Introduction
In animals, Selye defined stress to be a
non-specific general reaction upon different types of stimulation
(e.g., cold, surgical injury, hyperthermia) (1). Stress can lead to disease (or even
death) if it is sustained and of high intensity (2).
Heat stress (HS) has been researched widely because
of its negative effects. Research has shown that losses of US$2.4
billion occurred in the US poultry industry because of a lack of
heat-abatement equipment (3). High
temperature has been shown to elicit negative effects on broiler
chickens, including increased consumption of fodder as well as a
reduced growth rate and viability of chickens (4). It can also increase the abdominal fat
of broiler chickens, thereby reducing the quality of products
derived from them (5). Gathiram
et al discovered that HS can cause endotoxemia and lead to
the death of monkeys, suggesting that the damage causes by HS in
mammals may also have serious consequences (6). Oxidative damage to proteins and lipid
peroxidation induced by HS has also been reported (7).
Cardiac failure and stroke can be induced by varies
types of stress, including hyperthermia (6,8).
Sudden cardiac death can occur in broiler chickens because of
hyperthermia (9). Pathologic
examination of chicken hearts has shown vacuoles and karyopyknosis
after HS (10). Hyperthermia can
induce tissue damage (especially heart failure) in rats (11). Studies in dogs have shown that HS
can induce collapse of cardiovascular function and death (12). Therefore, the heart might be
damaged more easily than other organs in humans or livestock.
The heat shock protein (Hsp) family is a group of
proteins that are highly conserved. Hsps can be found in virtually
all organisms. They act as ‘cellular chaperones’ in animals under
normal physiologic or pathologic conditions. Stresses such as
hyperthermia, oxidative damage, or physical/chemical injury can
upregulate Hsp expression dramatically and rapidly (13). According to their molecular weight,
the Hsp family can be divided into small Hsps, Hsp40, Hsp60, Hsp70,
Hsp90, and Hsp110 (14). They can
combine with misfolded/unfolded proteins to help them fold in the
correct way. Also, denatured proteins can be removed by Hsps to
maintain the normal physiologic function of cells (15,16).
Hsp40 could sense oxidative stress to act as the first line of
defence against oxidative which was similar with Hsp27 (17,18).
Hsp60 was found to inhibit bax in mitochondrial and reduce the cell
apoptosis (19). Zhang's research
indicated Hsp90 induced by aspirin could alleviate the chicken
primary myocardial cells damage and apoptosis during HS (20).
Hsp70 is one of the most important members of the
HSP family, As well as being a molecular chaperone, it can
facilitate DNA repair in human bronchial epithelial cells if
exposed to toxins such as benzo-a-pyrene (21). Studies have shown that Hsp70
expression in healthy myocardial cells is quite low, but is
increased significantly after HS (22), suggesting that Hsp70 might be
induced readily by HS and has physiologic roles in cardiac cells
during HS. Hsp70 is effective for the survival of cells and tissues
by combining with hydrophobic regions in unfolded proteins in an
adenosine triphosphate (ATP)-dependent manner to stabilize the
unfolded state and then help proteins recover into a folded state.
Li et al discovered that Hsp70 overexpression by heat-shock
pretreatment can reduce the liver injury induced by carbon
tetrachloride and accelerate liver repair in rats, and that these
phenomena may be related to the anti-oxidative properties of Hsp70
(23). Research has also shown
that inhibition of Hsp70 expression by micro-injection of
monoclonal antibodies against Hsp70 can weaken the survival and
tolerance of fibroblasts during HS in vitro (24). Hutter et al demonstrated
that the infarct size caused by acute occlusion of the left main
coronary artery followed by reperfusion is decreased because of
Hsp72 overexpression in transgenic mice (25). Other studies have suggested that
Hsp70 has anti-apoptotic effects in several cell types. Wang et
al discovered that induction of Hsp70 expression by
geranylgeranylacetone suppresses HS-induced apoptosis of mouse
cardiomyocytes in vivo (26). Increased expression of Hsp70 by
mild HS can also increase resistance to subsequent severe HS in
neuronal cells (27). Those
results suggest that Hsp70 overexpression can alleviate apoptosis
during HS. Yurinskaya et al showed that Hsp70 can decrease
the percentage apoptosis induced by isoAsp7-Aβ (1–42)
(isoAsp7-Aβ (1–42) is the amyloid-β isoform with
isomerized aspartic acid residue at position 7) in human
neuroblastoma cells, and that this phenomenon is related to the
protein kinases JNK, ERK and PI3K (28). The cell-free system established by
Beere et al showed that Hsp70 can hinder the recruitment of
pro-caspase-9 to the apoptotic peptidase activating factor-1
(Apaf-1) apoptosome to execute its anti-apoptotic effects (29). Hsp70 can combine with the
death-associated protein kinase Apaf-1 to inhibit downstream
activation of Apaf-1 and caspase-9 to achieve its anti-apoptotic
function during stress (30,31).
Therefore, Hsp70 could be an indispensable protein in myocardial
cells in vivo and in vitro during HS.
Previously, we have shown that heart damage in
chickens is quite obvious after HS. Research teams of Wu noted that
levels of enzymes related to heart damage (e.g., creatine kinase)
(CK), CK-MB, lactate dehydrogenase (LDH) are elevated significantly
after HS in broiler chickens, suggesting that HS can induce damage
to myocardial cells (10). Hsp70
may have a protective role in rat myocardial cells in vivo
and in vitro because groups with low expression of Hsp70
have shown more severe damage than groups with high expression of
Hsp70 (32). The remarkable role
of Hsp70 has also been investigated in other types of stress, such
as transport stress. Hsp70 expression in the pig stomach has been
shown to be associated with protective functions (33). The cytoprotective role of Hsp70 has
been researched widely, but little is known about its effects in
the primary myocardial cells of chickens in vitro.
In the present study, we established a cellular
model of low expression of Hsp70 and also investigated Hsp70
expression in the primary myocardial cells of chickens using
quercetin. [Quercetin is a flavonoid known to inhibit Hsp70
expression and has been used against several types of cancer, such
as adenocarcinoma and leukemia, to target Hsp70 (34–36)]. The protective role of Hsp70 was
measured by detection of apoptosis and its related cascades,
pathologic damage, and other parameters in normal chicken primary
myocardial cells (CPMC) and a cellular model of low expression of
Hsp70 during HS in vitro.
Materials and methods
Culture of CPMC
All experiments were undertaken in accordance with
the guidelines of the Animal Ethics Committee of Jiangsu Province
(China). The study protocol was approved by the Animal Care and Use
Committee of Nanjing Agricultural University (Nanjing, China).
Specific pathogen-free embryonated embroys (12 days of age; Qian
Yuan Hao Biotechnology Company, Nanjing, China) were harvested.
Hearts were removed under sterile conditions and cut into pieces
before washing four times in phosphate-buffered saline (PBS).
Collagenase type I (1 mg/ml; Life Technologies, Carlsbad, CA, USA)
was added to digest heart fibers at 4°C for 14–16 h. The reaction
was terminated by addition of Dulbecco's modified Eagle's medium
(DMEM; Life Technologies) with 20% fetal bovine serum (FBS; Life
Technologies) before centrifugation at 1,000 × g for 10 min at 4°C.
Primmorphs were resuspended by DMEM containing 20% FBS, 100
units/ml penicillin and 100 units/ml streptomycin before culture in
cell culture plates in a humidified atmosphere of 5% CO2
and 95% air at 37°C. Cells were transferred to new cell culture
plates after 1 h and 0.1 mM 5-Bromo-2-deoxyuridine solution
(Sigma-Aldrich, St. Louis, MO, USA) was added to the culture
solution to inhibit the growth of fibroblast. Cells were cultivated
for 48 h.
