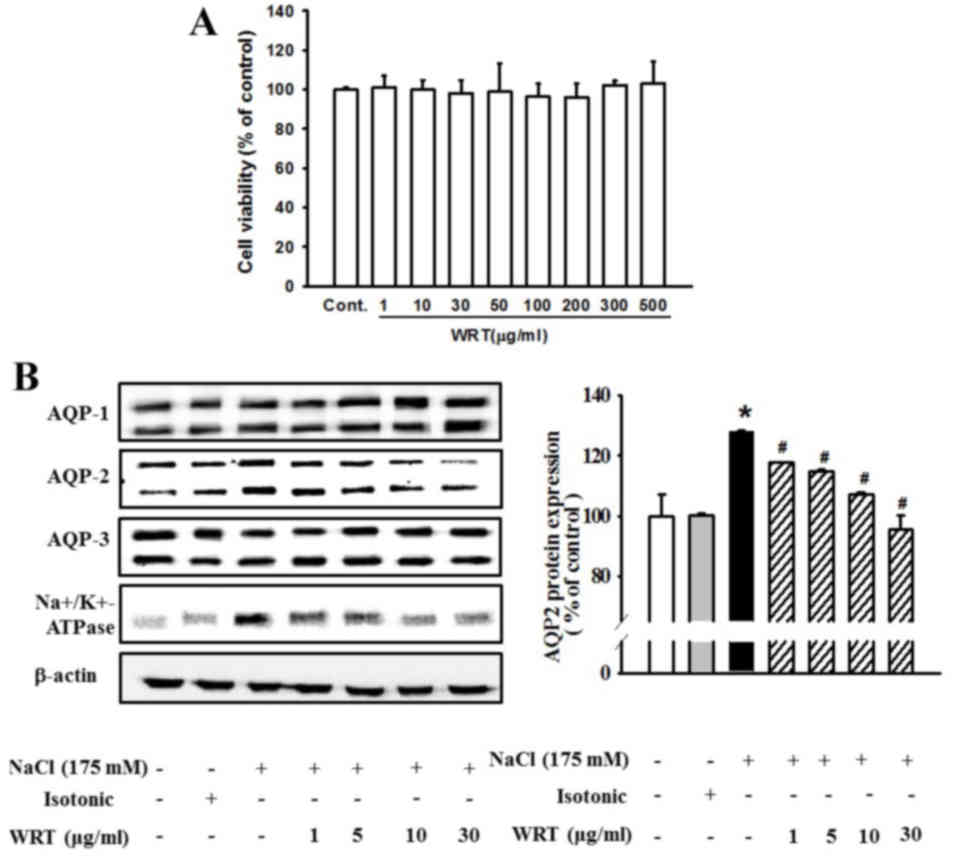
To assess the effect of WRT on cytotoxicity in
mIMCD-3 cells, the cells were incubated with WRT (1–500 µg/ml) for
24 h, and an MTT assay was performed. WRT show no cytotoxicity at
various concentrations (Fig. 1A).
Therefore, 30 µg/ml of WRT was used as the maximum dose in the
present study. Pretreatment with WRT significantly decreased the
hypertonic stress-induced expression of AQP2 (Fig. 1B). However, the expression levels
of AQP1 and AQP3 were not altered significantly by hypertonic
stress. The level of hypertonic stress-induced Na,K-ATPase
