Deep sea water improves hypercholesterolemia and hepatic lipid accumulation through the regulation of hepatic lipid metabolic gene expression
- Authors:
- Published online on: March 13, 2017 https://doi.org/10.3892/mmr.2017.6317
- Pages: 2814-2822
Abstract
Introduction
Hyperlipidemia is a lipid metabolism disorder, the prevalence of which has markedly increased in recent years. This disorder, which is caused by excessive consumption of food containing high levels of fat and cholesterol, is closely associated with hypertension, atherosclerosis (AS) and cardiovascular diseases (CVD) (1–3). It has previously been established that circulating low-density lipoprotein cholesterol (LDL-c), total cholesterol (TC) and triglycerides (TGs) are important risk factors in hypertension, AS and CVD (4–6). Increased levels of LDL-c, TC and TG in the blood weaken vessel walls and subsequently block blood flow, which may lead to myocardial infarction and stroke. Hypercholesterolemia may also be coupled with hepatic lipid accumulation. Increased lipid content in the liver induces chronic inflammation, which accelerates liver injury and may result in cirrhosis, liver failure and cancer. Accordingly, downregulation of increased LDL-c, TC and TG is required to prevent and treat these vascular and hepatic diseases.
It is well established that amelioration of lipid concentration in the blood prevents hypercholesterolemia and hepatic lipid accumulation. Lipid metabolism in the liver is controlled by fatty acid-synthesizing and energy expenditure enzymes, with decreased energy expenditure enzymes and increased fatty acid-synthesizing enzymes generally being observed in the livers of obese animal models fed a high-fat diet (HFD) and/or a high-cholesterol diet (HCD) (2,7–9). Furthermore, the hepatic expression levels of LDL receptor (LDLR) are associated with the concentration of circulating serum cholesterol in experimental animals fed a HFD and/or HCD (10). Previous studies have demonstrated that numerous candidates are able to decrease blood LDL-c, TC and TG levels via the suppression of lipogenic factors and the induction of lipolytic factors in obese animals, and several candidates improved lipid components in the blood via regulation of LDLR expression in hyperlipidemic rodents (2,7,9,11). These results indicated that regulation of lipid metabolism enzymes and LDLR are useful strategies for preventing liver fat accumulation and hypercholesterolemia.
Deep sea water (DSW) is considered a potent material that has food and medical applications. DSW contains abundant minerals, including magnesium (Mg), calcium (Ca), potassium (K) and zinc, which have important roles in cellular homeostasis and physiological responses (12–24). The beneficial effects of DSW on vascular diseases and metabolic disorders have been well demonstrated and these effects are thought to be associated with lipid metabolism (19,20). Hwang et al (19) demonstrated that DSW decreased body weight and improved lipid components in ob/ob mice; in addition, the differentiation of 3T3-L1 adipocytes was prevented by DSW (19,20). Although the beneficial effects of DSW in lipid metabolism have previously been investigated in several laboratories, the preventative effects of DSW on liver fat accumulation and hypercholesterolemia have not been fully investigated. Therefore, the present study aimed to determine the effects of DSW on liver fat accumulation and hypercholesterolemia in rats fed a HCD.
Materials and methods
Preparation of DSW
DSW was obtained from the Marine Deep Ocean Water Application Research Center in the Korea Institute of Ocean Science & Technology (Ansan, South Korea) from a depth of 500 m in the East Sea (Goseong, South Korea). Saline and minerals in DSW were removed and extracted by reverse osmosis filtration and electrodialysis (16). Extracted minerals were dissolved in desalinated DSW to generate hardness 4,000 (H4000) DSW containing 835.6 mg/l Mg, 279.9 mg/l Ca, 213.7 mg/l Na and 81.2 mg/l K (Mg:Ca concentration ratio, 3:1). H4000 DSW was serially diluted with desalinated DSW to prepare DSW of various hardness (400–2,000). The hardness of DSW was determined by the following formula: Total hardness=Ca hardness [2.5 × Ca concentration (mg/l)] + Mg hardness [4.1 × Mg concentration (mg/l)].
Animals and treatment
Animal experiments were conducted following approval by the Animal Use and Care Committee at Dongguk University (approval IACUC-2013-001; Gyeongju, Korea). A total of 42 male 5-week old Sprague-Dawley rats (120–130 g) with a normal diet (ND; 5L57, containing no cholesterol) were obtained from Orient Bio Inc. (Seongnam, Korea). The rats were housed under a 12 h light/dark cycle at 25±2°C and a relative humidity of 50±5%. The rats received the ND and tap water ad libitum for 1 week. Subsequently, rats received a HCD (D12336, Research Diets, Inc., New Brunswick, NJ, USA) with tap water or DSW of various hardness ad libitum for 6 weeks. The composition of the HCD is presented in Table I. Rats were randomly divided into 1 ND group and 6 experimental groups: Tap HCD, H0 HCD, H400 HCD, H800 HCD, H1500 HCD and H2000 HCD. Each group consisted of 6 rats. Body weight, and food and water intake were measured every 2–3 days during the experiment. After 6 weeks, animals were fasted for 24 h and subsequently sacrificed with ether by inhalation, then blood was collected to determine the lipid composition in each group.
Analysis of blood lipid components
TG, TC and high-density lipoprotein cholesterol (HDL-c) in the blood were enzymatically analyzed using commercial kits (AM157K, AM202K and AM203K respectively; Asan Pharmaceutical Co., Ltd., Seoul, Korea) according to the manufacturer's protocols. The LDL-c concentration was calculated using the Friedwald formula: LDL-c concentration=TC concentration-HDL-c concentration-TG/2.
Evaluation of liver damage indicators
Glutamate-oxaloacetate transaminase (GOT), glutamate-pyruvate transferase (GPT) and alkaline phosphatase (ALP) activity in the blood were assessed as indicators of liver damage. GOT and GPT activities were measured using a commercial kit (AM101K; Asan Pharmaceutical Co., Ltd.) based on the Reitman-Frankel method (25), whereas ALP activity was determined using a Kind-King method-based commercial kit (AM105S; Asan Pharmaceutical Co., Ltd.) according to the manufacturer's protocols.