Cell viability assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide salt
(MTT) assay was employed to determine cell viability when different
concentrations of quercetin (dissolved in dimethylsulfoxide (DMSO;
Sigma-Aldrich)) were added. Final concentration of DMSO in the
culture medium was 0.1%. CPMC were incubated in 96-well plates at
1×104 per well for 48 h. Then, quercetin (150, 200, 250,
300 µM) was added for 24 h. As a control solution, 0.1% DMSO was
added in triplicate. Then, 10 µl MTT (0.5 mg/l) was added to each
well, and the plate transferred to a system at 37°C to allow
formation of blue formazan crystals. The remaining MTT was removed
after 2 h and 150 µl DMSO added for 30 min. After shaking for 1 h,
absorbance was measured at 570 nm by a spectrophotometer (Infinite
200 PRO; Tecan, Geneva, Switzerland).
HS model in CPMC
Cells (3–4×106) were plated in cell
culture dishes (diameter, 60 mm). Then, cell plates were divided
randomly into the inhibitor group [HS+Quercetin; in which 200 µM
quercetin (Sigma-Aldrich) dissolved in DMSO was added to the
culture solution 1 h before HS. Final concentration of DMSO was
0.1%] and HS group (which was treated with the same amount of DMSO
as inhibitor group and a humidified atmosphere of 5% CO2
and 95% air at 42°C), quercetin group which was treated with
quercetin without HS. Duration of HS was 0, 1, 2, 3 and 5 h
(n=3).
Semi-quantitative measurement of
transcription of Hsp70 mRNA
Treated CPMC were collected by TRIzol Reagent (Life
Technologies) for measurement of total RNA according to
manufacturer instructions. Then, RNA samples were
reverse-transcribed into cDNA by a Transcript M-MLV kit (Life
Technologies) according to manufacturer instructions. All cDNA
samples were stored at −20°C. Sequences of Hsp70 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were supplied by
the gene bank of the National Center for Biotechnology Information.
Primers of target genes were designed by Primer Premier v5.0.
Primers of Hsp70 (forward and reverse) were AGC GTA ACA CCA CCA TTC
C and TGG CTC CCA CCC TAT CTC, and those for GAP DH were TGA AAG
TCG GAG TCA ACG GAT and ACGCTCCTGGAAGATAGTGAT, respectively.
Expected size of Hsp70 and GAPDH polymerase chain reaction (PCR)
product was 372 and 230 bp, respectively. A thermocycler (AB7300;
Life Technologies) was employed for quantitative PCR. Levels of
Hsp70 mRNA were normalized using the following formula:
RelativequantityofHsp70mRNA=2–ΔΔCq–ΔΔCq={[(CqHsp70mRNA–CqGAPDHmRNA)Heatstressgroup]–[(CqHsp70mRNA–CqGAPDHmRNA)Controlgroup]}
Expression of Hsp70 protein
Protein in CPMC of the HS group, HS+Quercetin group
and Quercetin group was collected by RIPA Lysis Buffer containing
1% phenyl methane sulfonyl fluoride according to manufacturer
instructions. The supernatant was collected by a pipette when the
mixture was centrifuged at 12,000 × g for 15 min. Protein
concentration was measured by a bicinchoninic acid (BCA) assay kit
(Life Technologies) according to manufacturer instructions. Each
sample was boiled for 15 min and then stored at −20°C. For sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, each track was
loaded with an equal amount of protein (30 µg). Then, the protein
was transferred onto a polyvinylidene fluoride (PVDF) membrane
(Bio-Rad, Hercules, CA, USA) at 120 V for 90 min. Then, 5% skimmed
milk powder dissolved in PBST was employed to block the PVDF
membrane at 37°C for 2 h. After washing with PBST, the PVDF
membrane was incubated with anti-chicken Hsp70 and GAPDH monoclonal
antibody (Abcam, Cambridge, UK) for 16 h at 4°C. After the first
incubation, the PVDF membrane was washed four times with PBST
before incubation with the corresponding peroxidase-conjugated goat
IgG antibody (Boster, Beijing, China). Luminous fluid was used to
detect the protein on the membrane. Bands on the developed film
were quantified using Quantity One v4.6.2 (Bio-Rad). Relative
amount of Hsp70 was normalized using the following formula:
RelativeamountofHsp70=(GrayvalueofHsp70–Grayvalueofbackground)/GrayvalueofGAPDH–Grayvalueofbackground)
Heart damage-related enzymes
Supernatant (3 ml) of CPMC were collected from each
group after HS. Activities of pathologic damage-associated enzymes
of myocardial cells (CK, CK-MB, LDH) were tested using kits
(Nanjing Jiancheng Biochemical Reagent, Nanjing, China) according
to their instructions.
Detection of CPMC apoptosis during
HS
CPMC were washed twice with pre-cooled FACS Buffer
after HS. Pancreatic enzymes without ethylenediamine tetra-acetic
acid were used to digest CPMC. After resuspension with 200 µl
binding buffer, 10 µl annexin V-fluorescein isothiocyanate and 15
µl propidium iodide were added. Flow cytometry was employed for all
samples within 1 h. FlowJo v7.6.1 was used for data analyses.
Activities of caspase-3, −8 and −9
after HS
CPMC were collected after HS. Protein concentration
in cells was measured by BCA kits. Cells were added to 96-well
plates, and each well contained 100–200 µg protein. Activities of
caspase-3, −8 and −9 (which are associated with apoptosis) were
detected using kits (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) according to their instructions.
Expression of cleaved caspase-3, AIF,
Bax, Bcl-2 and cytochrome c
Apoptosis-associated proteins in CPMC of the HS
group and HS+Quercetin group was detected according to western blot
by anti-chicken AIF, Cleaved caspase-3, Bax, Bcl-2 and cytochrome
c monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA).
Statistical analysis
Differences between experimental groups and the
control group were analyzed by one-way analysis of variance
followed by the least square difference multiple comparison test
using SPSS (IBM, Armonk, NY, USA). Results are the mean ± standard
deviation (SD). P<0.05 was considered significant and P<0.01
was considered highly significant. Unless indicated otherwise,
experiments were done in triplicate.
Results
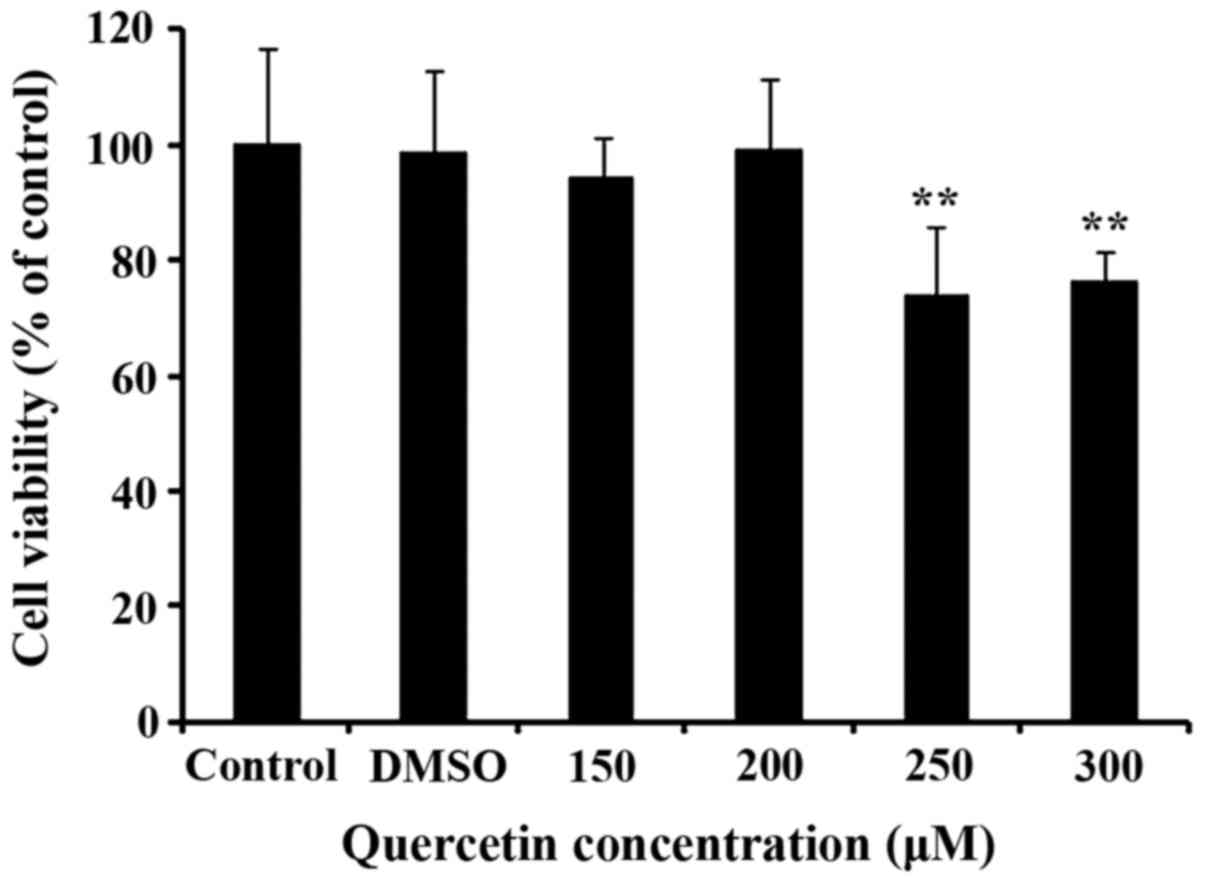
Cell viability after quercetin
treatment
The effect of different concentrations of quercetin
on CPMCs after 24 h is shown in Fig.