α1-subunit was also decreased by WRT pretreatment. In addition, the
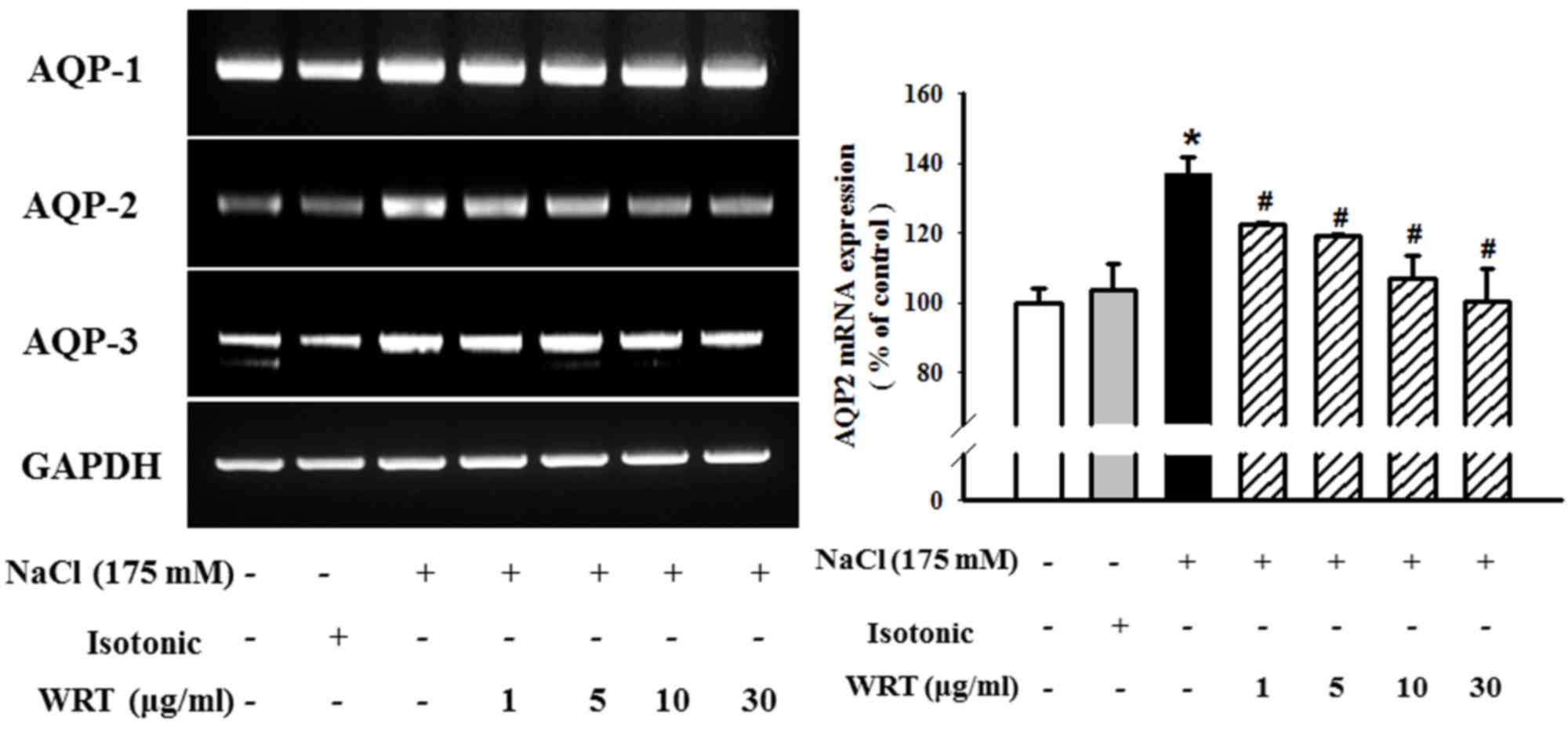
mRNA levels of AQP1, AQP2 and AQP3 were measured using RT-PCR
analysis. As shown in Fig. 2,
pretreatment with WRT (1–30 µg/ml) markedly decreased hypertonic
stress-induced mRNA levels of AQP2. However, no differences in AQP1
or AQP3 were observed between the hypertonic-stress group and WRT
group. Alterations in the mRNA levels corresponded closely to the
alterations in protein levels.
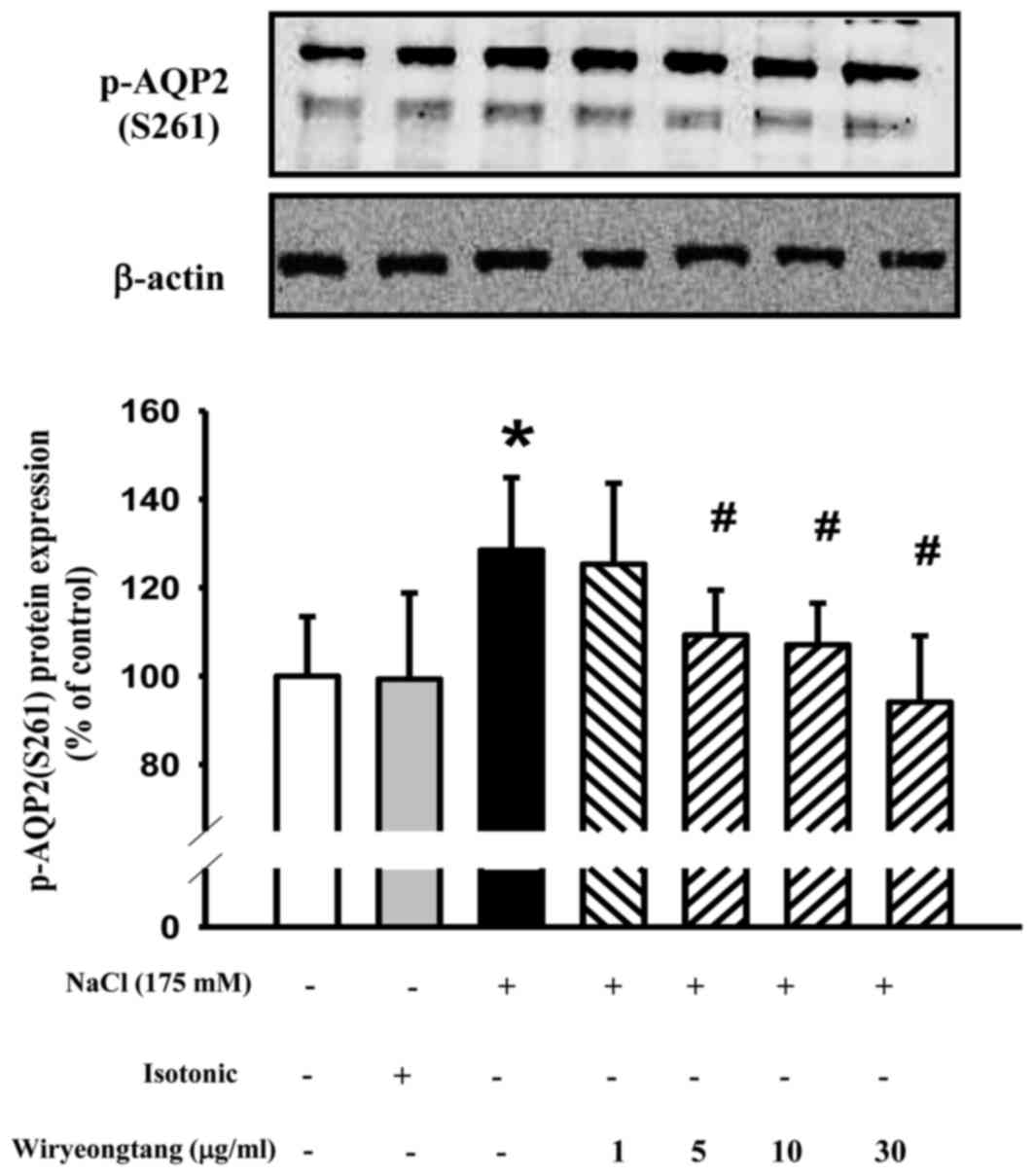
In addition to mediating expression, hypertonic
stress mediates the phosphorylation of AQP2. Pretreatment with WRT
