Introduction
Inflammation associated with cancer is a promising
target for the development of anticancer therapies. Cytokines,
chemokines and growth factors may have an important function in the
interaction between tumor cells and infiltrating leukocytes from
blood vessels. The existence of inflammatory components in the
microenvironment of neoplastic tissues frequently leads to
increased angiogenesis, resistance to hormones and inhibition of
adaptive anti-tumor immunity. The survival, proliferation and
subsequent invasion and metastasis of tumor cells is regulated by
inflammatory mediators present at the tumor site (1).
Primary inflammatory cytokines, including
interleukin-1 (IL-1) and tumor necrosis factor (TNF), expressed by
leukocytes and tumor infiltrating cells, are targets for anticancer
therapy. Anti-cytokine strategies against tumors have been
investigated with TNF inhibitors in certain inflammatory diseases
(2). Clinical trials with TNF-α
antagonists, alone or combined with other therapies, have been
performed in patients with cancer. In cases of advanced solid
cancer, TNF-α antagonists were well tolerated and exhibited
biological activity and partial response with renal carcinoma or
stable disease (3,4). Permanent activation of NF-κΒ
contributes actively in tumorigenesis by promoting cell cycle
progression and inhibiting apoptosis. NF-κΒ is activated through
TNF-α, thus inhibition of this factor may support cancer therapies
that target apoptosis (5,6). Inhibition of NF-κΒ is associated with
apoptosis and reduced cell growth, and this inhibition may be
beneficial in the treatment of cancer (7). The p38α mitogen-activated protein
kinase (p38α MAPK) may have tumor suppressor activity through
regulation of the p53 gene, which interferes with cell cycle
progression and induces apoptosis. However, it also exhibits
oncogenic activity associated with various processes, including
invasion, inflammation and angiogenesis, which are essential in
tumor development (8).
Plants are a major source of active substances that
are used in therapeutic medicine as their metabolites have great
structural diversity (9).
Anti-inflammatory compounds have been extracted from natural
products, including fruits, vegetables, spices and traditional
medicinal herbs, and these compounds have been gaining importance
as potential chemopreventive or therapeutic agents for cancer
(10). In the last 2 decades,
herbal products have been important candidates in the discovery of
novel drugs for cancer (11).
Drugs derived from natural products that have antibacterial,
anticoagulant, antiparasitic, immunosuppressive and anticancer
properties are able to treat 87% of categorized human diseases
(12). There were 12 natural
products and 32 derivatives of natural products among the 128
anticancer drugs released to the market between 1981 and 2010. They
were obtained from various sources, including plants and
microorganisms. Between 1940 and 2010, 175 small molecules were
released for the treatment of cancer and of those, 131 molecules
were developed from natural products (13). The investigation of novel plants
with anti-inflammatory, anti-tumor and anti-carcinogenic potential
is important and may enable the development of novel drugs for
cancer treatment. Several members of Myrtaceae family have been
previously investigated and various activities were observed,
including antioxidant (14),
anti-tumor (15–21) and anti-inflammatory activities
(22–27). To the best of our knowledge, there
are no existing studies investigating the activity of
Calyptranthes grandifolia. However, important activities
have been demonstrated in other members of the Calyptranthes
genus (28–35). Thus, this genus demonstrates
potential and may be beneficial in the treatment of inflammatory
and tumor processes. The objective of the present study was to
investigate the expression of genes associated with proliferation
and inflammation in cells of the Caco-2 cell line treated with
extracts from Calyptranthes grandifolia O.Berg.
Materials and methods
Plant material
Leaves of Calyptranthes grandifolia O.Berg
were collected in Lajeado, Rio Grande do Sul, in Southern Brazil
and identified by Professor Elisete Maria de Freitas. From this
material ethanol and hexane extracts were isolated according to the
following methodology.
Hexanic extract preparation
The leaves of the plant were dried in an incubator
with circulating air at 38°C for 24 h. Subsequently, leaves were
reduced to small fragments to increase the contact surface with the
extraction solution. The leaves of Calyptranthes grandifolia
O.Berg were packed with hexane solvent in an amber bottle at room
temperature for 72 h. Vacuum filtration was performed and the
filtrate was stored in an amber jar at room temperature until the
solvent was removed. Maceration of the leaves occurred for 2 weeks
and the solvent was changed twice. Subsequently, vacuum filtration
and removal of solvent was performed with the aid of a rotary
evaporator at 40°C. Finally, the extract obtained was stored in
amber bottles and refrigerated at 4±1°C until experiments were
performed.
Ethanolic extract preparation
Plant leaves were dried in an oven with circulating
air at 38°C for 24 h. Following this period, cold static soaking
was performed on fragments of leaves with 90% ethanol and the
material was placed in an amber bottle and kept at room temperature
for 7 days. Subsequent to the extraction period, vacuum filtration
and removal of solvent was performed with the aid of a rotary
evaporator at 40°C. The extract was placed in amber bottles and
refrigerated at 4±1°C until experiments were performed.
Dilution of extracts
Solubilization of the extract was performed with
dimethylsulfoxide (DMSO) so that the final concentration was
≤0.5%.
Cell culture
Caco-2 colorectal adenocarcinoma cell line (HTB-37;
American Type Culture Collection, Manassas, VA, USA) and RAW 264.7
murine macrophage cell line (TIB-71; American Type Culture
Collection) were cultivated in microwell plates (1×105 cells/well)
in an incubator (37°C, 5% CO2). Subsequently, treatment with plant
extract was performed at different concentrations (25, 50, 100 and
200 µg/ml), with incubation in culture medium Dulbecco's modified
Eagle's medium (DMEM, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (FBS,
Sigma-Aldrich; Merck KGaA) for 1 h at 37ºC. Subsequently,
lipopolysaccharides (LPS; 1 µg/ml) were added, and incubation was
performed at 37°C and 5% CO2 for 24 h (36). Subsequent to 24 h incubation at
different concentrations and treatments, extraction of total RNA
was performed on the Caco-2 cell line and the supernatant of RAW
264.7 cells was collected for ELISA assay. In addition to treatment
with extracts, each culture plate had a positive control, in which
cells were stimulated with LPS only, and a negative control with no
stimulation or treatment. A total of 5 different experiments were
performed for gene expression analysis.