1. Cell viability was reduced significantly by ~25% at 250 and
300 µM quercetin, but the other groups were not influenced.
According to these results, a quercetin concentration of 200 µM was
selected for experimentation.
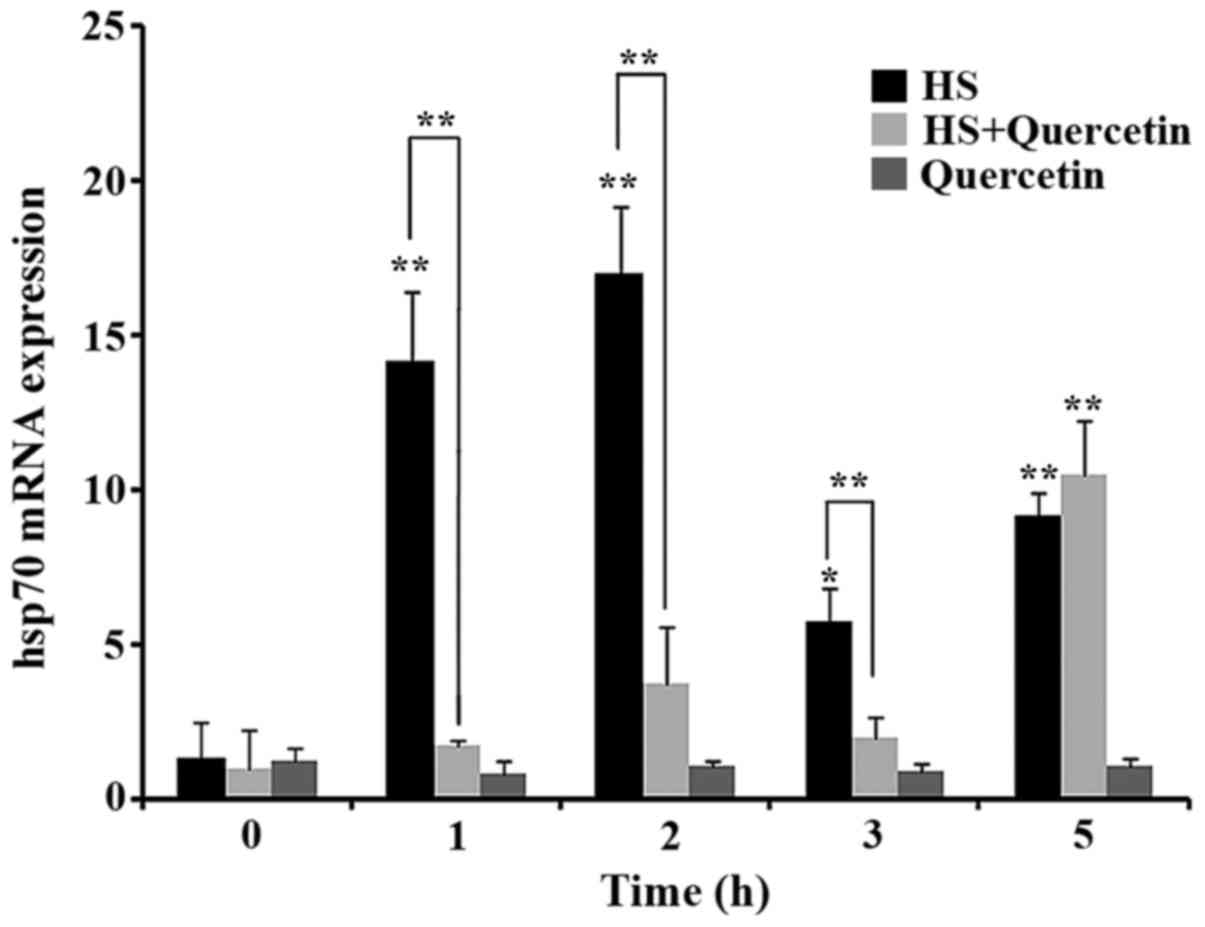
Transcription of Hsp70 mRNA during
HS
The transcription level of the RNA of the
housekeeping gene GAPDH did not change in response to HS. The
transcription level of Hsp70 mRNA increased significantly
(P<0.01) from the beginning of HS (1 h) in the HS group. In
contrast to the HS group, the transcription level of Hsp70 mRNA in
HS+Quercetin group did not change significantly (P>0.05) until 5
h (P<0.01). No obvious change was discovered in Quercetin group
(P>0.05). These results suggested that quercetin inhibited Hsp70
mRNA transcription during HS within 5 h (Fig. 2).