(1–30 µg/ml) for 30 min decreased the hypertonic stress-induced
phosphorylation of AQP2 (Fig.
3).
Effect of WRT on alterations in
osmolality under hypertonic stress
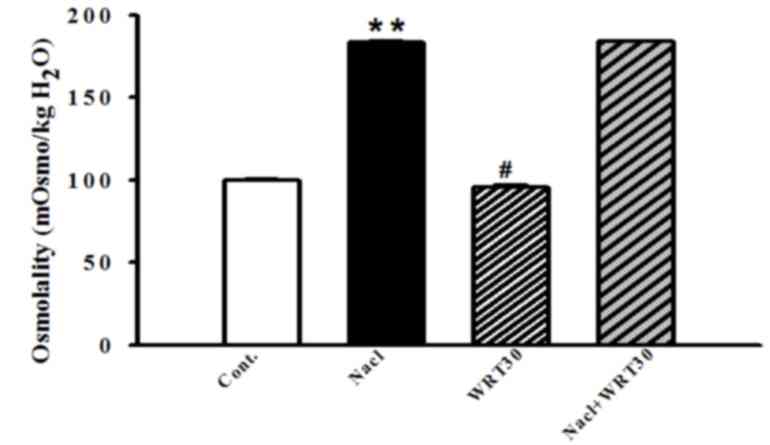
To examine whether osmotic alterations occurred, the
osmolality of the medium was measured. mIMCD-3 cells were
pretreated with WRT (1–30 µg/ml) for 30 min, and stimulated with
NaCl (175 mM) for 1 h. The osmolality was significantly increased
by hypertonic stress. WRT alone also altered osmolality, however,
WRT did not affect the osmolality of the NaCl-treated group
(Fig. 4).