Cell viability assay by alamar
Blue®
Caco-2 cells were plated at density of 1×105
cells/ml in 96-well plates containing 200 µl of DMEM low glucose
and 10% FBS, and incubated for 24 h in an atmosphere of 5% CO2, 90%
humidity and 37°C. Subsequently, cells were treated with
concentrations of 25, 50, 100 or 200 µg/ml per well of extract, and
incubated for 72 h. Following this period, treatment was removed
and a solution of 10% alamar Blue® dye was added per
well. The absorbance readings were performed following 6 h
incubation at 37°C, at a wavelength of 540 nm (oxidized) and 620 nm
(reduced) in an ELISA reader. As a negative control, cells were
placed only in culture medium and DMSO. The percentage of cell
viability was calculated using the following formula: % alamar
Blue® reduction = absorbance at 540 nm-(absorbance at
630 nm×correction factor) ×100. Correction factor was calculated by
staining the culture medium with no cells.
Cell viability assay by Trypan
Blue
Cells were removed from the plates with the aid of a
scraper and transferred to 15 ml centrifuge tubes (1×105
cells/centrifuge tube; Corning Incorporated, Corning, NY, USA),
which were centrifuged at 600 × g for 10 min at room temperature.
Following centrifugation, the supernatant was discarded and the
pellet was resuspended in 1 ml DMEM in a 1:10 dilution with Trypan
Blue dye, which stains non-viable cells. Finally, a total count of
viable cells was performed in a Neubauer chamber for subsequent
plating of viable Caco-2 and RAW264.7 cells (37).
RNA extraction
Total RNA extraction was performed by the
TRIzol® method (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions and purification
was performed by illustra RNAspin Mini kit (GE Healthcare Life
Sciences, Chalfont, UK). Total RNA was quantified using
aL-Quant® spectrophotometer (Loccus, São Paulo, Brazil)
with a 2 µl final product of RNA extraction, and absorbance was
read at 260 nm.
Complementary DNA (cDNA)
synthesis
Synthesis of cDNA was performed from 0.5 µg total
RNA, using poly-A tail complementary oligonucleotide primers and
the Superscript™ II Reverse Transcriptase kit according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). To each sample tube, 1 µl of dNTP mix and 1 µl OligodT was
added prior to incubation for 5 min at 65°C. Then, 9 µl of a mix
containing 10X PCR Buffer, 25 mM MgCl2, 0.1 M DTT and RNase OUT was
added and further incubated for 2 min at 42°C. A total of 1 µl of
Superscript II RT was added and incubated again at 42°C for 50 min
and 70°C for 15 min. Finally, 1 µl of RNase H was added and
incubated at 37°C for 20 min. At the end of synthesis, the cDNA was
stored at −20°C until amplification was performed by quantitative
polymerase chain reaction (qPCR).
qPCR
Gene expression analysis was performed by qPCR. The
results were normalized to β-actin (38,39)
and the efficiency of reactions was evaluated using the standard
curve of each gene analyzed. The qPCR results were expressed as the
relative quantification of amplified cDNA with respect to the
normalizer gene (40). Primers
used for amplification of specific cDNA fragments were selected
from the published sequence of each gene using online tool Primer3
v.0.4.0 (41). All primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.; Table I). DNA amplification and relative
quantification was performed using a StepOnePlus™ Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
Platinum® SYBR® Green qPCR SuperMix-UDG kit
(Invitrogen; Thermo Fisher Scientific, Inc.) in a total volume of
25 ml [12.5 µl SuperMix, 0.5 µl (50 µmol/l) Rox reference dye, 0.3
µl of each primer (10 µmol/l forward and 10 µmol/l reverse), 9.4 µl
H2O and 2.0 µl 1:20 diluted template cDNA] according to the
manufacturer's (SYBR® Green kit) instructions.
Amplification and reading of samples was performed in duplicate
with the following protocol for all genes: Initial incubation for 3
min at 94°C; followed by 45 cycles of 30 sec denaturation at 94°C;
30 sec annealing at 55°C and 30 sec extension at 60°C. To confirm
specificity of the reaction, a dissociation curve was performed for
each primer pair with melting temperature analysis of each
gene.
 | Table I.Oligonucleotide primers for
quantitative polymerase chain reaction. |
Table I.
Oligonucleotide primers for
quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) | Fragment size
(bp) |
|---|
| NF-κΒ |
| 209 |
|
Sense |
ACACCGTGTAAACCAAAGCC |
|
|
Antisense |
CAGCCAGTGTTGTGATTGCT |
|
| p38α |
| 243 |
|
Sense |
CAGTGGGATGCATAATGGCC |
|
|
Antisense |
GCATCTTCTCCAGCAAGTCG |
|
| TNF-α |
| 120 |
|
Sense |
CCCTGGTATGAGCCCATCTATC |
|
|
Antisense |
AAAGTAGACCTGCCCAGACTCG |
|
| β-actin |
| 140 |
|
Sense |
CTGGAACGGTGAAGGTGACA |
|
|
Antisense |
AAGGGACTTCCTGTAACAATGCA |
|
TNF-α cytokine release in RAW 264.7
cells by ELISA assay
Following 24 h incubation of RAW 264.7 cells with
LPS stimulation, 0.4 ml supernatant was collected and stored at
−80°C and used for quantification of the release of TNF-α
pro-inflammatory cytokine using the Mouse TNF alpha ELISA
Ready-SET-Go® ELISA kit (cat. no. 88-7324-86;
e-Bioscience, Inc., San Diego, CA, USA) according to the
manufacturer's instructions.