Electron microscopic analysis
Livers were pre-fixed with 0.1 M PBS containing 2.5% glutaraldehyde for 2 h at 4°C and subsequently washed with 0.1 M PBS three times for 15 min. The tissues were subsequently post-fixed by immersion in 2% osmium tetroxide solution for 2 h at 4°C followed by dehydration with ethanol. Tissues were embedded with epon-812 resin, sectioned at 100 nm thickness using a Leica Ultracut R (Leica Microsystems GmbH, Wetzlar, Germany) and double-stained with uranyl acetate and lead nitrate. Finally, tissues were visualized using a Hitachi H-7500 transmission electron microscope (Hitachi, Ltd., Tokyo, Japan) at 80 kV.
Semi-quantitative and quantitative (q) reverse transcription-polymerase chain reaction (RT-PCR)
The expression of fatty acid synthase (FAS), carnitine palmitoyltransferase-1 (CPT-1), sterol regulatory element binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor γ (PPARγ) was analyzed by semi-quantitative RT-PCR, and qPCR was used to analyze the expression of LDLR. Livers were rapidly frozen in liquid nitrogen and stored at −80°C. Total RNA in individual liver samples was extracted using an easy-BLUE™ Total RNA Extraction kit (17061; Intron Biotechnology, Inc., Seongnam, Korea) according to the manufacturer's protocol. cDNA synthesis was performed using PrimeScript™ 1st strand cDNA Synthesis kit (6110a; Takara Bio, Inc., Otsu, Japan) according to the manufacturer's protocols and amplification of PCR products for semi-quantitative RT-PCR was performed with 2 µl of cDNA in Ex Taq DNA polymerase mixture containing 2 mM MgCl2, 200 µM dNTP (Takara Bio, Inc.) and 0.2 µM of each forward and reverse primer (Bioneer Corporation, Daejeon, Korea) with a final reaction volume of 25 µl. The PCR cycling conditions were as follows: 95°C for 10 min (initial denaturation), 22–32 cycles at 95°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec (amplification) and 72°C for 10 min (final extension). All reactions were finished during the exponential phases. PCR products and 100 bp ladder (WelGene Co., Daegu, Korea) were subjected to agarose gel electrophoresis containing 0.5 µg/ml ethidium