Effect of WRT on hypertonic
stress-induced SGK1 and TonEBP
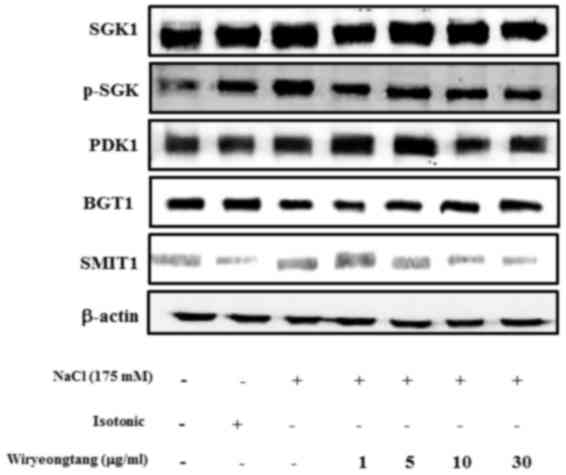
SGK1 is a signaling kinase, which is induced by
hypertonicity. Therefore, the inhibitory effect of WRT on the
hypertonic stress-induced expression of SGK1 was examined. As shown
in Fig. 5, hypertonicity was
associated with the phosphorylation of SGK1, However, WRT
attenuated the hypertonic stress-induced phosphorylation of Sgk1 in
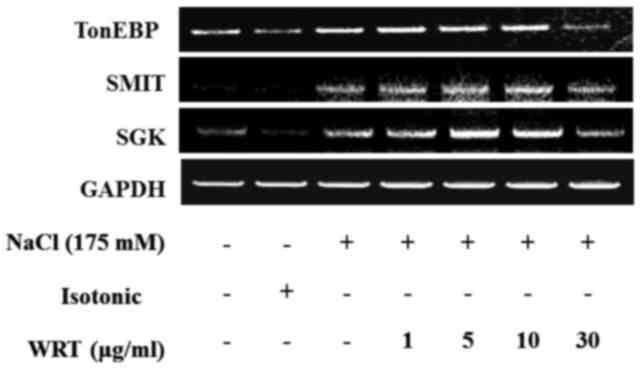
a dose-dependent manner. To evaluate alterations in the activity of
SGK1, SMIT and TonEBP, mRNA expression levels were measured in the
mIMCD-3 cells. Confluent mIMCD-3 cells were pretreated with WRT and
then treated with 175 mM NaCl. Hypertonic stress increased the mRNA
levels of SGK1, SMIT and TonEBP. However, WRT decreased these mRNA
expression levels in a dose-dependent manner (Fig. 6).
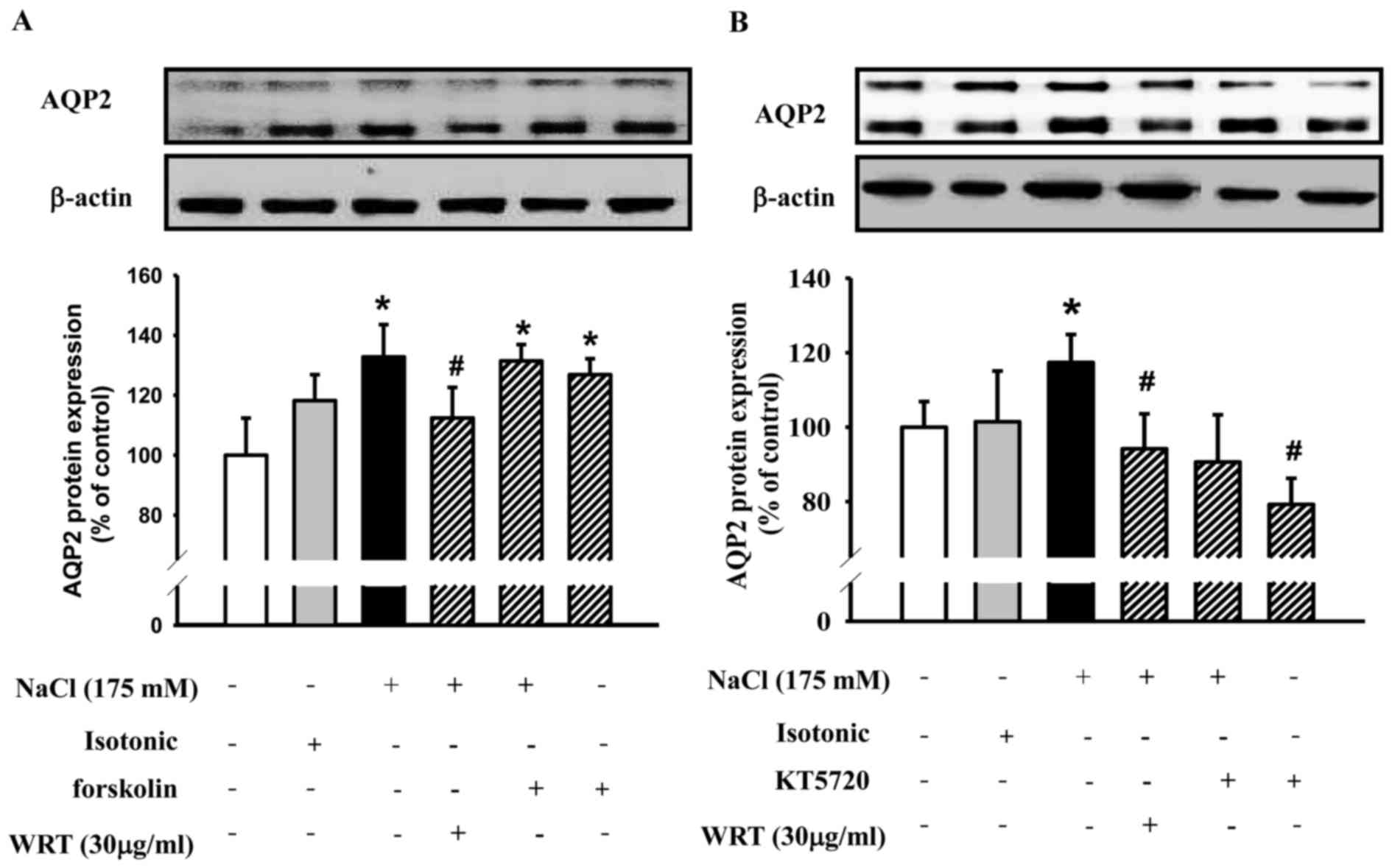
Involvement of the PKA signaling
pathways on the inhibitory effects of WRT in hypertonic
stress-induced expression of AQP2
To clarify the involvement of PKA signaling pathways
on the inhibitory effects of WRT in the hypertonic stress-induced