Statistical analysis
Data were tabulated and analyzed with descriptive
statistics using SPSS software (version 20.0; IBM SPSS, Armonk, NY,
USA) and GraphPad Prism (version 5.0; GraphPad Software, Inc., La
Jolla, CA, USA). Comparison between the controls was performed
using an unpaired Student's t-test and the effects of extracts were
analyzed by one-way analysis of variance followed by the Tukey
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
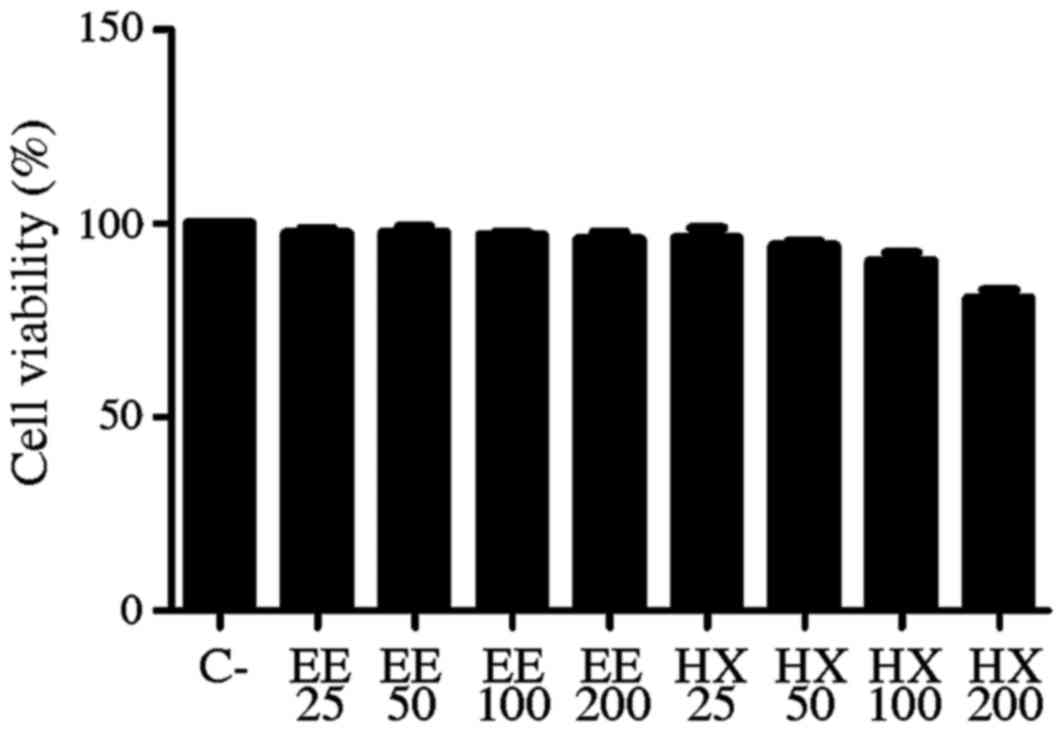
Cell viability by alamar
Blue®
It was observed that the ethanolic and hexane
extracts of Calyptranthes grandifolia O.Berg did not affect the
cell proliferation when compared with the negative control. The
negative control exhibited a viability of 62.6% (± 4.367), and for
ethanolic and hexane extracts at tested concentrations, the
percentages of viability were similar to the control (Fig. 1).
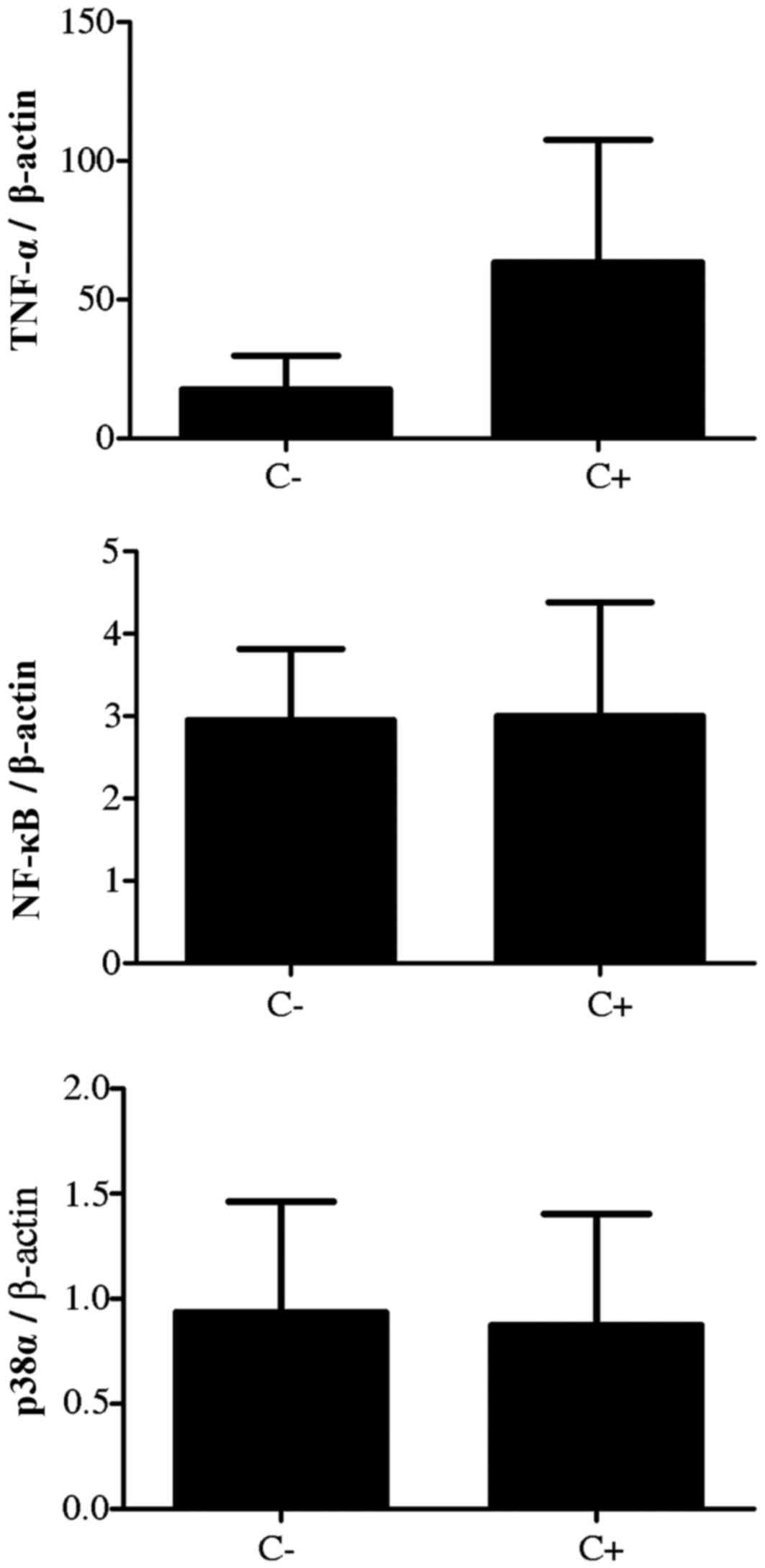
Gene expression analysis
For the analysis of the genes of interest, the
positive control consisted of Caco-2 cells that were stimulated
only by LPS with no extract treatment, and the negative control
consisted only of cells in culture. The comparison between negative
and positive controls was performed in order to verify whether
there was a difference in gene expression when stimulated by LPS.