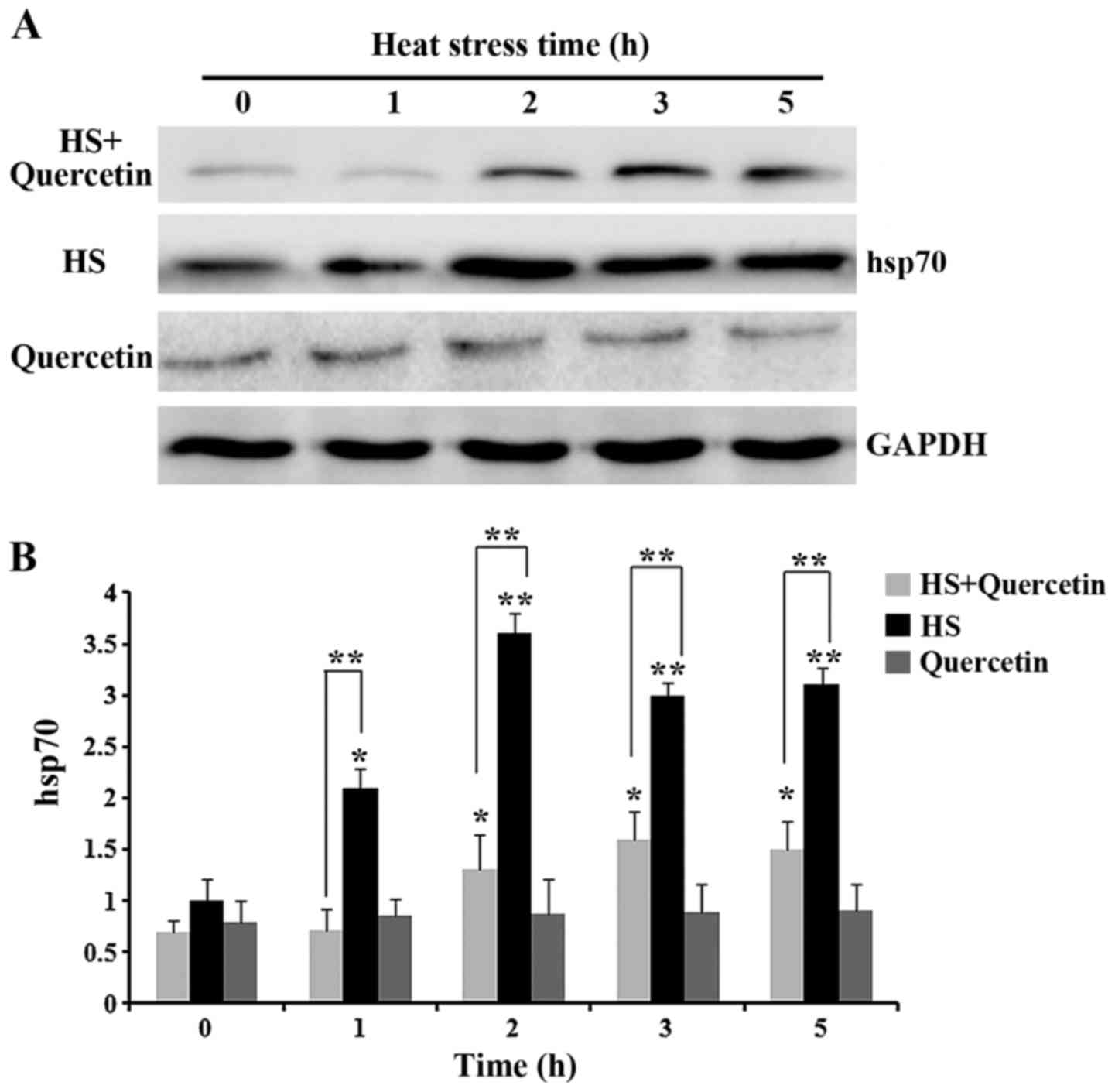
Hsp70 expression of during HS
Expression of Hsp70 in HS group, HS+Quercetin and
Quercetin group was normalized to that of the housekeeping gene
GAPDH (Fig. 3). At 0 h, Hsp70 had
low expression in CPMC, but then increased significantly
(P<0.05) from 1 h of HS and maintained high expression until 5 h
(P<0.01). After quercetin treatment, Hsp70 expression was
reduced highly significantly compared with that in the HS group.
Expression of Hsp70 in Quercetin group was quite low and no change
was discovered (P>0.05). These results suggested that quercetin
caused low expression of Hsp70 in CPMC within 5 h of HS, however,
quercetin failed to decreased the expression of Hsp70 without
HS.
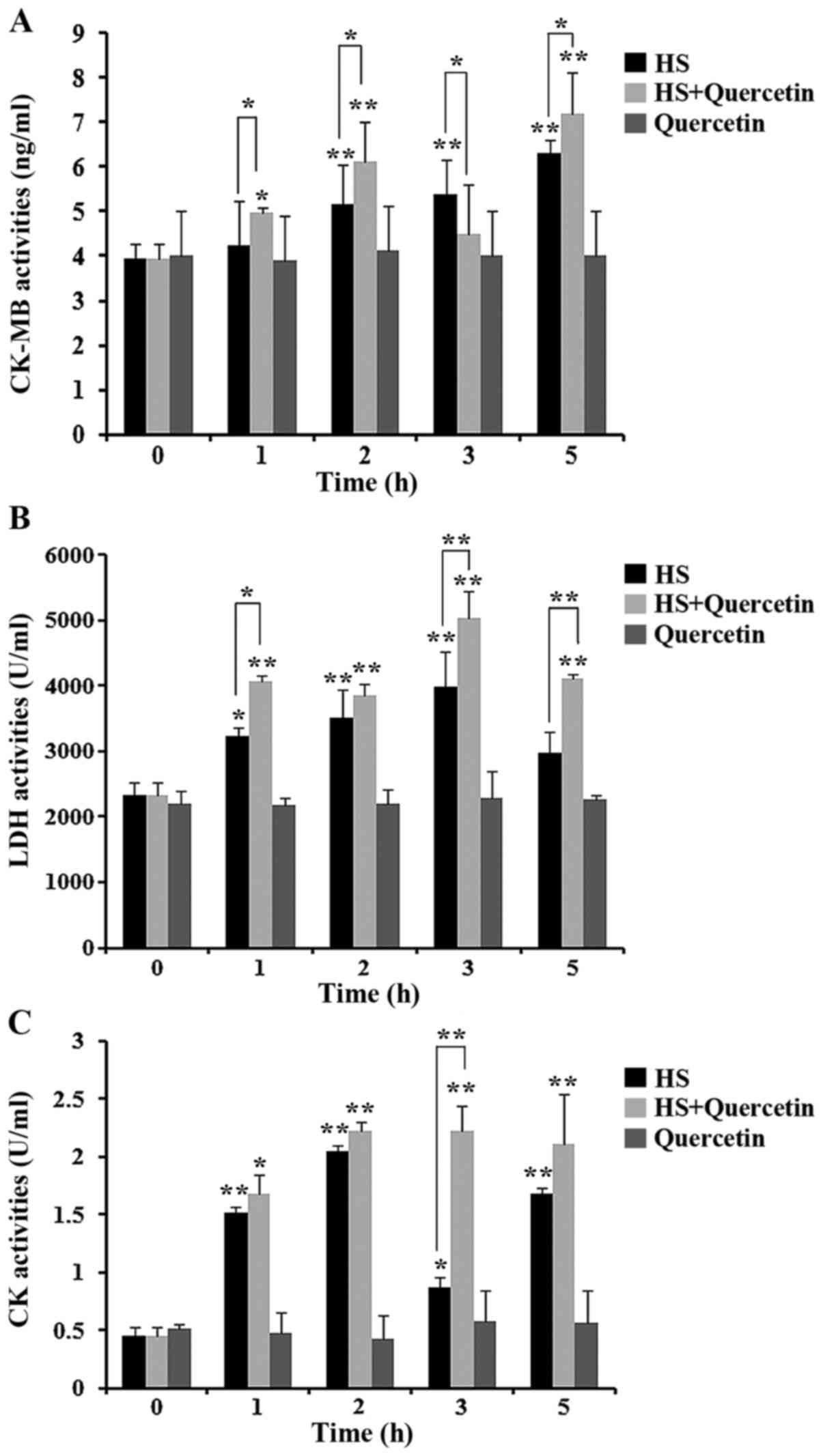
Levels of heart damage-associated
enzymes during HS
Levels of CK-MB, LDH and CK released from
heat-stressed CPMC were detected in the supernatants of all groups
(Fig. 4). After HS, levels of
these three enzymes were significantly higher (P<0.05) in HS
group and HS+Quercetin group. However when Hsp70 was inhibited in
HS+Quercetin group, CK-MB release was significantly greater
(P<0.05) than the HS group 1, 2 and 5 h after HS. Also LDH
levels were significantly higher in HS+Quercetin group at 1
(P<0.05), 3 and 5 h (P<0.01) compared with the HS group. CK
showed a similar trend to the other two enzymes. Between the two
treatment groups, CK release was threefold higher at 3 h than in
the HS group. No cell damage was discovered in Quercetin groups.
Enzyme activities suggested that damage to CPMC in the Hsp70
low-expression group (HS+Quercetin) was more than that in the HS
group.