expression of AQP2, the mIMCD-3 cells were pretreated with KT5720,
a cell permeable-specific competitive inhibitor of PKA, or with
WRT, and then treated with 175 mM NaCl. Following treatment with
forskolin, an adenylate cyclase activator, the expression of AQP2
was markedly enhanced (Fig. 7A).
The results showed that pretreatment with WRT exhibited a similar
effect to KT5720, which decreased the hypertonic stress-induced
expression of AQP2 (Fig. 7B).
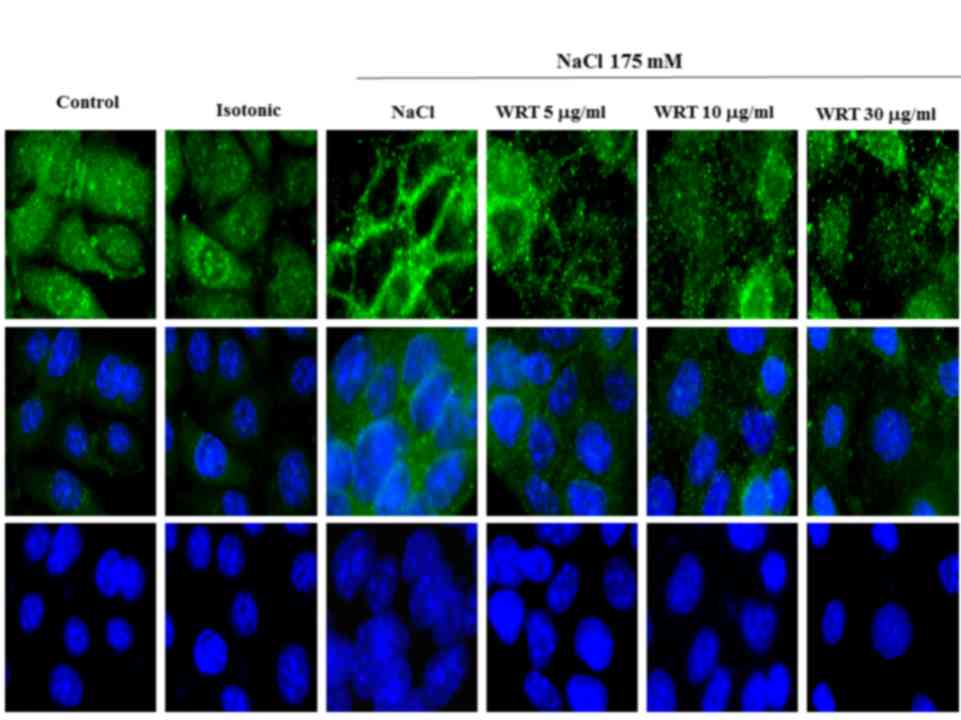
Effect of WRT on hypertonic
stress-induced increase of AQP2 trafficking
Hypertonic stress-induced water osmosis is dependent
on the insertion of vesicles containing AQP2 into the apical
membrane. To determine whether WRT decreases hypertonic
stress-induced trafficking of AQP2 to the apical membrane,
immunofluorescence was performed. As shown in Fig. 8, AQP2 was predominantly located
intracellularly in the control or isotonic-treated cells. Following
stimulating hypertonic stress-induced cells with 175 mM NaCl, AQP2
was localized to the apical membrane. However, pretreatment with
WRT decreased NaCl- induced AQP2 trafficking in mIMCD-3 cells.