bromide (Promega Corporation, Madison, WI, USA) and observed using i-MAX Gel Image Analysis System with CoreBio MFC software (CoreBio System Co., Ltd., Seoul, Korea). qPCR was performed using a QGreen™ SYBR Green Master Mix kit (Cellsafe Co. Ltd., Suwon, Korea) and the Eco Real-Time PCR system (Illumina, Inc., San Diego, CA, USA). The PCR cycling conditions were as follows: 95°C for 10 min followed by 45 cycles at 95°C for 10 sec, 60°C for 10 sec and 72°C for 30 sec. The relative intensity of the target genes was calculated using Eco™ software version 3.1.7 (Illumina, Inc., San Diego, CA, USA) by the ΔΔCq method (26). GAPDH was used as an internal control to normalize target gene expression. The PCR primer sequences for target genes are presented in Table II.
Statistical analysis
Values were presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance with SPSS software (version no. 22; SPSS, Inc., Chicago, IL, USA) followed by Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Changes in the lipid composition of blood in response to DSW treatment
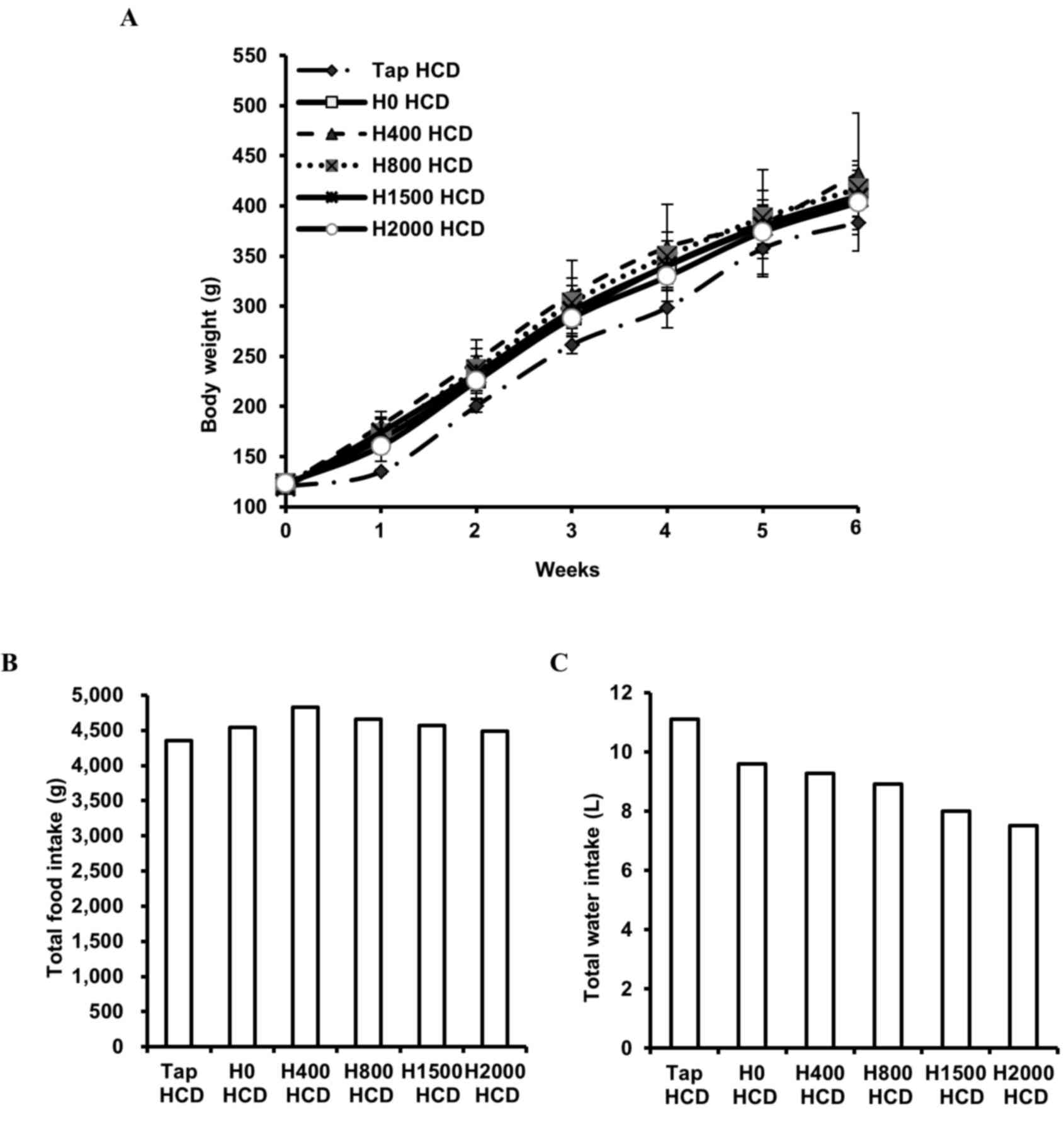
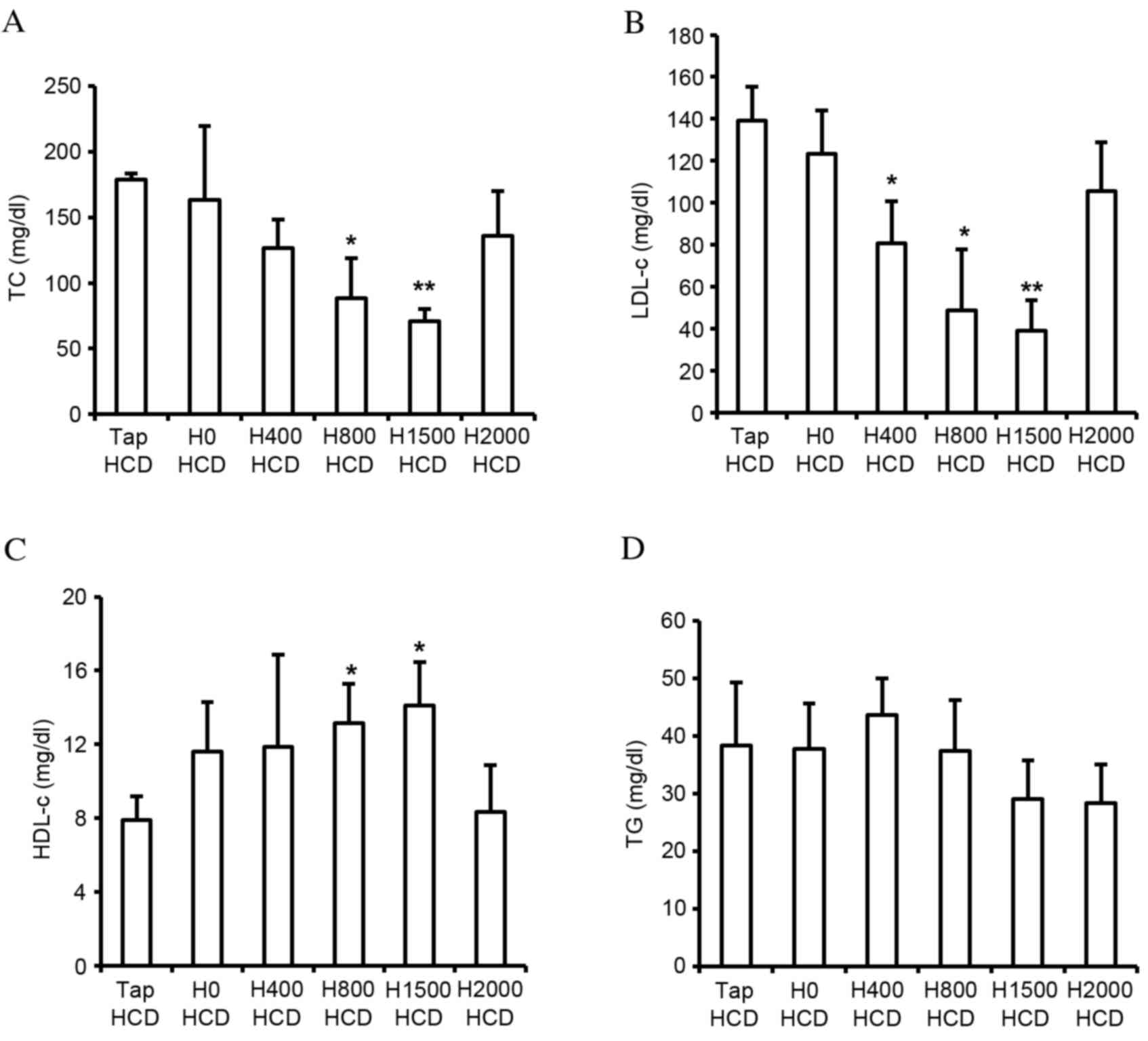
The present study monitored body weight, and food and water (tap water or DSW) intake, in rats fed a HCD. No significant differences in body weight (Fig. 1A) or food intake (Fig. 1B) were observed among the groups. However, reduced total water intake was observed in DSW groups in a hardness-dependent manner (Fig. 1C). In addition, blood lipid components were measured. Blood TC and LDL-c in rats fed a HCD were increased ~3.4- and 29.9-fold, respectively, and HDL-c was decreased ~4.8-fold compared with rats fed a ND (data not shown). Despite the decreased total water intake in DSW groups, significantly reduced levels of TC and LDL-c were observed in the H800 (P<0.05) and H1500 (P<0.01) HCD groups compared with the Tap HCD group (Fig. 2A and B). In addition, significantly increased HDL-c was detected in response to DSW in the H800 and H1500 HCD groups compared with in the Tap HCD group (P<0.05; Fig. 2C). However, no significant alterations in TG were observed among the groups (Fig. 2D).
