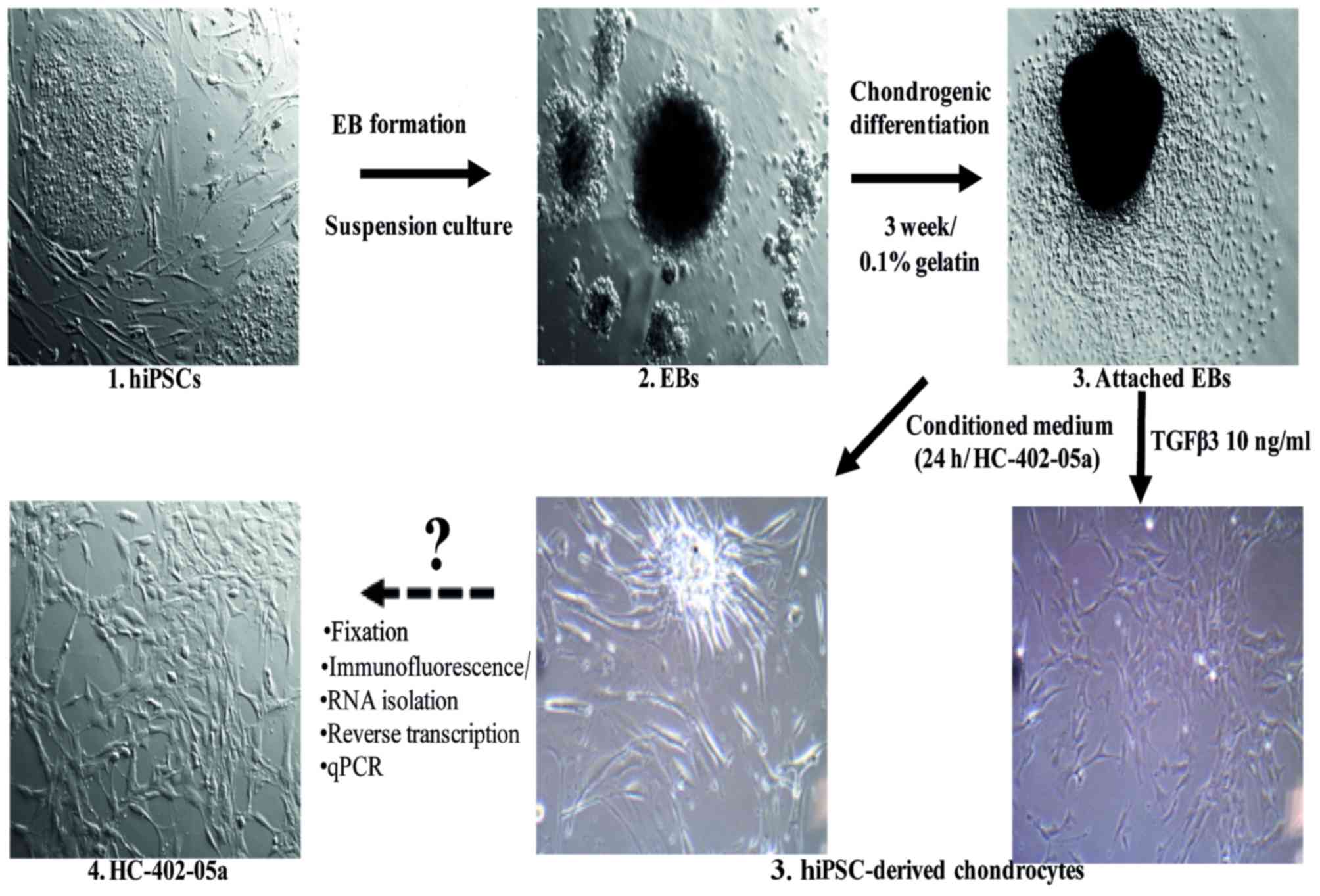
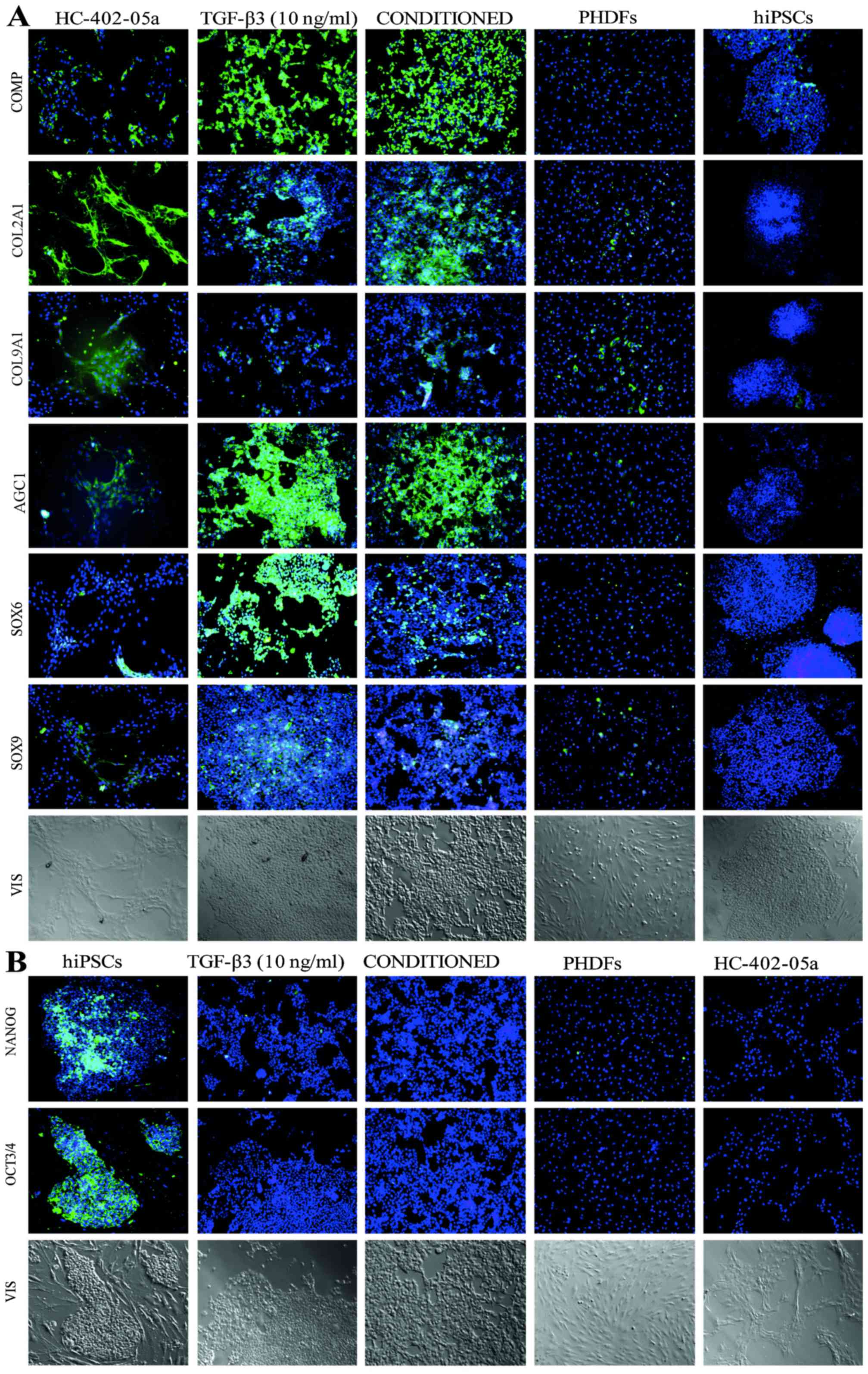
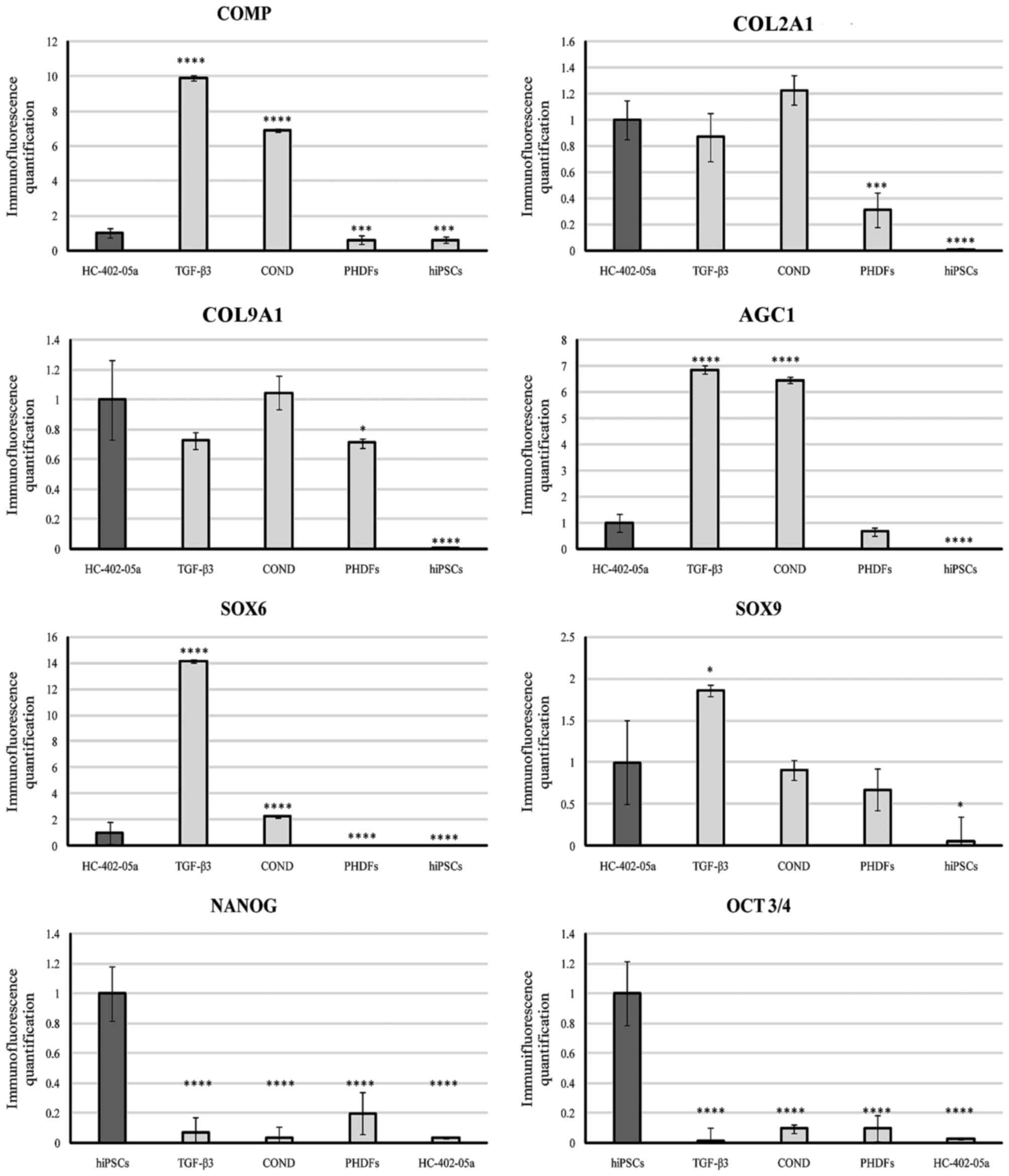
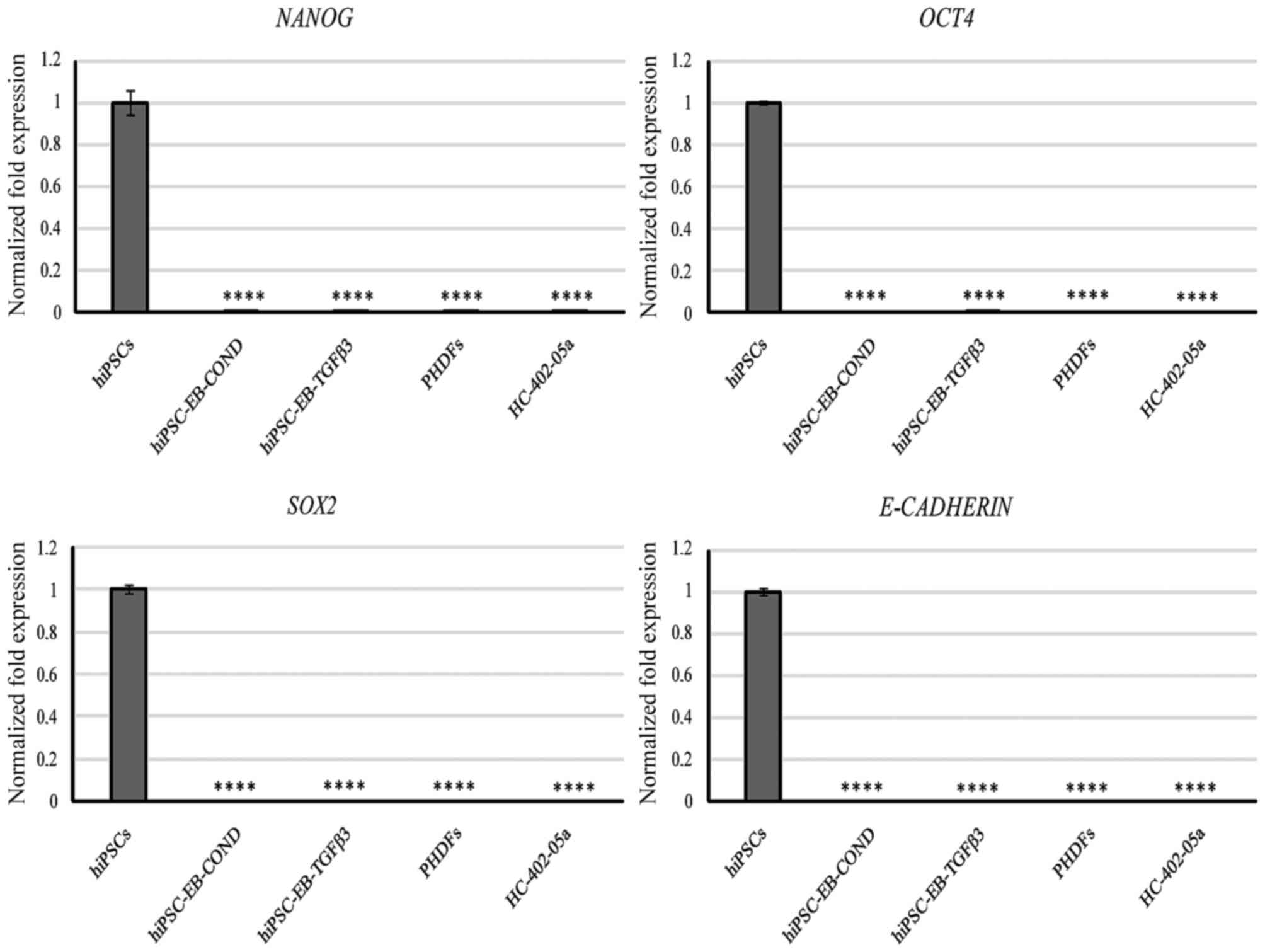
|
1
|
Liu T, Li Q, Wang S, Chen C and Zheng J:
Transplantation of ovarian granulosa-like cells derived from human
induced pluripotent stem cells for the treatment of murine
premature ovarian failure. Mol Med Rep. 13:5053–5058.
2016.PubMed/NCBI
|
|
2
|
Cao B, Li Z, Peng R and Ding J: Effects of
cell-cell contact and oxygen tension on chondrogenic
differentiation of stem cells. Biomaterials. 64:21–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lach M, Trzeciak T, Richter M, Pawlicz J
and Suchorska WM: Directeddifferentiation of induced pluripotent
stem cells into chondrogenic lineages for articular cartilage
treatment. J Tissue Eng. 5:20417314145527012014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren Y, Deng CL, Wan WD, Zheng JH, Mao GY
and Yang SL: Suppresive effects of induced pluripotent stem
cell-conditioned medium in in vitro hypertrophic scarring
fibroblast activation. Mol Med Rep. 11:2471–2476. 2015.PubMed/NCBI
|
|
5
|
Chen FH and Tuan RS: Mesenchymal stem
cells in arthritic diseases. Arthritic Res Ther. 10:2232008.