Discussion
WRT is a well-known traditional oriental medicine
used for the treatment of chronic edema, dysuresia and water
imbalance during in vitro hypertonic stress. WRT has a
beneficial effect on inhibiting the PKA-mediated expression of AQP2
water channels and accumulation of organic osmolytes under
hypertonic stress.
The osmotic gradient is required to drive the
reabsorption of water from the renal collecting ducts (16). Hypertonic stress (175 mM NaCl)
increased the expression levels of AQP2 in mIMCD-3 cells. Under
hypertonic conditions, the activation of cation channels and
osmolyte transport systems is possibly the most important mechanism
to prevent osmotic cell shrinkage (17). The major organic osmolytes found in
mammalian renal medulla consist of myo-inositol, betaine, sorbitol,
taurine and glycerphosphorylcholine. Several of these are actively
transported into the cell by membrane transporters, including the
sodium/myo-inositol cotransporter (SMIT), sodium/chloride/betaine
cotransporter (BGT1) and sodium/chloride/taurine cotransporter
(TauT). Sorbitol is synthesized from glucose by aldose reductase
(AR) (17). When cultured cells
are exposed to hypertonic conditions, the activity of AR is
upregulated and transporters lead to the cellular accumulation of
organic osmolytes. In the present study, the osmolyte-associated
protein expression of BGT1 was determined using western blot
analysis. However, pretreatment with WRT did not affect the
expression of BGT1.
The TonE/TonEBP pathway mediates tonicity-responsive
transcriptional regulation by stimulating the urea transporters and
possibly the AQP2 water channel (18,19).
TonEBP is an essential regulator in the urine concentrating
mechanism. In the present study, pretreatment with WRT decreased
the protein and mRNA expression levels of TonEBP and SMIT. Thus,
WRT regulated hypertonic stress-induced urine concentration via the
TonEBP signaling pathway in the IMDC. SGK, an aldosterone-induced
gene in mineralocorticoid target cells, regulates the epithelial
sodium channel. SGK activation by aldosterone is mediated through
mineralocorticoid receptors (MRs) (20).
ENaC transports Na+ and is essential for
salt and fluid homeostasis across epithelial tissues. Aldosterone
also causes the translocation of MR to the nucleus and the
phosphorylation of SGK-1 (21).
The functions of SGK and PKA are well understood and are known to
upregulate the activity of ENaC (22). SGK1 is known to be regulated by
hypertonic stress. In the present study, the mRNA expression of SGK
and protein and mRNA expression levels of phosphorylated-SGK were
decreased by WRT pretreatment in a dose-dependent manner. These
results demonstrated that WRT regulated SGK1-mediated sodium
channels against hypertonic stimulation, although there was no
direct evidence that ENaC was regulated by WRT in the IMDC. Thus,
further investigation is required to clarify the mechanism
underlying the effect of WRT in hypertonic stress-induced
natriuresis.
AQPs are a family of specialized water channels
expressed on heterogeneous cell types, of which at least three
major types are found in the central nervous system (23,24).
The AQP1 water channel is expressed in the proximal tubule and thin
descending limb of the loop of Henle (25). AQP3 and AQP4 are present in the
basolateral plasma membrane of collecting duct principal cells and
represent exit pathways for water reabsorbed apically via AQP2. In
addition, vasopressin regulates the osmotic transport of water
(26). Under hypertonic stress,
treatment with 175 mM NaCl (650 mosmol/kg) induced the expression
of AQP2 and transport to the apical membrane, demonstrating that
the renal collecting duct was involved in urine concentration.
Vasopressin promotes renal water reabsorption,
decreasing the excretion of free water to dilute plasma and lower
serum osmolality. Vasopressin is the primary hormone regulating
water conservation in mammals. The apical accumulation of AQP2 is
mediated by vasopression, a water channel protein, thus
facilitating water reabsorption by the kidney. Vasopressin binds to
the V2 receptor of IMCD cells, activates the heterotrimeric G
protein Gαs and stimulates adenylyl cyclases III and VI, which
convert ATP into cAMP (27).