Suppression of hepatic lipid accumulation
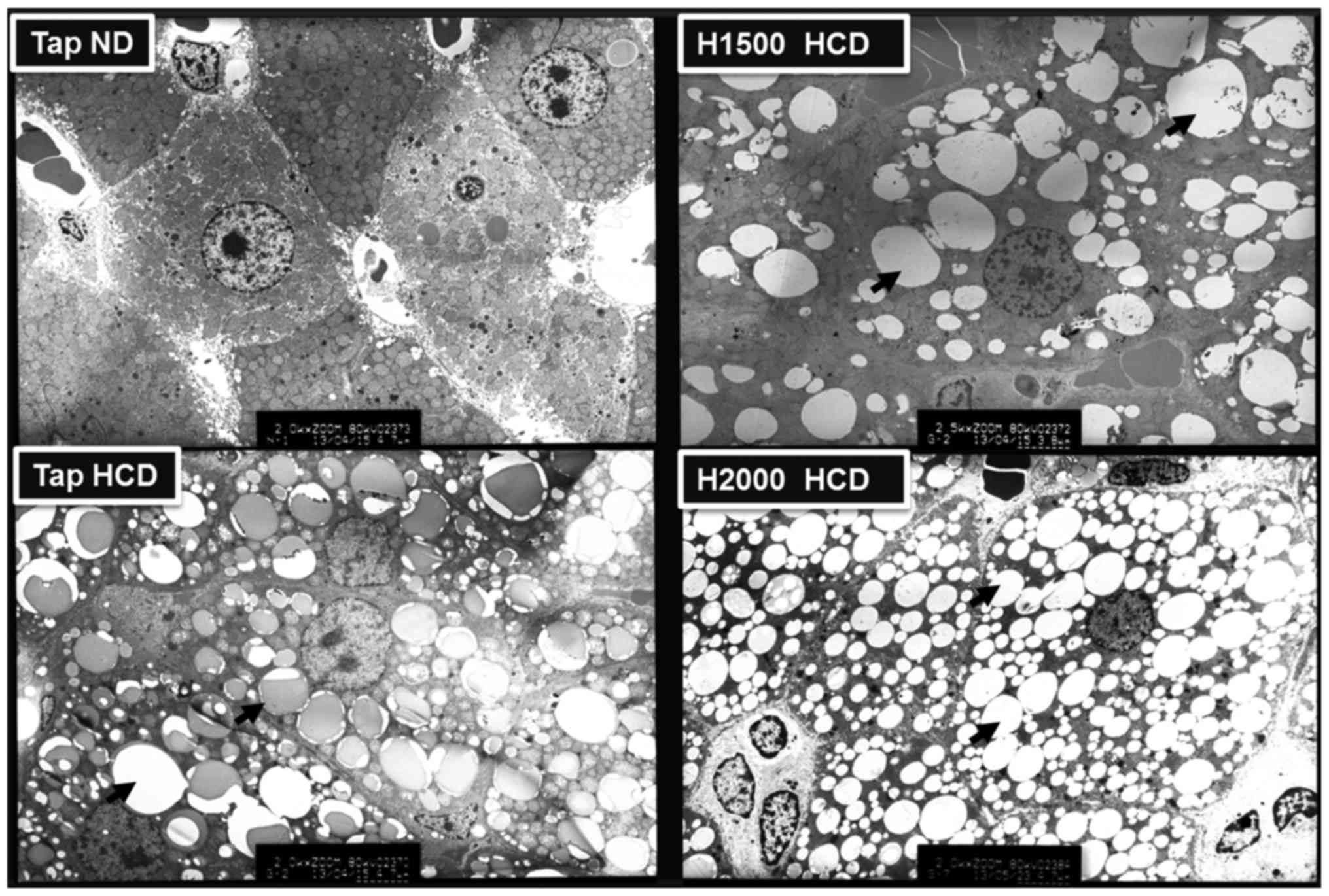
Metabolic diseases, including obesity, diabetes and hypercholesterolemia, may be induced by a HCD and are associated with hepatic lipid accumulation (27). Therefore, the present study analyzed the distribution of lipid droplets in rat liver cells using electron microscopy. The liver cells of rats fed a HCD exhibited numerous lipid droplets and the number of lipid droplets was visibly increased in HCD livers compared with ND-fed rat livers. However, the H1500 DSW HCD group exhibited fewer liver cell lipid droplets compared with the Tap HCD group (Fig. 3). Conversely, the H2000 group exhibited increased numbers of lipid droplets in liver cells compared with the H1500 DSW HCD group (Fig. 3). The results of electron microscopy corresponded to the blood TC, LDL-c and HDL-c levels observed in these groups.
Alleviation of liver injury indices
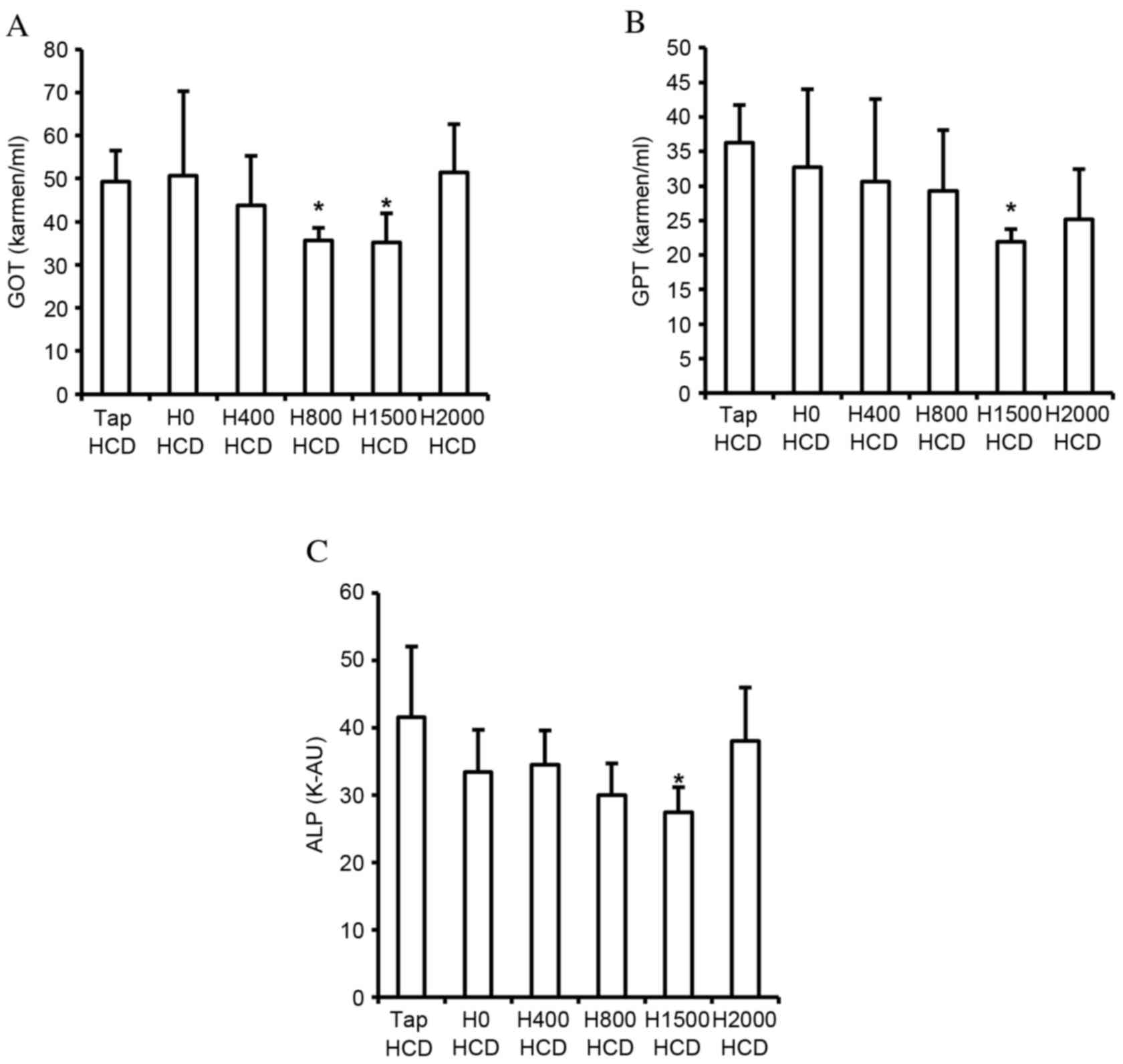
Lipid accumulation in the liver, and increased blood TC and LDL-c concentration, are associated with liver injury. The present study detected the suppressive effects of DSW on hepatic lipid accumulation, and the elevation of blood TC and LDL-c concentration. Therefore, the effects of DSW on liver injury indices in the blood, including GOT, GPT and ALP, were assessed. HCD-induced increased GOT, GPT and ALP levels in the blood were significantly decreased by H1500 DSW compared with the Tap HCD group (P<0.05; Fig. 4); however, H2000 DSW did not significantly reduce levels compared with the Tap HCD group (Fig. 4). The decrease in GOT, GPT and ALP levels corresponded with the decrease of hepatic lipid accumulation and blood TC and LDL-c levels.