View Article : Google Scholar
|
|
6
|
Kulcenty K, Wróblewska J, Mazurek S,
Liszewska E and Jaworski J: Molecular mechanisms of induced
pluripotency. Contemp Oncol (Pozn). 19:A22–A29. 2015.PubMed/NCBI
|
|
7
|
Suchorska WM, Augustyniak E and Łukjanow
M: Genetic stability of pluripotent stem cells during anti-cancer
therapies. Exp Ther Med. 11:695–702. 2016.PubMed/NCBI
|
|
8
|
Lee J, Taylor SE, Smeriglio P, Lai J,
Maloney WJ, Yang F and Bhutani N: Early induction of a
prechondrogenic population allows efficient generation of stable
chondrocytes from human induced pluripotent stem cells. FASEB J.
29:3399–3410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye J, Hong J and Ye F: Reprogramming rat
embryonic fibroblasts into induced pluripotent stem cells using
transposon vectors and their chondrogenic differentiation in
vitro. Mol Med Rep. 11:989–994. 2015.PubMed/NCBI
|
|
10
|
Fu C, Yan Z, Xu H, Zhang C, Zhang Q, Wei
A, Yang X and Wang Y: Isolation, identification and differentiation
of human embryonic cartilage stem cells. Cell Biot Int. 39:777–787.
2015. View Article : Google Scholar
|
|
11
|
Oldershaw RA, Baxter MA, Lowe ET, Bates N,
Grady LM, Soncin F, Brison DR, Hardingham TE and Kimber SJ:
Directed differentiation of human embryonic stem cells toward
chondrocytes. Nat Biotechnol. 28:1187–1194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toh WS and Cao T: Derivation of
chondrogenic cells from human embryonic stem cells for cartilage
tissue engineering. Methods Mol Biol. Jul 12–2014.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mardani M, Hashemibeni B, Ansar MM,
ZarkeshEsfahani SH, Kazemi M, Goharian V, Esmaeili N and Esfandiary
E: Comparison between chondrogenic markers of differentiated
chondrocytes from adipose deived stem cells and articular
chondrocytes in vitro. Iran J Basic Med Sci. 16:763–773.
2013.PubMed/NCBI
|
|
14
|
Lee HJ, Choi BH, Min BH and Park SR:
Changes in Surface markers of human mesenchymal stem cells during
the chondrogenic differentiation and dedifferentiation processes in
vitro. Arthritis Rheum. 60:2325–2332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Augustyniak E, Trzeciak T, Richter M,
Kaczmarczyk J and Suchorska W: The role of growth factors in stem
cell-directed chondrogenesis: A real hope for damaged cartilage
regeneration. Int Orthop. 39:995–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Augustyniak E, Suchorska WM, Trzeciak T
and Richter M: Gene expression profile in human induced pluripotent
stem cells: Chondrogenic differentiation in vitro, part B.
Mol Med Rep. 15:2402–2414. 2017.
|
|
17
|
Wróblewska J: A new method to generate
human induced pluripotent stem cells (iPS) and the role of the
protein KAP1 in epigenetic regulation of self-renewal. PhD
dissertation. Poznan University of Medical Sciences. http://www.wbc.poznan.pl/Content/373798/index.pdf2015.
|
|
18
|
Suchorska WM, Lach MS, Richter M,
Kaczmarczyk J and Trzeciak T: Bioimaging: An useful tool to monitor
differentiation of human embryonic stem cells into chondrocytes.
Ann Biomed Eng. 44:1845–1859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nejadnik H, Diecke S, Lenkov OD, Chapelin
F, Doing J, Tong X, Derugin N, Chan RC, Gaur A, Yang F, et al:
Improved approach for chondrogenic differentiation of human induced
pluripotent stem cells. Stem Cell Rev. 11:242–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta (CT)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodrigo I, Hill RE, Balling R, Münsterberg
A and Imai K: Pax1 and Pax9 activate Bapx1 to induce chondrogenic
differentiation in the sclerotome. Development. 130:473–482. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blake JA and Ziman MR: Pax genes:
Regulators of lineage specification and progenitor cell
maintenance. Development. 141:737–751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh P and Schwarzbauer JE: Fibronectin
and stem cel differentiation-lessons from chondrogenesis. J Cell
Sci. 125:3703–3712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gigout A, Jolicoeur M, Nelea M, Raynal N,
Farndale R and Buschmann MD: Chondrocyte aggregation in suspension
culture is GFOGER-GPP- and beta1 integrin-dependent. J Biol Chem.