Increased expression of TNF-α was observed following treatment with
LPS compared with the negative control, however, this was not
significantly different. In addition, the expression of NF-κB and
p38α were similar in negative and positive control groups;
expression was not altered by LPS treatment. Thus, the expression
of these genes was stable in the negative and positive control
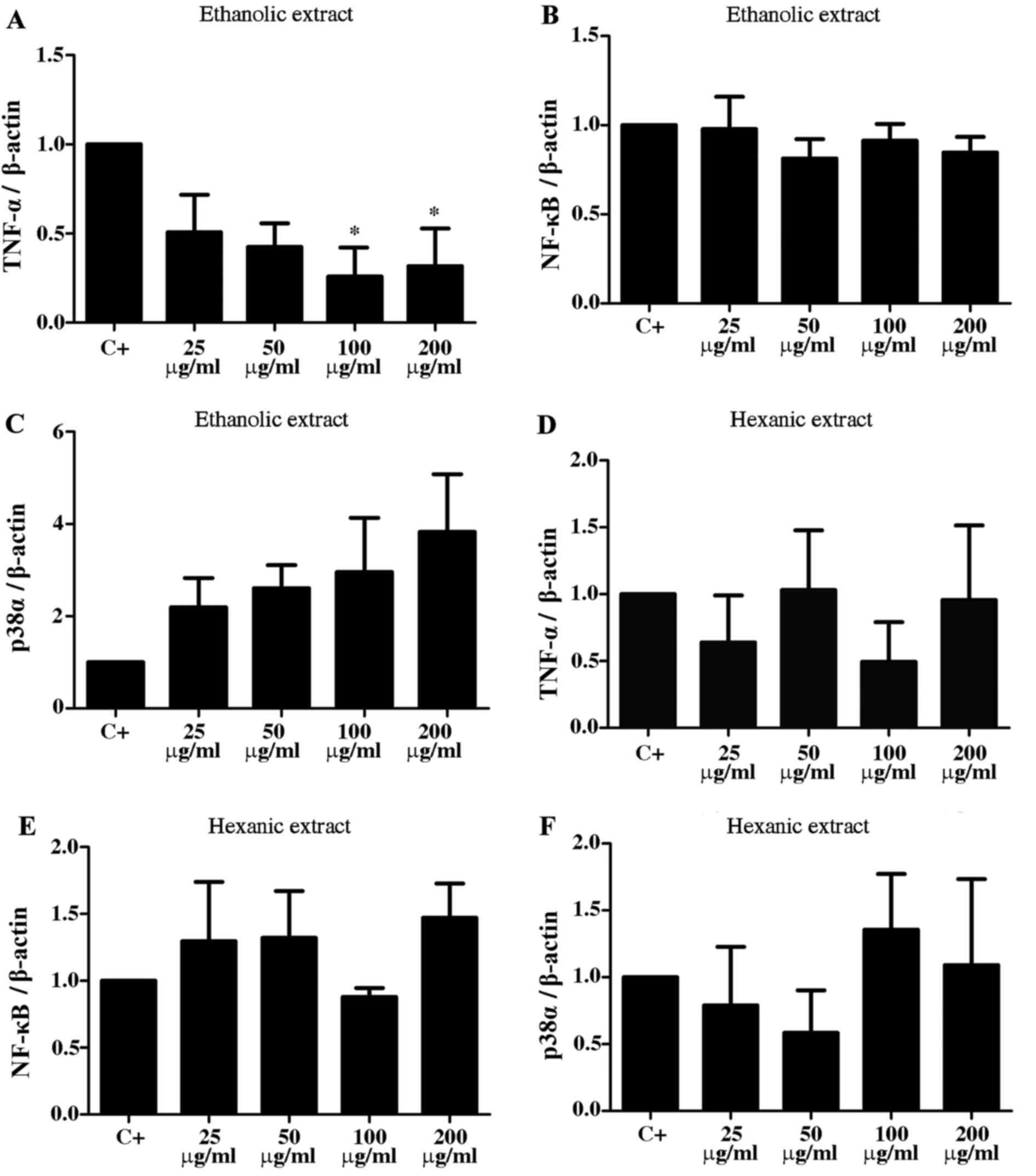
groups (Fig. 2). When evaluating
the effect of the ethanolic extract of Calyptranthes grandifolia on
gene expression, a decrease in TNF-α expression was observed, which
was significant at concentrations of 100 and 200 µg/ml, compared
with the positive control (P<0.025; Fig. 3A). Regarding NF-κΒ gene expression,
expression was similar for all treatments and there were no
significant changes (Fig. 3B). The
expression of p38α increased with increasing concentrations of
ethanolic extract, however, these increases were not significant
(Fig. 3C). The expression of
TNF-α, NF-κΒ and p38α with hexane extract treatment exhibited no
significant variation compared with the positive control (Fig. 3D-F).