Regulation of lipid metabolism-regulating gene expression in the liver
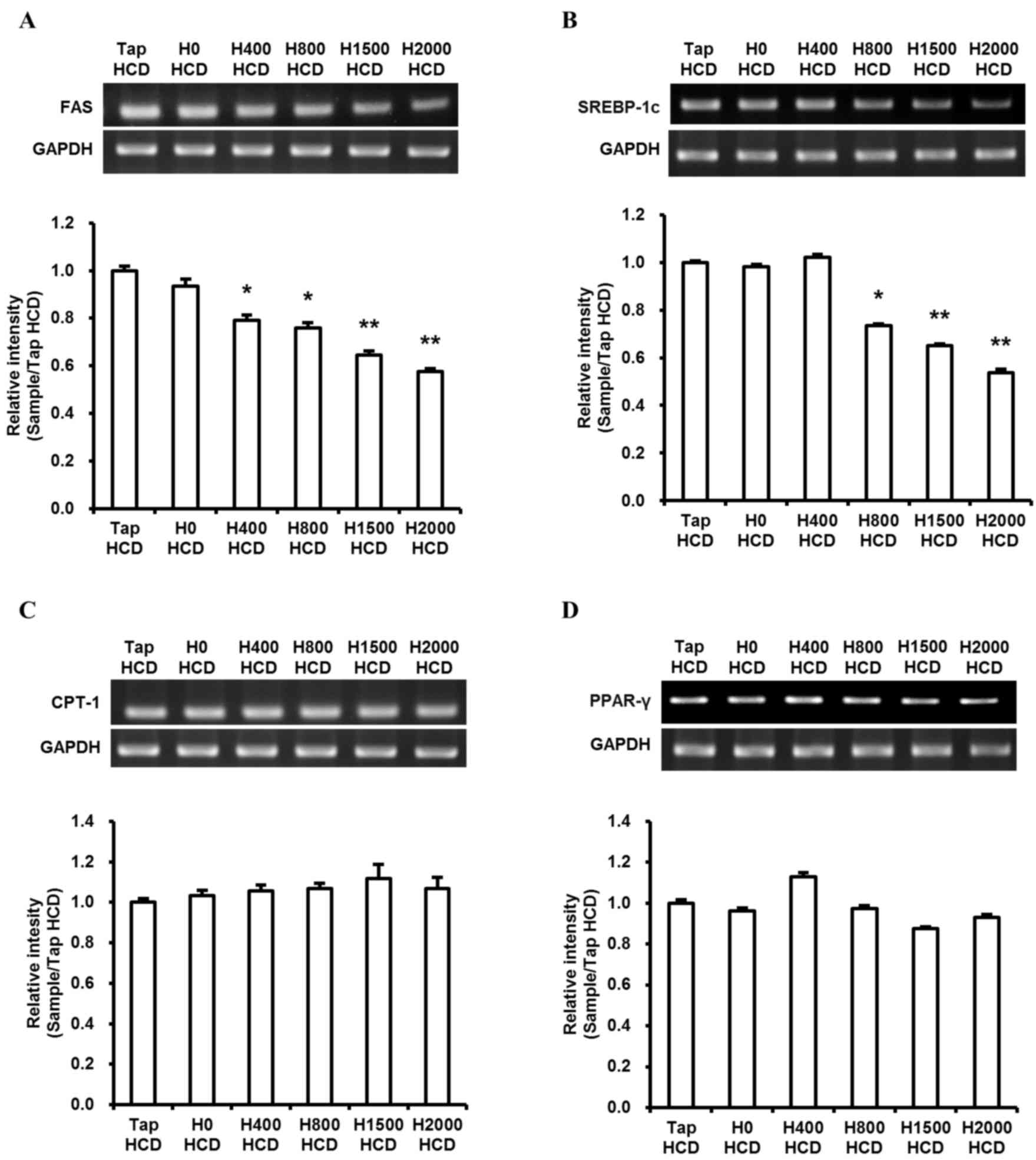
Lipid homeostasis in the liver is governed by the balance of expression between fatty acid-synthesizing enzymes and energy expenditure enzymes. Numerous studies have detected fat accumulation in the livers of HFD- and/or HCD-fed rodents (7,8,28). Furthermore, hepatic FAS, PPARγ and SREBP-1c expression in rodent livers have previously been demonstrated to be significantly increased by a HFD and/or HCD (8,11,16,28,29). Therefore, the present study investigated the difference in the expression of these genes between control and DSW groups in rats fed a HCD. In addition, the expression of CPT-1, an energy expenditure enzyme, was assessed. DSW groups exhibited significantly reduced levels of FAS and SREBP-1c expression in H800, H1500 and H2000 HCD groups compared with the Tap HCD group (P<0.05; Fig. 5A and B). However, no significant differences were observed in CPT-1 and PPARγ expression (Fig. 5C and D).