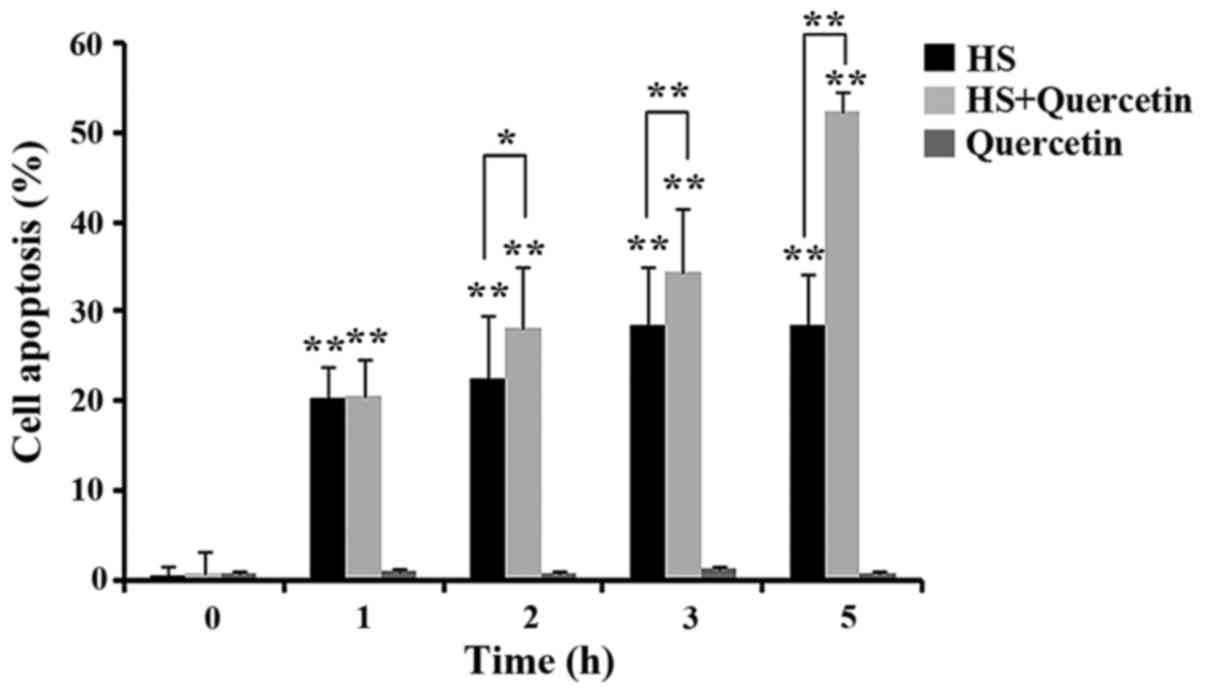
Apoptosis of CPMC during HS
Apoptosis in all experimental groups was shown in
Fig. 5. At 0 h, All of the groups
showed no obvious apoptosis. After 1 h of HS, apoptosis was
observed in HS- and quercetin-treated group, but there was no
significant difference between them. Percentage of apoptotic cells
in the inhibitor group was higher compared with the HS group at 2
(P<0.05), 3 and 5 h (P<0.01), and a considerable number of
non-viable apoptotic cells was observed at 5 h. No apoptosis was
discovered in quercetin groups without HS (P>0.05). These
results suggested that apoptosis became more severe in the group
which Hsp70 expression was inhibited.
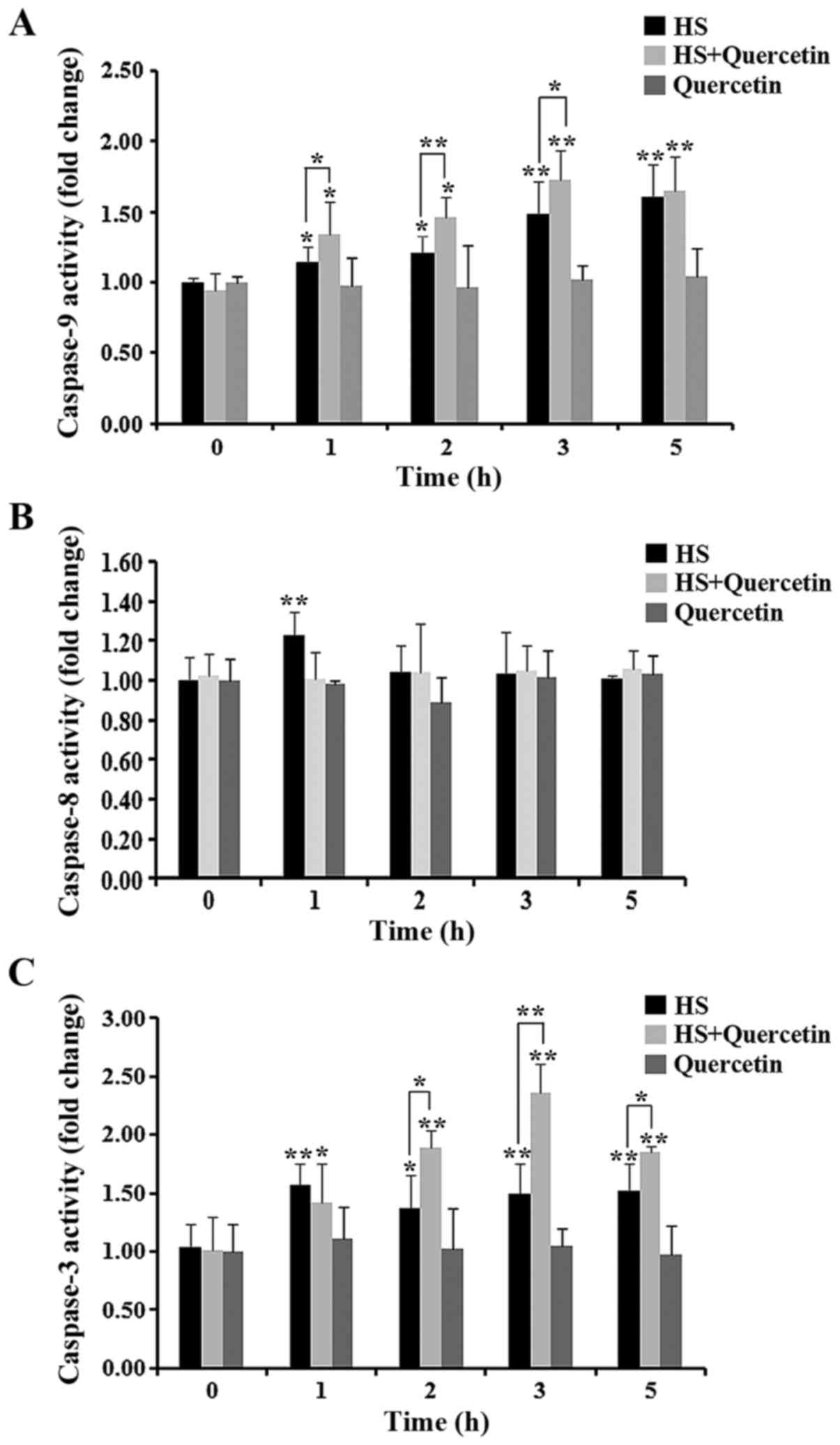
Levels of apoptosis-associated enzymes
during HS
Levels of the apoptosis-related enzymes caspase-9,
−8, and −3 are shown in Fig. 6.
Almost no change in caspase-8 activity was observed during HS in
all experimental groups. Levels of caspase-9 and caspase-3 were
elevated significantly at the start of HS. Quercetin-treated CPMC
showed higher levels of caspase-9 at 1, 2 and 3 h compared with the
HS group. Caspase-3 levels were also significantly (2 and 5 h) or
highly significantly (3 h) elevated in HS+Quercetin groups during
HS. When treated with quercetin alone, all of the enzymes showed no
change during 5 h.