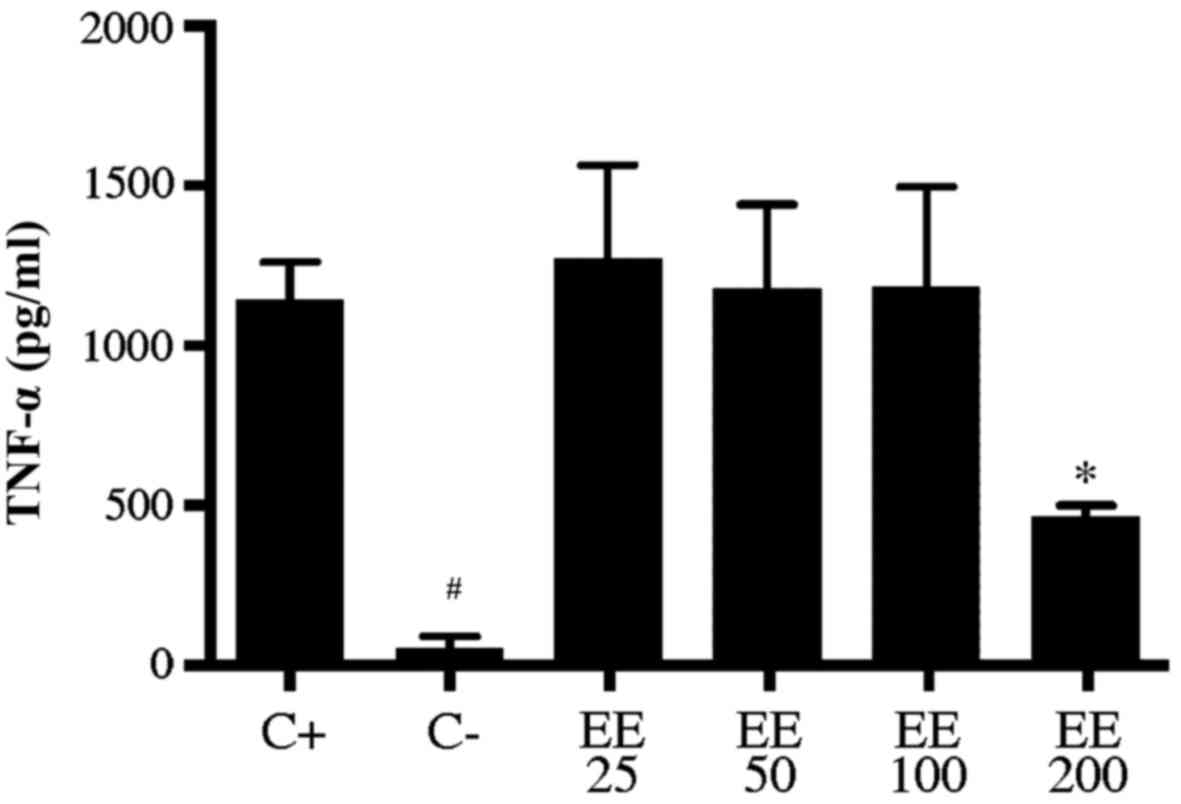
TNF-α cytokine release in RAW 264.7
cells by ELISA
In order to demonstrate the decrease in TNF-α in
gene expression, an ELISA colorimetric assay was performed to
evaluate the level of cytokine release. A macrophage lineage was
used, characterized by the release of inflammatory cytokines. The
inhibition of TNF-α was significant (P<0.05) with the ethanolic
extract at a concentration of 200 µg/ml (464.0±36.7 pg/ml) compared
with the positive control (1143.0±118.0 pg/ml; Fig. 4) and also when compared with a
concentration of 25 µg/ml (1272±293 pg/ml). Concentrations of 25
µg/ml (1272±293 pg/ml), 50 µg/ml (1179±261 pg/ml) and 100 µg/ml
(1185±312 pg/ml) exhibited no significant difference compared with
the positive control (Fig. 4). The
negative control (53.6±36.8 pg/ml) exhibited a significant
difference in TNF-α cytokine release compared with the positive
control (P<0.0001; Fig. 4) and
compared with concentrations of 25, 50 and 100 µg/ml (P<0.001;
Fig. 4). There were no significant
differences when treated with hexanic extract (data not shown).
Discussion
Stimulation by LPS increased TNF-α gene expression
in the positive control (stimulated with LPS) compared with the
negative control (no stimulation with LPS). The expression of p38α
and NF-κΒ did not change in the positive control compared with the
negative control. Herath et al (42) demonstrated that different serotypes
of LPS produce different responses upon cell stimulation. For
example, in human gingival fibroblasts there was no activation of
the NF-κΒ pathway by LPS 1435/1449. However, LPS1690 significantly
activated the pathway. Other pro-inflammatory genes also exhibit
modified activation by different LPS serotypes.
When evaluating the effect of Calyptranthes
grandifolia O.Berg extract treatment, on TNF-α gene expression, the
present study observed reduced expression compared with the
positive control at certain concentrations in both extracts.
However, only the ethanolic extract at concentrations of 100 and
200 µg/ml significantly reduced TNF-α expression compared with the
positive control. To the best of our knowledge, there are no
previous studies that have evaluated the effect of this plant on
TNF-α expression, however, Ferreira et al (43) evaluated the anti-inflammatory
activity of Campomanesia adamantium extracts (also a member of the
Myrtaceae family) and the production of nitric oxide (NO), TNF-α
and IL-10 in J774.A1 macrophages stimulated with LPS/interferon-γ.
Paw edema in mice was inhibited by oral administration of the
extracts, it inhibited NO and TNF-α production, and increased IL-10
production. Thus, the anti-inflammatory activity of the extract may
be associated with the inhibition of pro-inflammatory cytokines and
increased IL-10. Li et al (44) evaluated the activity of flavonoids
in RAW 264.7 cells and demonstrated the inhibition of NO, TNF-α,
IL-6 and IL-Ip, indicating an important role for flavanoids in
anti-inflammatory activity (44).
The present study observed no changes in NF-κΒ
expression between the different extracts and concentrations
tested. The activation of NF-κΒ in Caco-2 cells may be associated
with the state of cell differentiation. NF-κΒ signaling pathways or
IκΒ kinase do not exist in isolation, therefore, various mechanisms
integrate their activity with other signaling pathways (45). NF-κΒ is a protein that modulates
the apoptotic response as it is a transcription factor that
protects against and contributes to apoptosis (46). The nuclear factor is a central
regulator of immune responses and has an important role in the
expression of cytokine genes, including IL-2, IL-6, C-C motif
chemokine 2 and CD40 ligand. Furthermore, it is also involved in
the expression of genes associated with cell survival and
proliferation, including cyclin D1, cyclin D2, c-Myc, c-Myb,
cyclooxygenase-2, Bcl-2 and Bcl-xl (47,48).
Thus, NF-κΒ is considered as a tumor promoter and is frequently
identified as constitutively active in tumors (49–52).
NF-κΒ may not have been activated due to the high expression of
TNF-α, and thus by anti-inflammatory signals.
The present study observed no significant
differences in p38α gene expression between the various treatments
and concentrations. However, its expression did increase with
increasing concentrations of ethanolic extract, with the highest
expression at a concentration of 200 µg/ml. The activation of p38
isoforms is specifically controlled by different regulators and
they are co-activated by several combinations (53,54).