Regulation of hepatic LDLR gene expression
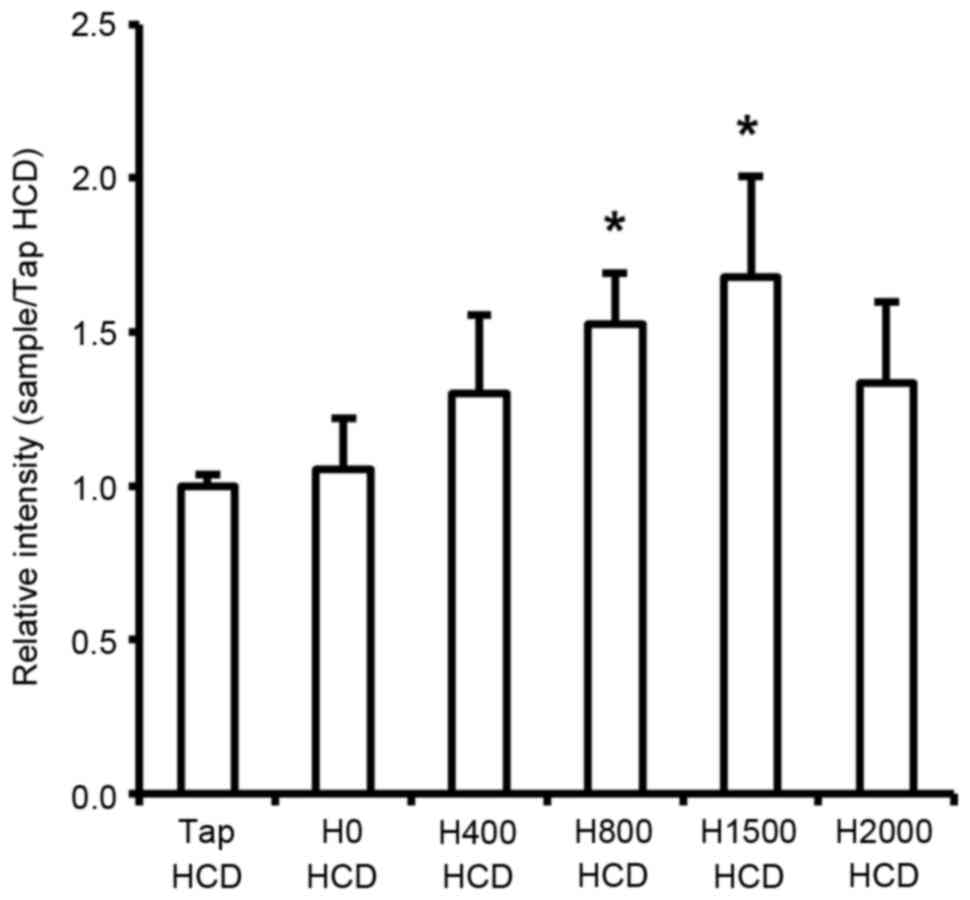
The present study demonstrated that serum TC and LDL-c levels were decreased in response to DSW in rats fed a HCD. Circulating serum cholesterol is primarily absorbed in the liver through hepatic LDLR-mediated endocytosis and is subsequently metabolized (30,31). Consequently, serum cholesterol levels should be associated with hepatic LDLR levels. Therefore, the present study investigated mRNA expression of LDLR in the liver of rats. The results revealed a significant increase in hepatic LDLR mRNA in rats fed a HCD in response to DSW at H800 and H1500 compared with the Tap HCD group (P<0.05; Fig. 6). However, although H2000 DSW also increased LDLR mRNA expression compared with in the Tap HCD group, the increase was not statistically significant (Fig. 6).
Discussion
Several studies have demonstrated the importance of minerals, including Mg and Ca, in lipid metabolism. For example, increased Mg intake was demonstrated to prevent hypercholesterolemia, lipid oxidation and oxidative damage (32,33). Conversely, growth inhibition in fetal mice was induced by altered lipid metabolism caused by maternal Mg deficiency and low levels of Mg in blood were observed in obese children from South India (34,35). In addition, a high Ca intake was associated with low serum levels of TC and LDL-c in humans (36). The present study demonstrated that the blood lipid composition in rats fed a HCD improved in response to DSW containing high levels of Mg and Ca (concentration ratio Mg:Ca=3:1; Fig. 2). The results indicated that DSW may reduce blood TC and LDL-c, and increase HDL-c, through increased blood Mg and Ca levels. However, the TC, LDL-c and HDL-c levels in rats treated with H2000 DSW, the highest hardness in this experiment, were not significantly altered (Fig. 2A-C). These results demonstrated that increased Mg levels in the blood may have an important role in the reduction of harmful cholesterol; however, the beneficial effects of excessive concentrations of Mg and Ca may be lower.
Increased levels of blood lipid components, including TG, TC and LDL-c, induced by a HFD and/or HCD may lead to liver fat accumulation. The hepatic accumulation of fat may be prevented by lowering blood levels of these lipid components. Previous studies (19,23,29) have demonstrated that DSW attenuated hepatic lipid accumulation in hamsters and mice. Furthermore, an increase of Mg and Ca in DSW led to the alleviation of hepatic lipid accumulation and oxidation in a concentration-dependent manner in hamsters fed HFDs (29). The results of the present study demonstrated that H1500 DSW prevented lipid accumulation in the liver; however, this decrease was not observed in the H2000 DSW group (Fig. 3). Therefore, although the association between hepatic lipid accumulation and mineral (Mg and Ca) content is unclear, the results of the present study indicated that the beneficial effects of DSW on hepatic lipid accumulation may be determined by the concentration of Mg and Ca in DSW.
Increased levels of liver injury indicators are associated with liver fat accumulation and increased serum TC and LDL-c. Chen et al (29) detected decreased GOT and GPT in hamsters fed a HFD/HCD for 6 weeks following treatment with DSW drinking water. In addition, the previous study demonstrated that the decrease was associated with a reduction in TC and TG concentration. The results of the present study are consistent with those of Chen et al (29; Fig. 4). High levels of GOT, GPT and ALP have been observed in patients with liver diseases, including hepatitis, cirrhosis, liver failure and liver cancer (37,38). Therefore, the suppression of increases in GOT, GPT and ALP levels may be important for the prevention of diet-induced hepatic diseases.
