|
1
|
Nickerson HD and Dutta S: Diabetic
complications: Current challenges and opportunities. J Cardiovasc
Transl Res. 5:375–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmidt AM: Recent highlights of ATVB:
Diabetes mellitus. Arterioscler Thromb Vasc Biol. 34:954–958. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porter KE and Riches K: The vascular
smooth muscle cell: A therapeutic target in type 2 diabetes? Clin
Sci. 125:167–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gray SP and Jandeleit-Dahm K: The
pathobiology of diabetic vascular complications-cardiovascular and
kidney disease. J Mol Med. 92:441–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chait A and Bornfeldt KE: Diabetes and
atherosclerosis: Is there a role for hyperglycemia? J Lipid Res.
50:(Suppl). S335–S339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
7
|
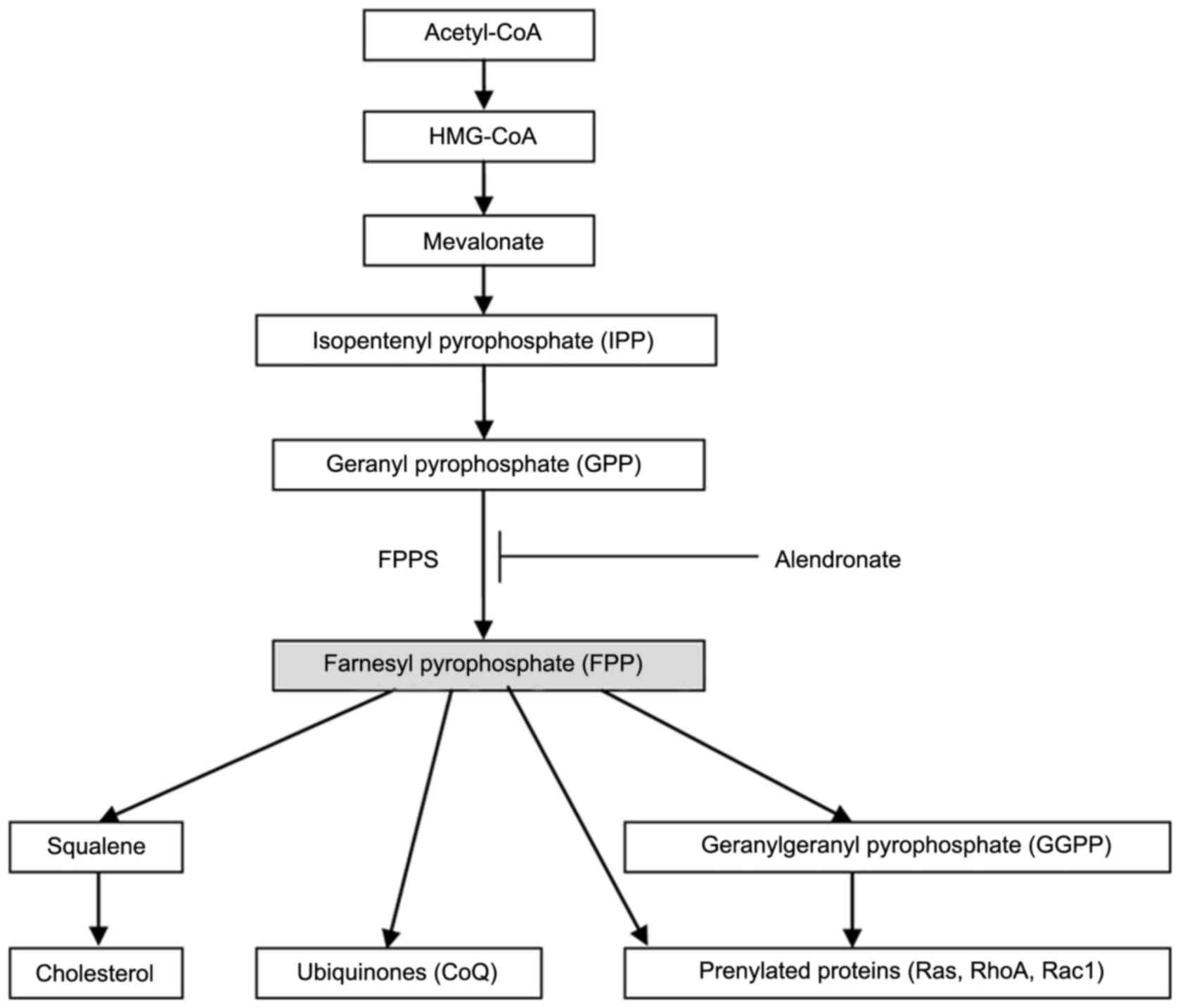
Dhar MK, Koul A and Kaul S: Farnesyl
pyrophosphate synthase: A key enzyme in isoprenoid biosynthetic
pathway and potential molecular target for drug development. N
Biotechnol. 30:114–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun S and McKenna CE: Farnesyl
pyrophosphate synthase modulations: A patent review (2006–2010).
Expert Opin Ther Pat. 21:1433–1451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buhaescu I and Izzedine H: Mevalonate
pathway: A review of clinical and therapeutical implications. Clin
Biochem. 40:575–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen GP, Zhang XQ, Wu T, Li L, Han J and
Du CQ: Alteration of mevalonate pathway in proliferated vascular
smooth muscle from diabetic mice: Possible role in
high-glucose-induced atherogenic process. J diabetes Res.