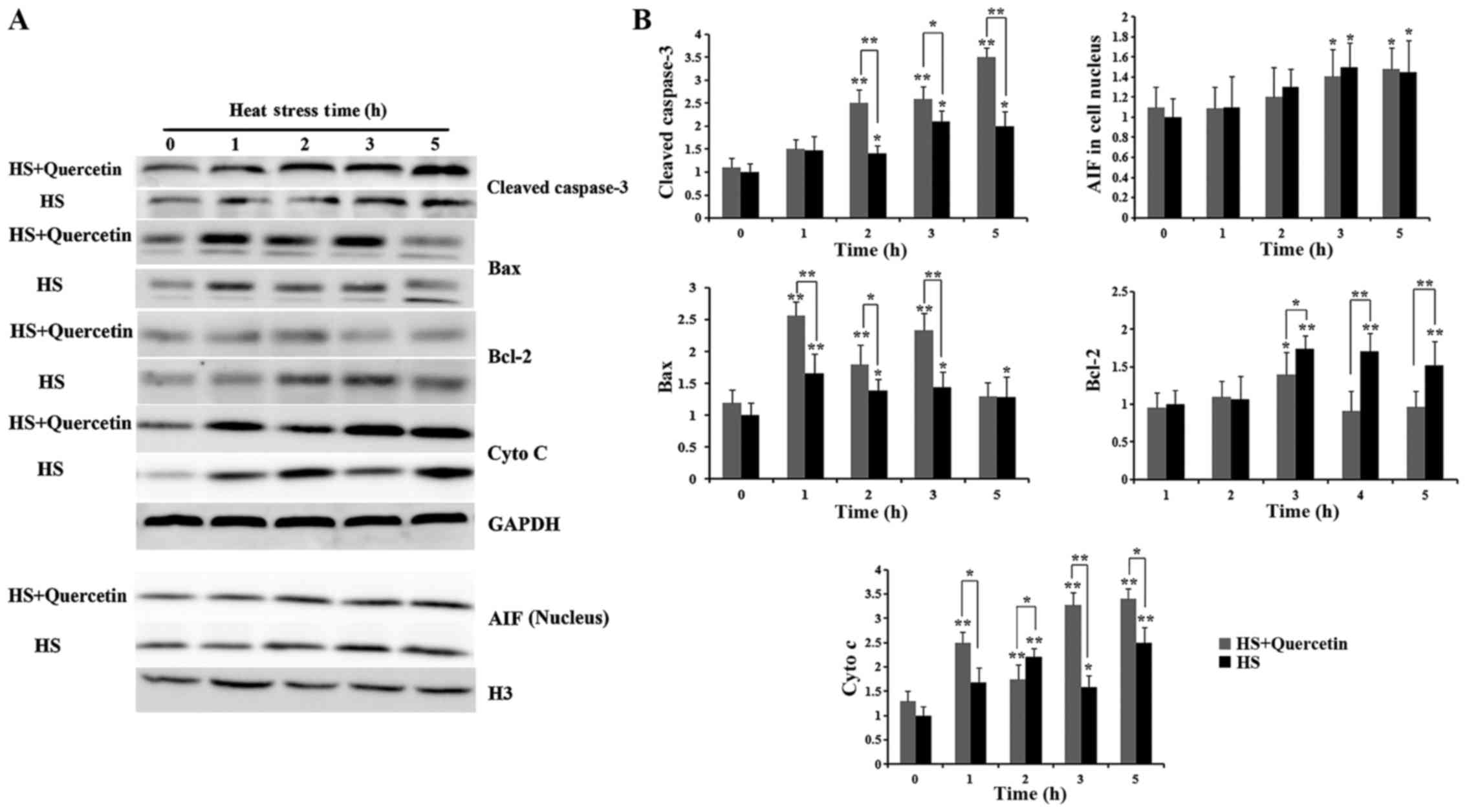
Levels of heart apoptosis-associated
proteins during HS
Levels of cleaved caspase-3, AIF, Bax and cytochrome
c are shown in Fig. 7.
Expression of cleaved caspase-3 was significantly elevated at the
start of HS in both groups, quercetin-treated CPMC was higher than
that in HS groups during HS at 2 (P<0.01), 3 (P<0.05) and 5 h
(P<0.01). As for AIF, though expression of AIF in cell nucleus
was elevated at 3 and 5 h (P<0.05), no obvious difference was
observed between two groups during HS. Expression of Bax in
Quercetin-treated CPMC was significantly elevated compared with HS
group at 1 (P<0.01), 2 (P<0.05) and 3 h (P<0.01).
Expression of Bcl-2 in HS+Quercetin group didn't show obvious
change during HS except 2 h and was lower in quercetin-treated
groups at 2 (P<0.05), 3 and 5 h (P<0.01). Cytochrome c
was upregulated by HS in both groups, however, levels in
HS+Quercetin (Hsp70 low expression) groups were significantly
higher at 1 (P<0.05), 3 (P<0.01) and 5 h (P<0.05) compared
with HS group.
Discussion
If cells suffer HS, intracellular proteins undergo
oxidation, and proteins that can ‘sense’ this change become
misfolded (37). Hsp70 can be
induced readily during severe stress and can act as a ‘sensor’ for
such misfolded proteins.
Studies have shown that Hsp70 is expressed in
several cell types under HS (38–40).
However, the importance of Hsp70 in myocardial cells (especially
those in poultry) under HS is not known. To ascertain if Hsp70 has
a protective role in chicken myocardia, we inhibited Hsp70
expression using quercetin in CPMC. Inhibition of the transcription
and translation of Hsps has been investigated (41,42).
Also, to ascertain if quercetin can affect CPMC viability, we used
the MTT assay. We found that CPMC were unchanged after using
quercetin (200 µM) and our results also showed quercetin was
no-harmful to CPMC without HS. Therefore, all further apoptosis and
heat stressed-damage were not induced by quercetin itself, but by a
shortage of Hsp70. Western blotting showed that Hsp70 expression
was obviously elevated at the start of HS in both groups. However,
Hsp70 expression in the quercetin-treated group was reduced
significantly compared with that in the HS group. PCR data revealed
that expression of Hsp70 mRNA was also reduced in the
quercetin-treated group according. These results suggest that 200
µM quercetin can obviously inhibit transcription of mRNA and
protein expression of Hsp70 during HS. These results also
demonstrate that a model of low expression of Hsp70 was
established.
CK, CK-MB and LDH are present in myocardial cells
and possess different biologic activities. Studies have shown that
these enzymes can be released from the cytoplasm if cells are
damaged. Thus, concentrations of these enzymes in cellular
supernatants can reflect the extent of cell damage (43). CK and CK-MB are associated with
energy transfer, muscle contraction, and ATP regeneration in cells,
and are regarded as specific diagnostic factors for myocardial
damage (44). Previously, we
showed that transport stress can cause pathologic damage to the
myocardial cells of piglets, and that LDH levels are a damage
criterion of myocardial cells (45). Here, we discovered that activities
of CK-MB and LDH in the quercetin-treated group were elevated
significantly, and that CK levels were also higher than those in
the HS group. These results confirmed our hypothesis that damage to
CPMC is associated with inhibition of Hsp70 expression.
Studies have demonstrated that HS can induce
oxidative stress in livestock (46,47).
Hsp70 can sense the redox status of organisms and upregulate the
level of oxidation of non-protein thiols (especially glutathione)
to execute an anti-oxidative effect (48,49).
On the basis of those studies, we hypothesized that inhibition of
Hsp70 expression may be associated with damage to CPMC during
HS.
Apoptotic cells were not observed under the non-HS
state in the HS group and HS+Quercetin group. When both groups were
exposed to HS, 20% apoptosis appeared from 1 h onwards. Apoptosis
increased with increasing duration of HS. In the Hsp70 low
expression group, significantly more apoptotic cells were observed
compared with the HS group at 2–3 h of HS, and >50% apoptosis
was noted in the Hsp70 low expression group at 5 h compared with
28% apoptosis in the HS group. Results of western blot also
indicated Cleaved caspase-3, the performer of cell apoptosis, was
higher in inhibitor group during HS, We hypothesized that reduced
expression of Hsp70 may be a key reason for the increase in
apoptosis of CPMC during HS. Detection of apoptosis-related enzymes
showed that caspase-8 activity was not elevated because of HS in
the HS group or HS+Quercetin group. However, activities of
caspase-3 and caspase-9 were increased during HS, and the
quercetin-treated group had higher levels than those in the HS
group during HS.