283:31522–31530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang J and Hall BK: Differential
expression of neural cell adhesion molecule (NCAM) during
osteogenesis and secondary chondrogenesis in the embryonic chick.
Int J Dev Biol. 39:519–528. 1995.PubMed/NCBI
|
|
26
|
Nakatani K, Tanaka H, Ikeda K, Sakabe M,
Kadoya H, Seki S, Kaneda K and Nakajima Y: Expression of NCAM in
activated portal fibroblasts during regeneration of the rat liver
after partial hepatectomy. Arch Histol Cytol. 69:61–72. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Francavilla C, Loeffler S, Piccini D, Kren
A, Christofori G and Cavallaro U: Neural cel adhesion molecule
regulates the cellular response to fibroblast growth factor. J Cell
Sci. 120:4388–4394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rainbow RS, Kwon H and Zeng L: The role of
Nkx3. 2 in chondrogenesis. Front Biol (Beijing). 9:376–381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawato Y, Hirao M, Ebina K, Shi K,
Hashimoto J, Honjo Y, Yoshikawa H and Myoui A: Nkx3.2 promotes
primary chondrogenic differentiation by upregulating
Col2a1transcription. PLoS One. 7:e347032012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi SW, Jeong DU, Kim JA, Lee B, Joeng
KS, Long F and Kim DW: Indian Hedgehog signaling triggers Nkx3.2
protein degradation during chondrocyte maturation. Biochem J.
443:789–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu CF and Lefebvre V: The transcription
factors SOX9 and SOX5/SOX6 cooperate genome-wide through
super-enhancer to drive chondrogenesis. Nucleic Acids Res.
43:8183–8203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han Y and Lefebvre V: L-Sox5 and Sox6
drive expression of the aggrecan gene in cartilage by securing
binding of Sox9 to a far-upstream enhancer. Mol Cell Biol.
28:4999–5013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamizu K, Schlessinger D and Ko MS: SOX9
accelerates ESC differentiation to three germ layer lineages by
repressing SOX2 expression through P21 (WAF1/CIP1). Development.
141:4254–4266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akiyama H, Lyons JP, Mori-Akiyama Y, Yang
X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR,
et al: Interactions between Sox9 and beta-catenin control
chondrocyte differentiation. Genes Dev. 18:1072–1087. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liao J, Hu N, Zhou N, Lin L, Zhao C, Yi S,
Fan T, Bao W, Liang X, Chen H, et al: Sox9 potentiates BMP-2
induced chondrogenic differentiation and inhibits BMP-induced
osteogenic differentiation. PLoS One. 9:e890252014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamda T, Sakai T, Hiraiwa H, Nakashima M,
Ono Y, Mitsuyama H and Ishiguro N: Surface markers and gene
expression to characterize the differentiation of monolayer
expanded human articular chondrocytes. Nagoya J Med Sci.
75:101–111. 2013.PubMed/NCBI
|
|
37
|
Sivan SS, Wachtel E and Roughley P:
Structure, function, aging and turnover of aggrecan in the
invertebral disc. Biochim Biophys Acta. 1840:3181–3189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kiani C, Chen L, Wu YJ, Yee AJ and Yang
BB: Structure and function of aggrecan. Cell Res. 12:19–32. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meng Q, Long X, Deng M, Cai H and Li J:
The expressions of IGF-1, BMP-2 and TGF-β1 in cartilage of condylar
hyperplasia. J Oral Rehabil. 38:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agriogiannis GD, Sifakis S, Patsouris ES
and Konstantinidou AE: Insulin-like growth factors in embryonic
fetal growth and skeletal development (Review). Mol Med Rep.
10:579–584. 2014.PubMed/NCBI
|
|
41
|
Fujita T, Azuma Y, Fukuyama R, Hattori Y,
Yoshida C, Koida M, Ogita K and Komori T: Runx2 induces osteoblast
and chondrocyte differentiation and enhances their migration by
coupling with P13K-Akt signaling. J Cell Biol. 166:85–95. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guntur AR and Rosen CJ: IGF-1 regulation
of key signaling pathway in bone. Bonekey Rep. 2:4372013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang YH, Chin CC, Ho HN, Chou CK, Shen
CN, Kuo HC, Wu TJ, Wu YC, Hung YC, Chang CC and Ling TY:
Pluripotency of mouse spermatogonial stem cells maintained by
IGF-dependent pathway. FASEB J. 23:2076–2087. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nicoll SB, Barak O, Csóka AB, Bhatnagar RS
and Stern R: Hyaluronidases and CD44 undergo differential
modulating during chondrogenesis. Biochem Biophys Res Commun.