Increased cAMP levels activate cAMP-dependent PKA and lead to
phosphorylation (27). PKA is
critical in water excretion through regulation of the production
and action of the antidiuretic hormone, vasopressin (28). AQP2 channels are then
phosphorylated and are rapidly redistributed from intracellular
vesicles to the apical membrane of collecting duct principal cells
(29). In hypertonic conditions,
pretreatment with WRT decreased the protein and mRNA expression
levels of AQP2. The Na,K-ATPase α1-subunit in basolateral membrane
was also regulated by WRT. These results suggested that WRT
decreased AQP2 trafficking to the membrane through inhibition of
cAMP/PKA signaling pathway and direct/indirect involvement of
TonEBP under hypertonic stress in mIMCD-3 cells. In addition, it
was suggested that the pathophysiology of hypertonic stress-induced
AQP2 was not limited to the ‘classical’ cAMP/PKA pathway in mIMCD-3
cells.
Forskolin, an adenylate cyclase activator, increases
intracellular levels of cAMP in a variety of cells (12,30).
In the present study, WRT decreased hypertonic stress-induced
levels of cAMP in a dose-dependent manner. Forskolin increased the
expression of AQP2 with/without NaCl stimulation. WRT exhibited a
similar effect as the PKA inhibitor, KT5720, which decreased the
hypertonic stress-induced expression of AQP2 suggesting that WRT
inhibited hypertonic stress-induced expression of AQP2 via the
classical cAMP/PKA pathway in mIMCD-3 cells (31).
In conclusion, pretreatment of cells with WRT
decreased the hypertonic stress-induced phosphorylation of SGK. As
a result, the phosphorylation of AQP2 was decreased and this led to
increased urine volume. Taken together, the implications of the
present study in IMCD cells were that WRT had the beneficial effect
in regulating water balance against in vitro hypertonic
stress. The classical cAMP/PKA and TonEBP/SGK1 signaling pathways
were involved in hypertonic stress-induced expression of AQP2.
Thus, WRT may inhibit these signaling pathways, resulting in the
regulation of renal homeostasis.
Acknowledgements
This study was supported by the National Research
Foundation of Korea grant funded by the Korean Government (MSIP;
grant no. 2008–0062484).
References
|
1
|
Klussmann E, Maric K and Rosenthal W: The
mechanisms of aquaporin control in the renal collecting duct. Rev
Physiol Biochem Pharmacol. 141:33–95. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee SM, Lee YJ, Yoon JJ, Kang DG and Lee
HS: Effect of Poria cocos on hypertonic stress-induced water
channel expression and apoptosis in renal collecting duct cells. J
Ethnopharmacol. 141:368–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Day RE, Kitchen P, Owen DS, Bland C,
Marshall L, Conner AC, Bill RM and Conner MT: Human aquaporins:
Regulators of transcellular water flow. Biochim Biophys Acta.
1840:1492–1506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nielsen S, Frøkiaer J, Marples D, Kwon TH,
Agre P and Knepper MA: Aquaporins in the kidney: From molecules to
medicine. Physiol Rev. 82:205–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Storm R, Klussmann E, Geelhaar A,
Rosenthal W and Maric K: Osmolality and solute composition are
strong regulators of AQP2 expression in renal principal cells. Am J
Physiol Renal Physiol. 284:F189–F198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hozawa S, Holtzman EJ and Ausiello DA:
cAMP motifs regulating transcription in the aquaporin 2 gene. Am J
Physiol. 270:C1695–C1702. 1996.PubMed/NCBI
|
|
7
|
Umenishi F, Narikiyo T, Vandewalle A and
Schrier RW: cAMP regulates vasopressin-induced AQP2 expression via
protein kinase A-independent pathway. Biochim Biophys Acta.
1758:1100–1105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasler U, Jeon US, Kim JA, Mordasini D,
Kwon HM, Féraille E and Martin PY: Tonicity-responsive enhancer
binding protein is an essential regulator of aquaporin-2 expression
in renal collecting duct principal cells. J Am Soc Nephrol.
17:1521–1531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon ED, Jung KY, Edsall LC, Kim HY,
García-Pérez A and Burg MB: Osmotic regulation of synthesis of
glycerol phosphocholine from phosphatidylcholine in MDCK cells. Am
J Physiol. 268:C402–C412. 1995.PubMed/NCBI
|
|
10
|
Bell LM, Leong ML, Kim B, Wang E, Park J,
Hemmings BA and Firestone GL: Hyperosmotic stress stimulates
promoter activity and regulates cellular utilization of the serum-
and glucocorticoid-inducible protein kinase (Sgk) by a p38
MAPK-dependent pathway. J Biol Chem. 275:25262–25272. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Grigsby CL, Law CS, Ni X, Nekrep
N, Olsen K, Humphreys MH and Gardner DG: Tonicity-dependent
induction of Sgk1 expression has a potential role in
dehydration-induced natriuresis in rodents. J Clin Inves.