2015:3792872015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russell RG: Bisphosphonates: The first 40
years. Bone. 49:2–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen GP, Li L, Yang Y, Fu M, Yao L, Wu T,
Zhang XQ and Hu SJ: Chronic inhibition of Farnesyl pyrophosphate
synthase improves endothelial function in spontaneously
hypertensive rats. Biochem Pharmacol. 80:1684–1689. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehrhof FB, Schmidt-Ullrich R, Dietz R and
Scheidereit C: Regulation of vascular smooth muscle cell
proliferation: Role of NF-kappaB revisted. Circ Res. 96:958–964.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen GP, Yao L, Lu X, Li L and Hu SJ:
Tissue-specific effects of atorvastatin on
3-hydroxy-3-methylglutarylcoenzyme A reductase expression and
activity in spontaneously hypertensive rats. Acta Pharmacol Sin.
29:1181–1186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lang JK, Gohil K and Packer L:
Simultaneous determination of tocopherols, ubiquinols, and
ubiquinones in blood, plasma, tissue homogenates, and subcellular
fractions. Anal Biochem. 157:106–116. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC,
Huang SH, Tan CS, Whiteman M, Lu J and Moore PK: Hydrogen sulfide
and its possible roles in myocardial ischemia in experimental rats.
J Appl Physiol (1985). 102:261–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu S, Liu Z and Liu P: Targeting hydrogen
sulfide as a promising therapeutic strategy for atherosclerosis.
Int J Cardiol. 172:313–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vandiver M and Snyder SH: Hydrogen
sulfide: A gasotransmitter of clinical relevance. J Mol Med (Berl).
90:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gadalla MM and Synder SH: Hydrogen sulfide
as a gasotransmitter. J Neurochem. 113:14–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whiteman M, Le Trionnaire S, Chopra M, Fox
B and Whatmore J: Emerging role of hydrogen sulfide in health and
disease: Critical appraisal of biomarkers and pharmacological
tools. Clin Sci. 121:459–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mani S, Untereiner A, Wu L and Wang R:
Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid
Redox Signal. 20:805–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiao W, Chaoshu T, Hongfang J and Junbao
D: Endogenous hydrogen sulfide is involved in the pathogenesis of
atherosclerosis. Biochem Biophys Res Commun. 396:182–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elsey DJ, Fowkes RC and Baxter GF:
Regulation of cardiovascular cell function by hydrogen sulfide
(H(2)S). Cell Biochem Funct. 28:95–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martelli A, Testai L, Marino A, Breschi
MC, Da Settimo F and Calderone V: Hydrogen sulphide:
Biopharmacological roles in the cardiovascular system and
pharmaceutical perspectives. Curr Med Chem. 19:3325–3326. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beltowski J and Jamroz-Wisniewska A:
Modulation of H(2)S metabolism by statins: A new aspect of
cardiovascular pharmacology. Antioxid Redox Signal. 17:81–94. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Dai M and Wang Y: Paeonol inhibits
proliferation of vascular smooth muscle cells stimulated by high
glucose via Ras-Raf-ERK1/2 signaling pathway in coculture model.
Evid Based Complement Alternat Med. 2014:4842692014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Liu J, Li L, Liu L and Wu L: Role
of Ras/PKCzeta/MEK/ERK1/2 signaling pathway in angiotensin
II-induced vascular smooth muscle cell proliferation. Regul Pept.
128:43–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishiko K, Sakoda T, Akagami T, Naka T, Doi
T, Tsujino T, Masuyama T and Ohyanagi M: Hyperglycemia induced cell
growth and gene expression via the serum response element through
RhoA and Rho-kinase in vascular smooth muscle cells. Prep Biochem
Biotechnol. 40:139–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawada N, Li Y and Liao JK: Novel aspects
of the roles of Rac1 GTPase in the cardiovascular system. Curr Opin
Pharmacol. 10:116–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu LH, Wang L, Wang D, Jiang H, Tang QZ,
Yan L, Bian ZY, Wang XA and Li H: Puerarin attenuates high-glucose
and diabetes-induced vascular smooth muscle cell proliferation by
blocking PKCbeta2/Rac1-dependent signaling. Free Radic Biol Med.
48:471–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McTaggart SJ: Isoprenylated proteins. Cell
Mol Life Sci. 63:255–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roskoski R Jr: Protein prenylation: A
pivotal posttranslational process. Biochem Biophys Res Commun.
303:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Casey PJ: Protein lipidation in cell
signaling. Science. 268:221–225. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ylitalo R, Mönkkönen J, Urtti A and
Ylitalo P: Accumulation of bisphosphonates in the aorta and some
other tissues of healthy and atherosclerotic rabbits. J Lab Clin
Med. 127:200–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ylitalo R, Kalliovalkama J, Wu X,
Kankaanranta H, Salenius JP, Sisto T, Lähteenmäki T, Ylitalo P and
Pörsti I: Accumulation of bisphosphonates in human artery and their
effects on human and rat arterial function in vitro. Pharmacol
Toxicol. 83:125–131. 1998. View Article : Google Scholar : PubMed/NCBI
|