The p38α MAPK activates other kinases and therefore regulates
diverse cellular responses. Thus, p38 signaling may be associated
with inflammation, the cell cycle, cell death, development,
differentiation, senescence and tumorigenesis (55). The anti-tumor effect of various
chemotherapeutic drugs is based on apoptosis through p38α
activation. However, it is important to note that this kinase is
associated with various responses, and may also be involved in
resistance to chemotherapy in certain types of tumors (56). Campbell et al (57) demonstrated that a modification of
p38α MAPK inhibited TNF-α production in macrophages induced by LPS,
post-transcriptionally. Furthermore, p38 negatively regulated the
expression of NF-κΒ, which allows transcriptional control of TNF-α
as the nuclear factor is required for its expression (57). Despite the pro-apoptotic role of
p38α, p38α MAPK has also been previously associated with an
anti-apoptotic activity (58–61).
Comes et al (62)
demonstrated that p38α induced the survival of colorectal cancer
cells by inhibiting autophagy. Another mechanism involved in
p38α-induced survival is the activation of the activating
transcription factor 6α-Ras homolog enriched in brain-mechanistic
target of rapamycin (ATF6α-Rheb-mTOR) pathway, which promoted the
survival of dormant tumor cells in vivo (63). Although the present study observed
no significant differences in the mRNA levels of p38α and NF-κΒ,
further studies are required with larger sample sizes, as well as
studies that investigate the expression at the protein level, to
verify the presence and activation of these proteins. The increase
in mRNA levels is not directly proportional to the amount of
protein translated, due to transcriptional and post-translational
modifications.
The extracts included in the present study did not
affect the cell viability when compared with the negative control.
According to the US National Cancer Institute, an extract may be
considered active or cytotoxic when it presents cytotoxicity with
IC50 values <30 µg/ml (half maximal inhibitory concentration)
(64). In this aspect, cytotoxic
extracts maybe potential candidates for anti-carcinogenic studies
(65). However, it is important to
note that these statements should be tested in other cell lines in
order to observe potential selective cytotoxicity.
The antioxidant activity was also determined in
ethanolic and hexane extracts of Calyptranthes grandifolia by the
research group (data not shown). This activity was evaluated by
antioxidant activity testing, by capturing the free radical
2,2-diphenyl-1-picryl-hidrazila (66). The results demonstrated that the
hexane extract exhibited no antioxidant activity, however, the
ethanolic extract exhibited a dose dependent antioxidant activity.
The observed antioxidant activity may be associated with a
potential anti-inflammatory activity of the extract, based on the
results observed for gene expression and TNF-α cytokine release.
The ethanolic extract had significant antioxidant activity compared
with hexane, which corresponds with the decrease of TNF-α gene
expression.
Based on the significant reduction of TNF-α when
Caco-2 cells were treated with the ethanolic extract of
Calyptranthes grandifolia O.Berg, the significant inhibition of
TNF-α cytokine release in RAW 264.7 murine macrophages, antioxidant
activity of the extract and the lack of effects on cell viability,
it is indicated that this extract may have anti-inflammatory
potential. However, further studies are required to elucidate the
signaling pathway that may be activated. Given the variation
between experiments and potential post-transcriptional regulation,
the analysis of other genes is required in order to assess the
potential pathways implicated. Furthermore, it is important to
analyze protein expression in order to confirm that the expressed
genes in Caco-2 cells are translated and determine the respective
levels of translation.
In conclusion, Calyptranthes grandifolia ethanolic
extract at concentrations of 100 and 200 µg/ml significantly
reduced TNF-α gene expression in the Caco-2 cell line. There were
no significant differences in p38α and NF-κΒ gene expression. Cells
treated with hexane extract exhibited no significant variations in
the expression of the genes investigated at any of the
concentrations. Ethanolic extract at 200 µg/ml significantly
inhibited TNF-α pro-inflammatory cytokine release. The extracts
were not considered to be cytotoxic and are not candidates for
anti-carcinogenic studies in this lineage. However, other studies
using different cell lines maybe performed to identify selective
cytotoxicity. The results of the present study indicate that
ethanolic extract has an anti-inflammatory potential by decreasing
expression of TNF-α. Thus, it is important to investigate its
genotoxicity and to conduct in vivo analysis to confirm its
anti-inflammatory potential.
References
|
1
|
Germano G, Allavena P and Mantovani A:
Cytokines as key component of cancer-related inflammation.
Cytokine. 43:374–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartlett DL, Ma G, Alexander HR, Libutti
SK and Fraker DL: Isolated limb reperfusion with tumor necrosis
factor and melphalan in patients with extremity melanoma after
failure of isolated limb perfusion with chemotherapeutics. Cancer.
80:2084–2090. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madhusudan S, Muthuramalingam SR,
Braybrooke JP, Wilner S, Kaur K, Han C, Hoare S, Balkwill F and
Ganesan TS: Study of etanercept, a tumor necrosis factor-alpha
inhibitor, in recurrent ovarian cancer. J ClinOncol. 23:5950–5959.
2005. View Article : Google Scholar
|
|
4
|
Harrison ML, Obermueller E, Maisey NR,
Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, et
al: Tumor necrosis factor alpha as a new target for renal cell
carcinoma: Two sequential phase II trials of infliximab at standard
and high dose. J ClinOncol. 25:4542–4549. 2007. View Article : Google Scholar
|
|
5
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beg AA and Baltimore D: An essential role
for NF-kappaB in preventing TNF-alpha-induced cell death. Science.
274:782–784. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stroh C, Held J, Samraj AK and
Schulze-Osthoff K: Specific inhibition of transcription factor
NF-kappaB through intracellular protein delivery of IkappaBalpha by
the Herpes virus protein VP22. Oncogene. 22:5367–5373. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dolado I and Nebreda AR: Regulation of
tumorigenesis by p38α MAP kinase. Topics Curr Genet. 20:99–128.
2008. View Article : Google Scholar
|
|
9
|
Brandão HN, David JP, Couto RD, Nascimento
JAP and David JM: Química e farmacologia de quimioterápicos
antineoplásicos derivados de plantas. Quím Nova. 33:1359–1369.
2010. View Article : Google Scholar
|
|
10
|
Aravindaram K and Yang NS:
Anti-inflammatory plant natural products for cancer therapy. Planta
Med. 76:1103–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhanot A, Sharma R and Noolvi MN: Natural
sources as potential anti-cancer agents: A review. Int J Phytomed.
3:9–26. 2011.
|
|
12
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Souza-Moreira TM, Moreira RRD, Sacramento
LVS and Pietro RCLR: Histochemical, phytochemical and biological
screening of Pliniacauliflora (Mart.) Kausel (Myrtaceae) leaves.