292:819–825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Takahashi N, Knudson CB, Thankamony S,
Ariyoshi W, Mellor L, Im HJ and Knudson W: Induction of CD44
cleavage in articular chondrocytes. Arthritis Rheum. 62:1338–1348.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Quintanilla RH Jr, Asprer JC, Vaz C,
Tanavde V and Lakshmipathy U: CD44 is a negative cell surface
marker for pluripotent stem cell identification during human
fibroblast reprogramming. PLoS One. 9:e854192014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Acharya PS, Majumdar S, Jacob M, Hayden J,
Mrass P, Weninger W, Assoian RK and Puré E: Fibroblast migration is
mediated by CD44-dependent TGF beta activation. J Cell Sci.
121:1393–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Halem-Smith H, Calderon R, Song Y, Tuan RS
and Chen FH: Cartilage oligomerix matrix protein enhances matrix
assembly during chondrogenesis of human mesenchymal stem cells. J
Cell Biochem. 113:1245–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tseng S, Reddi AH and Di Cesare PE:
Cartilage oligomeric matrix protein (COMP): A biomeraker of
arthritis. Biomark Insights. 4:33–44. 2009.PubMed/NCBI
|
|
50
|
Ghert MA, Qi WN, Erickson HP, Block JA and
Scully SP: Tenascin-C expression and distribution in cultured human
chondrocytes and chondrosarcoma cells. J Orthop Res. 20:834–841.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Murphy LI, Fischer D, Chiquet-Ehrismann R
and Mackie EJ: Tenascin-C induced stimulation of chondrogenesis is
dependent on the presence of the C-terminal fibrinogen-like
globular domain. FEBS Lett. 480:189–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Trebaul A, Chan EK and Midwood KS:
Regulation of fibroblast migration by tenascin-C. BiochemSoc Trans.
35:695–697. 2007. View Article : Google Scholar
|
|
53
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Involvement of the Wnt
signaling pathway in feeder-free culture of human induced
pluripotent stem cells. Mol Med Rep. 12:6797–6800. 2015.PubMed/NCBI
|
|
54
|
Qiu D, Ye S, Ruiz B, Zhou X, Liu D, Zhang
Q and Ying QL: Klf2 and Tfcp2l1, Two Wnt/β-catenin targets, act
synergistically to induce and maintain naive pluripotency. Stem
Cell Reports. 5:314–322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Marucci L, Pedone E, Di Vicino U,
Sanuy-Escribano B, Isalan M and Cosma MP: β-catenin fluctuates in
mouse ESCs and is essential for Nanog-mediated reprogramming of
somatic cells to pluripotency. Cell Rep. 8:1686–1696. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Davidson KC, Adams AM, Goodson JM,
McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ and Moon
RT: Wnt/β-catenin signaling promotes differentiation, not
self-renewal, of human embryonic stem cells and is repressed by
Oct4. Proc Natl Acad Sci USA. 109:4485–4490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Modarresi R, Lafond T, Roman-Blas JA,
Danielson KG, Tuan RS and Seghatoleslami MR: N-cadherin mediated
distribution of beta-catenin alters MAP kinase and BMP-2 signaling
on chondrogenesis-related gene expression. J Cell Biochem.
95:53–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang Y, Zhang X, Du K, Yang F, Shi Y,
Huang J, Tang T, Chen D and Dai K: Inhibition of β-catenin
signaling in chondrocytes induces delayed fracture healing in mice.
J Orthop Res. 30:304–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li TF, Chen D, Wu Q, Chen M, Sheu TJ,
Schwarz EM, Drissi H, Zuscik M and O'Keefe RJ: Transforming growth
factor-beta stimulates cyclin D1 expression through activation of
beta-catenin signaling in chondrocytes. J BiolChem.
281:21296–21304. 2006.
|