Caspase-dependent apoptosis acts via two
pathways. The extrinsic pathway is characterized by activation of
death receptors on cell surfaces and involves caspase-8 activation
(50). The intrinsic
(mitochondrial pathway) involves activation of caspase-9 and
caspase-3 (51,52). AIF was also involved in
mitochondrial pathway, It was released from mitochondria to cell
nucleus under various stressed conditions which can directly lead
to apoptosis via a caspase-independent way, Hsp70 has been reported
that could sequester released AIF from the mitochondria to cell
nucleus (53). In the present
study, the activity of caspase-8 did not show significant changes
in the HS group or HS+Quercetin group, suggesting that inhibition
of Hsp70-induced apoptosis may not be via the death receptor
pathway in CPMC during HS. Activities of caspase-3 and caspase-9
were elevated significantly, which suggested that apoptosis induced
by low expression of Hsp70 might be related to the mitochondrial
pathway. Expression of AIF in two groups performed a similar trend,
but no differences were detected between them, so we inferred that
aggravated cell apoptosis in quercetin-treated groups was
uncorrelated with AIF, Gordon et al discovered that
upregulated expression of Hsp70 can inhibit p53-mediated apoptosis
and reduce expression of Bax, which can induce cytochrome c
to release from mitochondria (54), Bcl-2 was another member of Bcl-2
family involved in the regulation of cell apoptosis, and it had
been much accounted of because of its anti-apoptosis effect by
decreasing the releasing of cytochrome c (55). Previous studies indicated that
Hsp70 could alleviate apoptosis by upregulating Bcl-2 (56,57).
We discovered that Bax in the quercetin-treated group were elevated
significantly, Levels of Bcl-2 in the quercetin-treated group were
significantly reduced. Studies also have shown that various types
of stress (including HS) can trigger apoptosis (58,59).
cytochrome c was regarded as the downstream of Bax and
Bcl-2, Hsp70 can inhibit release of cytochrome c, which
activates Apaf-1 to inhibit formation of the apoptosome and
subsequent activation of caspase-9 (49), Also, direct combination with Apaf-1
can inhibit events downstream of Apaf-1 activation (33). Data showed that cytochrome c
was elevated during HS, however, expression in quercetin-treated
group were elevated significantly.
The present study suggests that Hsp70 may have a
protective role against HS in CPMC. Aggravated apoptosis induced by
low expression of Hsp70 may be associated with the upregulation of
Bax, cytochrome c and negative regulation of Bcl-2 involved
in mitochondrial pathway during HS. However, the specific apoptotic
pathway that mediated by Hsp70 in CPMC during HS needs further
investigation.
Acknowledgements
The current study was supported by grants from the
National Key Basic Research Program of China (973 Program; grant
no. 2014CB138502), the National Natural Science Foundation of China
(grant no. 31602027), the National Natural Science Foundation of
China (grant no. 31672520), the National Natural Science Foundation
Of China (grant no. 31372403), Jiangsu Natural Science Foundation
of China (grant no. BK20160732), China Postdoctoral Science
Foundation (2016M591860), the Priority Academic Program Development
of Jiangsu Higher Education Institutions, Graduate Research and
Innovation Projects in Jiangsu Province and the Sino-German
Agricultural Cooperation Project of the Federal Ministry of Food,
Agriculture and Consumer Production, Berlin, Germany.
References
|
1
|
Selye H: A syndrome produced by diverse
nocuous agents. Nature. 138:321936. View
Article : Google Scholar
|
|
2
|
Geraert PA, Guillaumin S and Leclercq B:
Are genetically lean broilers more resistant to hot climate? Brit
Poultry Sci. 34:643–653. 1993. View Article : Google Scholar
|
|
3
|
St-Pierre NR, Cobanov B and Schnitkey G:
Economic losses from heat stress by US livestock industries. J
Dairy Sci. 86:E52–E77. 2003. View Article : Google Scholar
|
|
4
|
Teeter RG and Belay T: Broiler management
during acute heat stress. Anim Feed Sci Tech. 58:127–142. 1996.
View Article : Google Scholar
|
|
5
|
N'dri AL, Mignon-Grasteau S, Sellier N,
Beaumont C and Tixier-Boichard M: Interactions between the naked
neck gene, sex, and fluctuating ambient temperature on heat
tolerance, growth, body composition, meat quality, and sensory
analysis of slow growing meat-type broilers. Livest Sci. 110:33–45.
2007. View Article : Google Scholar
|
|
6
|
Gathiram P, Gaffin SL, Brock-Utne JG and
Wells MT: Time course of endotoxemia and cardiovascular changes in
heat-stressed primates. Aviat Space Environ Med. 58:1071–1074.
1987.PubMed/NCBI
|
|
7
|
Zhao W, Wisniewski M, Wang W, Liu J and
Liu Y: Heat-induced oxidative injury contributes to inhibition of
Botrytis cinerea spore germination and growth. World J Microbiol
Biotechnol. 30:951–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gathiram P, Wells MT, Raidoo D, Brock-Utne
JG and Gaffin SL: Portal and systemic plasma lipopolysaccharide
concentrations in heat-stressed primates. Circ Shock. 25:223–230.
1988.PubMed/NCBI
|
|
9
|
Yu JM, Bao ED, Yan JY and Lei L:
Expression and localization of Hsps in the heart and blood vessel
of heat-stressed broilers. Cell Stress Chaperones. 13:327–335.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu D, Xu J, Song EB, Tang S, Zhang XH,
Kemper N, Hartung J and Bao ED: Acetyl salicylic acid protected
against heat stress damage in chicken myocardial cells and may
associate with induced Hsp27 expression. Cell Stress Chaperones.
20:687–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gisolfi CV, Matthes RD, Kregel KC and
Oppliger R: Splanchnic sympathetic nerve activity and circulating
catecholamines in the hyperthermic rat. J Appl Physiol (1985).
70:1821–1826. 1991.PubMed/NCBI
|
|
12
|
Rai UC and Ambwany P: Cardiovascular
changes during varied thermal stress. Indian J Physiol Pharmacol.
24:119–125. 1980.PubMed/NCBI
|
|
13
|
De Maio A: Heat shock proteins: Facts,
thoughts, and dreams. Shock. 11:1–12. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z and Srivastava P: Heat-shock
proteins. Curr Protoc Immunol. 58:1T:A.1T.1–A.1T.6. 2004.
|
|
15
|
Clarke AR: Molecular chaperones in protein
folding and translocation. Curr Opin Struc Biol. 6:43–50. 1996.
View Article : Google Scholar
|
|
16
|
Young JC: Mechanisms of the Hsp70
chaperone system. Biochem Cell Biol. 88:291–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Préville X, Salvemini F, Giraud S,
Chaufour S, Paul C, Stepien G, Ursini MV and Arrigo AP: Mammalian
small stress proteins protect against oxidative stress through
their ability to increase glucose-6-phosphate dehydrogenase
activity and by maintaining optimal cellular detoxifying machinery.
Exp Cell Res. 247:61–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruddock LW and Klappa P: Oxidative stress:
Protein folding with a novel redox switch. Curr Biol. 9:R400–R402.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song E, Tang S, Xu J, Yin B, Bao E and
Hartung J: Lenti-siRNA Hsp60 promote bax in mitochondria and
induces apoptosis during heat stress. Biochem Biophys Res Commun.
481:125–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Qian Z, Zhu H, Tang S, Wu D,
Zhang M, Kemper N, Hartung J and Bao E: HSP90 gene expression
induced by aspirin is associated with damage remission in a chicken
myocardial cell culture exposed to heat stress. Br Poult Sci.
57:462–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duan Y, Huang S, Yang J, Niu P, Gong Z,
Liu X, Xin L, Currie W and Wu T: HspA1A facilitates DNA repair in
human bronchial epithelial cells exposed to Benzo [a] pyrene and
interacts with casein kinase 2. Cell Stress Chaperones. 19:271–279.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beckmann RP, Mizzen LE and Welch WJ:
Interaction of Hsp 70 with newly synthesized proteins: Implications
for protein folding and assembly. Science. 248:850–854. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li SQ, Wang DM, Shu YJ, Wan XD, Xu ZS and
Li EZ: Proper heat shock pretreatment reduces acute liver injury
induced by carbon tetrachloride and accelerates liver repair in
mice. J Toxicol Pathol. 26:365–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riabowol KT, Mizzen LA and Welch WJ: Heat
shock is lethal to fibroblasts microinjected with antibodies
against hsp70. Science. 242:433–436. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hutter JJ, Mestril R, Tam EK, Sievers RE,
Dillmann WH and Wolfe CL: Overexpression of heat shock protein 72
in transgenic mice decreases infarct size in vivo. Circulation.