RevBras Farmacogn. 20:48–53. 2010. View Article : Google Scholar
|
|
15
|
Zhang P, Zhang E, Xiao M, Chen C and Xu W:
Enhanced chemical and biological activities of a newly
biosynthesized eugenol glycoconjugate, eugenol α-D-glucopyranoside.
Appl Microbiol Biotechnol. 97:1043–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rizzo LY, Longato GB, Ruiz AL, Tinti SV,
Possenti A, Vendramini-Costa DB, Sartoratto A, Figueira GM, Silva
FL, Eberlin MN, et al: In vitro, in vivo and in silico analysis of
the anticancer and estrogen-like activity of guava leaf extracts.
Curr Med Chem. 21:2322–2330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sultana B, Anwar F, Mushtaq M, Aslam M and
Ijaz S: In vitro antimutagenic, antioxidant activities and total
phenolics of clove (Syzygiumaromaticum L.) seed extracts. Pak J
Pharm Sci. 27:893–899. 2014.PubMed/NCBI
|
|
18
|
Liu H, Schmitz JC, Wei J, Cao S, Beumer
JH, Strychor S, Cheng L, Liu M, Wang C, Wu N, et al: Clove extract
inhibits tumor growth and promotes cell cycle arrest and apoptosis.
Oncol Res. 21:247–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye CL, Liu Y and Wei DZ: Antioxidant and
anticancer activity of
3′-formyl-4′,6′-dihydroxy-2′-methoxy-5′-methylchalcone and
(2S)-8-formyl-5-hydroxy-7-methoxy-6-methylflavanone. J Pharm
Pharmacol. 59:553–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aisha AF, Ismail Z, Abu-Salah KM, Siddiqui
JM, Ghafar G and Majid Abdul AM: Syzygium campanulatum korth
methanolic extract inhibits angiogenesis and tumor growth in nude
mice. BMC Complement Altern Med. 13:1682013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pascoal AC, Ehrenfried CA, Lopez BG, de
Araujo TM, Pascoal VD, Gilioli R, Ruiz AL, Carvalho JE, Stefanello
ME and Salvador MJ: Antiproliferative activity and induction of
apoptosis IN PC-3 cells by the chalcone cardamonin from
Campomanesia adamantium (Myrtaceae) in a bioactivity-guided study.
Molecules. 19:1843–1855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michel MCP, Guimarães AG, Paula CA,
Rezende SA, Sobral MEG and Guimarães DAS: Extracts from the leaves
of Campomanesia velutina inhibits production of LPS/INF-γ induced
inflammatory mediators in J774A.1 cells and exerts
anti-inflammatory and antinociceptive effects in vivo. Rev Bras
Farmacogn. 23:927–936. 2013. View Article : Google Scholar
|
|
23
|
Jeong D, Yang WS, Yang Y, Nam G, Kim JH,
Yoon DH, Noh HJ, Lee S, Kim TW, Sung GH and Cho JY: In vitro and in
vivo anti-inflammatory effect of Rhodomyrtus tomentosa methanol
extract. J Ethnopharmacol. 146:205–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Flores G, Dastmalchi K, Wu SB, Whalen K,
Dabo AJ, Reynertson KA, Foronjy RFD, Armiento JM and Kennelly EJ:
Phenolic-rich extract from the Costa Rican guava
(Psidiumfriedrichsthalianum) pulp with antioxidant and
anti-inflammatory activity. Potential for COPD therapy. Food Chem.
141:889–895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Serafino A, Vallebona Sinibaldi P,
Andreola F, Zonfrillo M, Mercuri L, Federici M, Rasi G, Garaci E
and Pierimarchi P: Stimulatory effect of Eucalyptus essential oil
on innate cell-mediated immune response. BMC Immunol. 9:2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santos FA, Rao VSN and Silveira ER:
Anti-inflammatory and analgesic activities of the essential oil of
Psidium guianense. Fitoterapia. 68:65–68. 1997.
|
|
27
|
Sharma R, Kishore N, Hussein A and Lall N:
Antibacterial and anti-inflammatory effects of Syzygiumjambos L.
(Alston) and isolated compounds on acne vulgaris. BMC Complement
Altern Med. 13:2922013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gourdeau H, Leblond L, Hamelin B,
Desputeau C, Dong K, Kianicka I, Custeau D, Boudreau C, Geerts L,
Cai SX, et al: Antivascular and antitumor evaluation of
2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a
novel series of anticancer agents. Mol Cancer Ther. 3:1375–1384.
2004.PubMed/NCBI
|
|
29
|
Chetan BS, Nimesh MS, Manish PP and Ranjan
GP: Microwave assisted synthesis of novel 4H-chromene derivatives
bearing phenoxypyrazole and their antimicrobial activity assess. J
Serb ChemSoc. 77:1165–1174. 2012. View Article : Google Scholar
|
|
30
|
Mladenović M, Mihailović M, Bogojević D,
Matić S, Nićiforović N, Mihailović V, Vuković N, Sukdolak S and
Solujić S: In vitro antioxidant of selected
4-Hydroxy-chromene-2-one derivatives-SAR, QSAR and DFT studies. Int
J MolSci. 12:2822–2841. 2011. View Article : Google Scholar
|
|
31
|
Cheng JF, Ishikawa A, Ono Y, Arrhenius T
and Nadzan A: Novel chromene derivatives as TNF-alpha inhibitors.
Bioorg Med Chem Lett. 13:3647–3650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thareja S, Verma A, Kalra A, Gosain S,
Rewatkar PV and Kokil GR: Novel chromeneimidazole derivatives as
antifungal compounds: Synthesis and in vitro evaluation. Acta Pol
Pharm. 67:423–427. 2010.PubMed/NCBI
|
|
33
|
Correa RGC, Silva ML, Maia JGS, Gottlieb
OR, Mourão JC, Marx MC, Morais AA, Moura LL and Magalhães MT: Acta
Amaz. 2:531972.
|
|
34
|
Silva ML, Luz AIR, Zoghbi MGB, Ramos LS
and Maia JGS: Essential oil variation in Calyptranthes spruceana.
Phytochem. 23:2515–2516. 1984. View Article : Google Scholar
|
|
35
|
Maia JGS, Zoghbi MGB and Andrade EHA:
Aromatic Plants in the Amazon and their Essential Oils. Emílio
Goeldi Paraense Museum (Adolpho Ducke Series): Belém.