PPARγ and SREBP-1c are transcriptional regulators of lipid metabolism enzymes. Previous studies (28,39) have demonstrated that hepatic PPARγ and SREBP-1c expression were increased in rodents fed a HFD and/or HCD, and that suppression of PPARγ and SREBP-1c gene expression reduced fat accumulation and blood TC and LDL-c levels in livers of mice. Furthermore, decreased lipid deposits in hepatocytes were observed when SREBP-1c silencing was performed in vitro (40). In the present study, DSW suppressed liver fat accumulation and reduced the HCD-induced increases in TC and LDL-c levels in the blood and FAS and SREBP-1c transcription; however, no effects on CPT-1 and PPARγ expression were observed (Fig. 5). Although Chen et al (29) demonstrated that serum lipid component levels were improved in response to DSW drinking water, no effects were observed on FAS and SREBP-1c expression in response to DSW (29). The ratio of Mg:Ca in DSW drinking water in Chen et al (29) was 4-5:1; however, DSW in the present investigation was 3:1. Therefore, the dissimilarity in the effects of DSW on FAS and SREBP-1c expression may be caused by differences in the ratio of Mg:Ca. The results of the present study indicated that DSW may prevent lipid accumulation in the liver via suppression of FAS expression regulated by SREBP-1c, without the induction of CPT-1 transcription, and may be more effective at preventing liver fat accumulation and increases in TC and LDL-c levels.
Previous studies (31,41–43) have demonstrated an association between decreasing serum cholesterol and increasing LDLR expression in the liver in response to various materials. The present study demonstrated that H800 and H1500 DSW decreased serum LDL-c concentrations, and that this decrease was accompanied by the induction of LDLR expression in rats fed a HCD (Figs. 2B and 6). Therefore, the present study indicated that the hypocholesterolemic effects of DSW may be mediated by LDLR. However, although decreases in the expression levels of FAS and SREBP-1c were observed (Fig. 5), H2000 DSW did not prevent liver fat accumulation or improve serum lipid component levels (Figs. 2 and 3). In addition, LDLR expression was not significantly increased by H2000 DSW compared with in the Tap HCD group (Fig. 6). Although it is unclear why H2000 DSW does not affect liver fat accumulation, serum TG, TC and LDL-c levels, and hepatic LDLR expression, it may be hypothesized that these effects may be associated with Mg and Ca concentration. Consequently, the results of the present study indicated that H1500 DSW may be most suitable for preventing liver fat accumulation and hypercholesterolemia.
In conclusion, the present study assessed the effects of DSW on HCD-induced hepatic lipid accumulation and hypercholesterolemia in rats. The results demonstrated that DSW decreased TG, TC, LDL-c, GOT, GPT and ALP levels in the blood, and reduced lipid accumulation in the liver. Furthermore, the mRNA expression levels of FAS and SREBP-1c were downregulated, whereas the expression of LDLR was upregulated by DSW. Combined, these results indicated that DSW may have the potential to prevent hepatic lipid accumulation and may exert blood cholesterol-lowering activity via the inhibition of fatty acid synthesis in the liver and enhancement of LDL-c clearance in the blood, caused by increased hepatic LDLR expression. The present study indicated that DSW is a candidate for the prevention of hypercholesterolemia and hepatic lipid accumulation.
Acknowledgements
This work was financially supported by the National R&D Project ‘Development of New Application Technology For Deep Sea Water Industry’ supported by the Ministry of Oceans and Fisheries of the Republic of Korea.
References
|
Ma Y, Wang W, Zhang J, Lu Y, Wu W, Yan H and Wang Y: Hyperlipidemia and atherosclerotic lesion development in Ldlr-deficient mice on a long-term high-fat diet. PLoS One. 7:e358352012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Wu C, Wu H, Sheng L, Su Y, Zhang X, Luan H, Sun G, Sun X, Tian Y, et al: Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS One. 8:e619222013. View Article : Google Scholar : PubMed/NCBI | |
|
Daniels SR: Management of hyperlipidemia in pediatrics. Curr Opin Cardiol. 27:92–97. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Cholesterol Treatment Trialists' (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al: Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376:1670–1681. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP and Lloyd-Jones DM: Lifetime risks of cardiovascular disease. N Engl J Med. 366:321–329. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Harchaoui KE, Visser ME, Kastelein JJ, Stroes ES and Dallinga-Thie GM: Triglycerides and cardiovascular risk. Curr Cardiol Rev. 5:216–222. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Yang ZH, Miyahara H, Takeo J, Hatanaka A and Katayama M: Pollock oil supplementation modulates hyperlipidemia and ameliorates hepatic steatosis in mice fed a high-fat diet. Lipids Health Dis. 10:1892011. View Article : Google Scholar : PubMed/NCBI | |
|
Yao Z, Liu XC and Gu YE: Schisandra chinensis Baill, a Chinese medicinal herb, alleviates high-fat-diet-inducing non-alcoholic steatohepatitis in rats. Afr J Tradit Complement Altern Med. 11:222–227. 2013.PubMed/NCBI | |
|
Carrier B, Wen S, Zigouras S, Browne RW, Li Z, Patel MS, Williamson DL and Rideout TC: Alpha-lipoic acid reduces LDL-particle number and PCSK9 concentrations in high-fat fed obese Zucker rats. PLoS One. 9:e908632014. View Article : Google Scholar : PubMed/NCBI | |
|
Singh AB, Kan CF, Shende V, Dong B and Liu J: A novel posttranscriptional mechanism for dietary cholesterol-mediated suppression of liver LDL receptor expression. J Lipid Res. 55:1397–1407. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kim H, Bartley GE, Arvik T, Lipson R, Nah SY, Seo K and Yokoyama W: Dietary supplementation of chardonnay grape seed flour reduces plasma cholesterol concentration, hepatic steatosis, and abdominal fat content in high-fat diet-induced obese hamsters. J Agric Food Chem. 62:1919–1925. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kim S, Chun SY, Lee DH, Lee KS and Nam KS: Mineral-enriched deep-sea water inhibits the metastatic potential of human breast cancer cell lines. Int J Oncol. 43:1691–1700. 2013.PubMed/NCBI | |
|
Katsuda S, Yasukawa T, Nakagawa K, Miyake M, Yamasaki M, Katahira K, Mohri M, Shimizu T and Hazama A: Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull. 31:38–44. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Lee KS, Shin JS, Kwon YS, Moon DS and Nam KS: Suppression of cancer progression and metastasis in HT-29 human colorectal adenocarcinomas by deep sea water. Biotechnol Bioproc Eng. 18:194–200. 2013. View Article : Google Scholar | |