94:1408–1411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Yuan B, Dong W, Yang B, Yang Y,
Lin X and Gong G: Induction of heat-shock protein 70 expression by
geranylgeranylacetone shows cytoprotective effects in
cardiomyocytes of mice under humid heat stress. PLos One.
9:e935362014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mailhos C, Howard MK and Latchman DS: Heat
shock protects neuronal cells from programmed cell death by
apoptosis. Neuroscience. 55:621–627. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yurinskaya MM, Mitkevich VA, Kozin SA,
Evgen'ev MB, Makarov AA and Vinokurov MG: HSP70 protects human
neuroblastoma cells from apoptosis and oxidative stress induced by
amyloid peptide isoAsp7-Aβ (1–42). Cell Death Dis. 6:e19772015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beere HM, Wolf BB, Cain K, Mosser DD,
Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM and Green DR:
Heat-shock protein 70 inhibits apoptosis by preventing recruitment
of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saleh A, Srinivasula SM, Balkir L, Robbins
PD and Alnemri ES: Negative regulation of the Apaf-1 apoptosome by
Hsp70. Nat Cell Biol. 2:476–483. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ravagnan L, Gurbuxani S, Susin SA, Maisse
C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JF, Garrido C
and Kroemer G: Heat-shock protein 70 antagonizes apoptosis-inducing
factor. Nat Cell Biol. 3:839–843. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen HB, Zhang XC, Cheng YF, Abdelnasir A,
Tang S, Kemper N, Hartung J and Bao ED: Association of heat shock
protein 70 expression with rat myocardial cell damage during heat
stress in vitro and in vivo. Genet Mol Res. 14:1994–2005. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang M, Lv YJ, Yue Z, Islam A, Rehana B,
Bao E and Hartung J: Effects of transportation on expression of
Hsp90, Hsp70, Hsp27 and αB-crystallin in the pig stomach. Vet Rec.
169:3122011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aghdassi A, Phillips P, Dudeja V,
Dhaulakhandi D, Sharif R, Dawra R, Lerch MM and Saluja A: Heat
shock protein 70 increases tumorigenicity and inhibits apoptosis in
pancreatic adenocarcinoma. Cancer Res. 67:616–625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Larocca LM, Ranelletti FO, Maggiano N,
Rutella S, La Barbera EO, Rumi C, Serra F, Voso MT, Piantelli M,
Teofili L and Leone G: Differential sensitivity of leukemic and
normal hematopoietic progenitors to the killing effect of
hyperthermia and quercetin used in combination: Role of heat-shock
protein-70. Int J Cancer. 73:75–83. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei YQ, Zhao X, Kariya Y, Fukata H,
Teshigawara K and Uchida A: Induction of apoptosis by quercetin:
Involvement of heat shock protein. Cancer Res. 54:4952–4957.
1994.PubMed/NCBI
|
|
37
|
Kalmar B and Greensmith L: Induction of
heat shock proteins for protection against oxidative stress. Adv
Drug Deliver Rev. 61:310–318. 2009. View Article : Google Scholar
|
|
38
|
Marber MS, Mestril R, Chi SH, Sayen MR,
Yellon DM and Dillmann WH: Overexpression of the rat inducible
70-kD heat stress protein in a transgenic mouse increases the
resistance of the heart to ischemic injury. J Clin Invest.
95:1446–1456. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salo DC, Donovan CM and Davies KJ: HSP70
and other possible heat shock or oxidative stress proteins are
induced in skeletal muscle, heart and liver during exercise. Free
Radic Biol Med. 11:239–246. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Skidmore R, Gutierrez JA, Guerriero V Jr
and Kregel KC: HSP70 induction during exercise and heat stress in
rats: Role of internal temperature. Am J Physiol. 268:R92–R97.
1995.PubMed/NCBI
|
|
41
|
Nakanoma T, Ueno M, Iida M, Hirata R and
Deguchi N: Effects of quercetin on the heat-induced cytotoxicity of
prostate cancer cells. Int J Urol. 8:623–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujita M, Nagai M, Murata M, Kawakami K,
Irino S and Takahara J: Synergistic cytotoxic effect of quercetin
and heat treatment in a lymphoid cell line (OZ) with low HSP70
expression. Leuk Res. 21:139–145. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haagensen L, Jensen DH and Gesser H:
Dependence of myosin-ATPase on structure bound creatine kinase in
cardiac myfibrils from rainbow trout and freshwater turtle. Comp
Biochem Physiol A Mol Integr Physiol. 150:404–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Britton CV, Hernandez A and Roberts R:
Plasma creatine kinase isoenzyme determinations in infants and
children. Characterization in normal patients and after cardiac
catheterization and surgery. Chest. 77:758–760. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu L, Bao ED, Zhao R and Hartung J:
Expression of heat shock protein 60 in the tissues of transported
piglets. Cell Stress Chaperones. 14:61–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ganaie AH, Shanker G, Bumla NA, Ghasura
RS, Mir NA, Wani SA and Dudhatra GB: Biochemical and physiological
changes during thermal stress in bovines. J Vet Sci Technol.
4:10001262013.
|
|
47
|
Nisar NA, Sultana M, Waiz HA, Para PA and
Dar SA: Oxidative stress-threat to animal health and production.
Int J Livest Res. 3:76–83. 2013.
|
|
48
|
Musch MW, Kapil A and Chang EB: Heat shock
protein 72 binds and protects dihydrofolate reductase against
oxidative injury. Biochem Biophys Res Commun. 313:185–192. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zou J, Salminen WF, Roberts SM and Voellmy
R: Correlation between glutathione oxidation and trimerization of
heat shock factor 1, an early step in stress induction of the Hsp
response. Cell Stress Chaperones. 3:130–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zou H, Li Y, Liu X and Wang X: An APAF-1·
cytochrome c multimeric complex is a functional apoptosome that
activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matsumori Y, Northington FJ, Hong SM,
Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR and Liu
J: Reduction of caspase-8 and-9 cleavage is associated with
increased c-FLIP and increased binding of Apaf-1 and Hsp70 after
neonatal hypoxic/ischemic injury in mice overexpressing Hsp70.
Stroke. 37:507–512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gordon SA, Abou-Jaoude W, Hoffman RA,
McCarthy SA, Kim YM, Zhou X, Zhang XR, Simmons RL, Chen Y, Schall L
and Ford HR: Nitric oxide induces murine thymocyte apoptosis by
oxidative injury and a p53-dependent mechanism. J Leukoc Biol.
70:87–95. 2001.PubMed/NCBI
|
|
55
|
Fröhlich M, Jaeger A, Weiss DG and
Kriehuber R: Inhibition of BCL-2 leads to increased apoptosis and
delayed neuronal differentiation in human ReNcell VM cells in
vitro. Int J Dev Neurosci. 48:9–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard
RG, Sapolsky RM, Yenari MA and Steinberq GK: Gene transfer of HSP72
protects cornu ammonis 1 region of the hippocampus neurons from
global ischemia: Influence of Bcl-2. Ann Neurol. 52:160–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee
JE and Giffard RG: Antiapoptotic and anti-inflammatory mechanisms
of heat-shock protein protection. Ann N Y Acad Sci. 1053:74–83.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yin Y, Hawkins KL, Dweolf WC and
Morgentaler A: Heat stress causes testicular germ cell apoptosis in
adult mice. J Androl. 18:159–165. 1997.PubMed/NCBI
|
|
59
|
Isom SC, Prather RS and Rucker EB III:
Heat stress-induced apoptosis in porcine in vitro fertilized and
parthenogenetic preimplantation-stage embryos. Mol Repord Dev.
74:574–581. 2007. View Article : Google Scholar
|