2001.(Portuguese).
|
|
36
|
Nanthakumar NN, Fusunyan RD, Sanderson I
and Walker WA: Inflammation in the developing human intestine: A
possible pathophysiologic contribution to necrotizing
enterocolitis. Proc Natl Acad Sci USA. 97:6043–6048. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. 2001. View Article : Google Scholar
|
|
38
|
Yang J, Yang Q, Yu S and Zhang X:
Evaluation and validation of suitable reference genes for reverse
transcription-quantitative polymerase chain reaction studies in
cholangiocarcinoma patients and cell lines. Oncol Lett.
11:2673–2681. 2016.PubMed/NCBI
|
|
39
|
Piana C, Wirth M, Gerbes S, Viernstein H,
Gabor F and Toegel S: Validation of reference genes for qPCR
studies on Caco-2 cell differentiation. Eur J Pharm Biopharm.
69:1187–1192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rozen S and Skaletsky H: Primer3 on the
WWW for general users and for biologist programmers. Methods Mol
Biol. 132:365–386. 2000.PubMed/NCBI
|
|
42
|
Herath TD, Darveau RP, Seneviratne CJ,
Wang CY, Wang Y and Jin L: Tetra- and penta-acylated lipid A
structures of Porphyromonasgingivalis LPS differentially activate
TLR4-mediated NF-κB signal transduction cascade and
immuno-inflammatory response in human gingival fibroblasts. PLoS
One. 8:e584962013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferreira LC, Grabe-guimarães A, de Paula
CA, Michel MC, Guimarães RG, Rezende SA, de Souza Filho JD and
Saúde-Guimarães DA: Anti-inflammatory and antinociceptive
activities of Campomanesia adamantium. J Ethnopharmacol. 9:100–108.
2013. View Article : Google Scholar
|
|
44
|
Li LJ, Yu LJ, Li YC, Liu MY and Wu ZZ: In
vitro anti-inflammatory and free radical scavenging activities of
flavans from Ilex centrochinensis. Zhongguo Zhong Yao Za Zhi.
40:1523–1528. 2015.(In Chinese). PubMed/NCBI
|
|
45
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Foo SY and Nolan GP: NF-kappaB to the
rescue: RELs, apoptosis and cellular transformation. Trends Genet.
15:229–235. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clément JF, Meloche S and Servant MJ: The
IKK-related kinases: From innate immunity to oncogenesis. Cell Res.
18:889–899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 23:461–466. 2004.
View Article : Google Scholar
|
|
50
|
Wolska A, Lech-Maranda E and Robak T:
Toll-like receptors and their role in hematologic malignancies.
CurrMol Med. 9:324–335. 2009.
|
|
51
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu MC, Wang YP, Mikhail A, Qiu WR and Tan
TH: Murine p38-delta mitogen activated protein kinase, a
developmentally regulated protein kinase that is activated by
stress and proinflammatory cytokines. J BiolChem. 274:7095–7102.
1999.
|
|
54
|
Enslen H, Raingeaud J and Davis RJ:
Selective activation of p38 mitogen-activated protein (MAP) kinase
isoforms by the MAP kinase kinases MKK3 and MKK6. J BiolChem.
273:1741–1748. 1998.
|
|
55
|
Zarubin T and Han J: Activation and
signaling of the p38 MAP kinase pathway. Cell Res. 15:11–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Porras A and Guerrero C: Role of p38α in
apoptosis: Implication in cancer development and therapy. Atlas
Genet Cytogenet Oncol Haematol. 15:316–326. 2011.
|
|
57
|
Campbell J, Ciesielski CJ, Hunt AE,
Horwood NJ, Beech JT, Hayes LA, Denys A, Feldmann M, Brennan FM and
Foxwell BM: A novel mechanism for TNF-α regulation by p38 MAPK:
Involvement of NF-kappaB with implications for therapy in
rheumatoid arthritis. J Immunol. 173:6928–6937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Héron-Milhavet L and Leroith D:
Insulin-like growth factor I induces MDM2-dependent degradation of
p53 via the p38 MAPK pathway in response to DNA damage. J BiolChem.
3:15600–15606. 2002.
|
|
59
|
Okamoto S, Krainc D, Sherman K and Lipton
SA: Antiapoptotic role of the p38 mitogen-activated protein
kinase-myocyte enhancer factor 2 transcription factor pathway
during neuronal differentiation. Proc Natl AcadSci USA.
20:7561–7566. 2000. View Article : Google Scholar
|
|
60
|
Park JM, Greten FR, Li ZW and Karin M:
Macrophage apoptosis by anthrax lethal factor through p38 MAP
kinase inhibition. Science. 20:2048–2051. 2002. View Article : Google Scholar
|
|
61
|
Zhang X, Shan P, Alam J, Davis RJ, Flavell
RA and Lee PJ: Carbon monoxide modulates Fas/Fas ligand, caspases,
and Bcl-2 family proteins via the p38alpha mitogen-activated
protein kinase pathway during ischemia-reperfusion lung injury. J
BiolChem. 13:22061–22070. 2003.
|
|
62
|
Comes F, Matrone A, Lastella P, Nico B,
Susca FC, Bagnulo R, Ingravallo G, Modica S, Lo Sasso G, Moschetta
A, et al: A novel cell type-specific role of p38alpha in the
control of autophagy and cell death in colorectal cancer cells.
Cell Death Differ. 14:693–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schewe DM and Aguirre-Ghiso JA:
ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor
cells in vivo. Proc Natl Acad Sci USA. 29:10519–10524. 2008.
View Article : Google Scholar
|
|
64
|
Geran RI, Greenberg NH, McDonald MM,
Schumacher AM and Abott BJ: Protocols for screening chemical agents
and natural products against animal tumour and other biological
systems. Cancer Chemother Rep. 3:17–19. 1972.
|
|
65
|
Suffness M and Pezzuto JM: Assays related
to cancer drug discovery. In: Hostettmann K (ed), Methods in Plant
Biochemistry. Assays for Bioactivity. 6:71–133. 1990.
|
|
66
|
Mensor LL, Menezes FS, Leitão GG, Reis AS,
Santos TC, Coube CS and Leitão SG: Screening of Brazilian plant
extracts for antioxidant activity by the use of DPPH free radical
method. Phytother Res. 15:127–130. 2001. View Article : Google Scholar : PubMed/NCBI
|