119:1647–1658. 2009. View
Article : Google Scholar
|
|
12
|
Riedlinger JE, Tan PW and Lu W: Ping wei
san, a Chinese medicine for gastrointestinal disorders. Ann
Pharmacother. 35:228–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin E, Ho L, Lin MS, Huang MH and Chen WC:
Wu-Ling-San formula prophylaxis against recurrent calcium oxalate
nephrolithiasis-a prospective randomized controlled trial. Afr J
Tradit Complement Altern Med. 10:199–209. 2013.PubMed/NCBI
|
|
14
|
Yun GY: Oriental Herbal Formula Science.
Publisher myeongbo; Seoul: pp. 198–204. 1985
|
|
15
|
Lee YP, Lee YJ, Lee SM, Yoon JJ, Kim HY,
Kang DG and Lee HS: Effect of atractylodes macrocephala on
hypertonic stress-induced water channel protein expression in renal
collecting duct cells. Evid Based Complement Alternat Med.
2012:6508092012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knepper MA, Kwon TH and Nielsen S:
Molecular physiology of water balance. N Engl J Med. 372:1349–1358.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wehner F: Cell volume-regulated cation
channels. Contrib Nephrol. 152:25–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeon US, Kim JA, Sheen MR and Kwon HM: How
tonicity regulates genes: Story of TonEBP transcriptional
activator. Acta Physiol (Oxf). 187:241–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakayama Y, Peng T, Sands JM and Bagnasco
SM: The TonE/TonEBP pathway mediates tonicity-responsive regulation
of UT-A urea transporter expression. J Biol Chem. 275:38275–38280.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Náray-Fejes-Tóth A and Fejes-Tóth G: The
sgk, an aldosterone induced gene in mineralocorticoid target cells,
regulates the epithelial sodium channel. Kidney Int. 57:1290–1294.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salyer SA, Parks J, Barati MT, Lederer ED,
Clark BJ, Klein JD and Khundmiri SJ: Aldosterone regulates Na(+),
K(+) ATPase activity in human renal proximal tubule cells through
mineralocorticoid receptor. Biochim Biophys Acta. 1833:2143–2152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baines D: Kinases as targets for ENaC
regulation. Curr Mol Pharmacol. 6:50–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vella J, Zammit C, Di Giovanni G, Muscat R
and Valentino M: The central role of aquaporins in the
pathophysiology of ischemic stroke. Front Cell Neurosci. 9:1082015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tait MJ, Saadoun S, Bell BA and
Papadopoulos MC: Water movements in the brain: Role of aquaporins.
Trends Neurosci. 31:37–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhai XY, Fenton RA, Andreasen A, Thomsen
JS and Christensen EI: Aquaporin-1 is not expressed in descending
thin limbs of short-loop nephrons. J Am Soc Nephrol. 18:2937–2944.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Balkom BW, van Raak M, Breton S,
Pastor-Soler N, Bouley R, van der Sluijs P, Brown D and Deen PM:
Hypertonicity is involved in redirecting the aquaporin-2 water
channel into the basolateral, instead of the apical, plasma
membrane of renal epithelia cells. J Biol Chem. 278:1101–1107.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boone M and Deen PM: Physiology and
pathophysiology of the vasopressin-regulated renal water
reabsorption. Pflugers Arch. 456:1005–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilbert ML, Yang L, Su T and McKnight GS:
Expression of a dominant negative PKA mutation in the kidney
elicits a diabetes insipidus phenotype. Am J Physiol Renal Physiol.
308:F627–F638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Breyer JA, Bain RP, Evans JK, Nahman NS
Jr, Lewis EJ, Cooper M, McGill J and Berl T: Predictors of the
progression of renal insufficiency patients with insulin-dependent
diabetes and overt diabetic nephropathy the collaborative study
group. Kidney Int. 50:1651–1658. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoyer PB, Fitz TA and Niswender GD:
Hormone-independent activation of adenylate cyclase in large
steroidogenic ovine luteal cells does not result in increased
progesterone secretion. Endocrinology. 114:604–608. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yip KP and Sham JS: Mechanisms of
vasopressin-induced intracellular Ca2+ oscillations in rat inner
medullary collecting duct. Am J Physiol Renal Physiol.
300:F540–F548. 2011. View Article : Google Scholar : PubMed/NCBI
|