|
Fu ZY, Yang FL, Hsu HW and Lu YF: Drinking deep seawater decreases serum total and low-density lipoprotein-cholesterol in hypercholesterolemic subjects. J Med Food. 15:535–541. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ha BG, Shin EJ, Park JE and Shon YH: Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice. Mar Drugs. 11:4193–4212. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Hataguchi Y, Tai H, Nakajima H and Kimata H: Drinking deep-sea water restores mineral imbalance in atopic eczema/dermatitis syndrome. Eur J Clin Nutr. 59:1093–1096. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Hsu CL, Chang YY, Chiu CH, Yang KT, Wang Y, Fu SG and Chen YC: Cardiovascular protection of deep-seawater drinking water in high-fat/cholesterol fed hamsters. Food Chem. 127:1146–1152. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hwang HS, Kim HA, Lee SH and Yun JW: Anti-obesity and antidiabetic effects of deep sea water on ob/ob mice. Mar Biotechnol (NY). 11:531–539. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Hwang HS, Kim SH, Yoo YG, Chu YS, Shon YH, Nam KS and Yun JW: Inhibitory effect of deep-sea water on differentiation of 3T3-L1 adipocytes. Mar Biotechnol (NY). 11:161–168. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Li PC, Pan CH, Sheu MJ, Wu CC, Ma WF and Wu CH: Deep sea water prevents balloon angioplasty-induced hyperplasia through MMP-2: An in vitro and in vivo study. PLoS One. 9:e969272014. View Article : Google Scholar : PubMed/NCBI | |
|
Miyamura M, Yoshioka S, Hamada A, Takuma D, Yokota J, Kusunose M, Kyotani S, Kawakita H, Odani K, Tsutsui Y and Nishioka Y: Difference between deep seawater and surface seawater in the preventive effect of atherosclerosis. Biol Pharm Bull. 27:1784–1787. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Sheu MJ, Chou PY, Lin WH, Pan CH, Chien YC, Chung YL, Liu FC and Wu CH: Deep sea water modulates blood pressure and exhibits hypolipidemic effects via the AMPK-ACC pathway: An in vivo study. Mar Drugs. 11:2183–2202. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshioka S, Hamada A, Cui T, Yokota J, Yamamoto S, Kusunose M, Miyamura M, Kyotani S, Kaneda R, Tsutsui Y, et al: Pharmacological activity of deep-sea water: Examination of hyperlipemia prevention and medical treatment effect. Biol Pharm Bull. 26:1552–1559. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Reitman S and Frankel S: A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63. 1957. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Vuppalanchi R and Chalasani N: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 49:306–317. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, Suzuki Y, Saito H, Kohgo Y and Okumura T: Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 336:215–222. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Chen IS, Chang YY, Hsu CL, Lin HW, Chang MH, Chen JW, Chen SS and Chen YC: Alleviative effects of deep-seawater drinking water on hepatic lipid accumulation and oxidation induced by a high-fat diet. J Chin Med Assoc. 76:95–101. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Ma PT, Gil G, Südhof TC, Bilheimer DW, Goldstein JL and Brown MS: Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci USA. 83:8370–8374. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Yasunobu Y, Hayashi K, Shingu T, Nomura K, Ohkura Y, Tanaka K, Kuga Y, Nomura S, Ohtani H, Nishimura T, et al: Reduction of plasma cholesterol levels and induction of hepatic LDL receptor by cerivastatin sodium (CAS 143201-11-0, BAY w 6228), a new inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, in dogs. Cardiovasc Drugs Ther. 11:567–574. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Abad C, Vargas FR, Zoltan T, Proverbio T, Piñero S, Proverbio F and Marín R: Magnesium sulfate affords protection against oxidative damage during severe preeclampsia. Placenta. 36:179–185. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Olatunji LA and Soladoye AO: Increased magnesium intake prevents hyperlipidemia and insulin resistance and reduces lipid peroxidation in fructose-fed rats. Pathophysiology. 14:11–15. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Gupta M, Solanki MH, Chatterjee PK, Xue X, Roman A, Desai N, Rochelson B and Metz CN: Maternal magnesium deficiency in mice leads to maternal metabolic dysfunction and altered lipid metabolism with fetal growth restriction. Mol Med. 20:332–340. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Niranjan G, Anitha D, Srinivasan AR, Velu VK, Venkatesh C, Babu MS, Ramesh R and Saha S: Association of inflammatory sialoproteins, lipid peroxides and serum magnesium levels with cardiometabolic risk factors in obese children of South Indian population. Int J Biomed Sci. 10:118–123. 2014.PubMed/NCBI | |
|
Jacqmain M, Doucet E, Després JP, Bouchard C and Tremblay A: Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am J Clin Nutr. 77:1448–1452. 2003.PubMed/NCBI | |
|
Miyake S: The mechanism of release of hepatic enzymes in various liver diseases. II. Altered activity ratios of GOT to GPT in serum and liver of patients with liver diseases. Acta Med Okayama. 33:343–358. 1979.PubMed/NCBI | |
|
Cremers J, Drent M, Driessen A, Nieman F, Wijnen P, Baughman R and Koek G: Liver-test abnormalities in sarcoidosis. Eur J Gastroenterol Hepatol. 24:17–24. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Morán-Salvador E, López-Parra M, García-Alonso V, Titos E, Martínez-Clemente M, González-Périz A, López-Vicario C, Barak Y, Arroyo V and Clària J: Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 25:2538–2550. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Deng Q, Li X, Fu S, Yin L, Zhang Y, Wang T, Wang J, Liu L, Yuan X, Sun G, et al: SREBP-1c gene silencing can decrease lipid deposits in bovine hepatocytes cultured in vitro. Cell Physiol Biochem. 33:1568–1578. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Chang XX, Yan HM, Xu Q, Xia MF, Bian H, Zhu TF and Gao X: The effects of berberine on hyperhomocysteinemia and hyperlipidemia in rats fed with a long-term high-fat diet. Lipids Health Dis. 11:862012. View Article : Google Scholar : PubMed/NCBI | |
|
Benn T, Kim B, Park YK, Yang Y, Pham TX, Ku CS, Farruggia C, Harness E, Smyth JA and Lee JY: Polyphenol-rich blackcurrant extract exerts hypocholesterolaemic and hypoglycaemic effects in mice fed a diet containing high fat and cholesterol. Br J Nutr. 113:1697–1703. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y, Peng L, Yang LC, Xu XD, Li WJ, Luo XM and Jin X: Wedelolactone regulates lipid metabolism and improves hepatic steatosis partly by AMPK Activation and Up-regulation of expression of PPARα/LPL and LDLR. PLoS One. 10:e01327202015. View Article : Google Scholar : PubMed/NCBI |