Introduction
Primary hepatocellular carcinoma (HCC) is one of the
most predominant diseases worldwide, particularly in China
(1,2). Hepatitis B virus (HBV), hepatitis C
virus (HCV), aflatoxin B1, water pollution and alcoholism all
contribute to the development of liver cancer (3). In China, the leading cause of liver
cancer is cirrhosis due to HBV infection (4–6). The
nonstructural HBV X protein (HBx) is comprised of 154 amino acids,
with a molecular mass of ~17.5 kDa (7). It is a key regulatory protein of HBV,
and has been associated with HBV infection, replication,
pathogenesis and potentially carcinogenesis (8,9).
Recently, Kouwaki et al (10) reported that HBx regulates HBV
replication by interacting with jumonji-C domain containing-5
(JMJD5), a novel binding partner to HBx, which facilitates HBV
replication via the hydroxylase activity of JMJD5. In addition,
anti-HBx short hairpin RNAs have been demonstrated to effectively
inhibit HBV replication in vivo by targeting conserved
sequences in the oncogenic HBx open reading frame (11). Kim et al (12) revealed that HBx altered host gene
expression, leading to the development of HCC in transgenic mice.
Notably, a previous study revealed that enhanced cell motility was
alleviated by mutations in the proline rich domain located in HBx,
which provided novel insights on the underlying mechanism of HCC
development associated with chronic HBV infection (13). However, the exact underlying
mechanism of HBx in HCC development and progression remains
unclear.
MicroRNAs (miRNAs) are a newly identified class of
conserved RNA molecules 21 to 23 nucleotides in length, and
regulate the stability or translational efficiency of target mRNAs.
miRNAs serve important roles in the progression and metastasis of
liver cancer (14).
Epithelial-mesenchymal transition (EMT) has been recognized as a
critical event that initiates cancer invasion and metastasis. It
involves the loss of tumor cell polarity and is associated with
alterations in intercellular adhesion, cytoskeletal reorganization
and cellular signaling pathway. Yin et al (15) reported that liver
metastasis-associated miRNAs, including serum miRNA (miR)-126,
miR-141 and miR-21, may be novel biomarkers for the clinical
diagnosis of early stage liver-metastatic colorectal cancer. A
previous study determined that miR-21 may act as a key regulator of
fibrogenic EMT in hepatocytes via PTEN/Akt pathway (16). Zhang et al (17) additionally demonstrated that
miR-21, miR-17 and miR-19a, induced by a phosphatase of
regenerating liver-3, are involved in the proliferation and
invasion of colon cancer cells. A number of studies have revealed
that miR-21 is overexpressed in human tumors and that it may be
involved in the regulation of metastasis in several tumor types,
including melanoma (18), breast
cancer (19,20), keratinocytes (21), colorectal cancer (22) and HCC (23). However, it is unclear whether
miR-21 is involved in the progression and metastasis of
HBV-positive liver cancers.
The aim of the present study was to investigate the
role of miR-21 in HBx-positive liver cancer cells, and to determine
the association between HBx and miRNAs in the development and
metastasis of liver cancer cells. The results of the present study
may provide novel ideas and effective targets for future HCC
therapy.
Materials and methods
Cell line and agents
The human HCC cell lines, MHCC97H and MHCC-97L, were
obtained from the Liver Cancer Institute of Fudan University
(Shanghai, China). The SMMC-7721 liver cancer and immortalized L02
human liver cell lines were used as negative controls and were
purchased from Cell Resource Center of Shanghai Institutes for
Biological Sciences (Chinese Academy of Sciences, Shanghai, China).
The liver cancer cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1%
penicillin and 1% streptomycin. DMEM and FBS were purchased from
Hyclone; GE Healthcare Life Sciences (Logan, UT, USA).
MTT assay
MTT assay was performed as previously described
(24,25). Briefly, the SMMC-7721 human liver
cancer cells at a density of 3,000 cells/well were transfected with
HBx-small interfering (si)RNA1, HBx-siRNA2 or HBx-siRNA3 for 48, 72
or 96 h, respectively. Proliferation in the different groups was
detected by MTT assay. In the other treatment group, MHCC-97H cells
were plated into 96-well plates at a density of 3,000 cells/well
and treated with miR-21 mimics or miR-21 inhibitors for 48, 72 or
96 h. The cell viability was determined by MTT assay.
Transfection
Three pairs of siRNA specific to HBx were designed
and synthesized by Jima Corporation (Shanghai, China). Scramble
siRNA was used as the negative control (NC) for siRNA experiments.
Transfection of cells was performed using Oligofectamine
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The Opti-MEM medium was
replaced 6 h following transfection. RNA was extracted 48 and 72 h
following siRNA treatment for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and cell lysates were prepared
for western blot analysis.
The sequences for the chemically synthesized
HBx-siRNAs were as follows: HBx-siRNA1 forward,
5′-GGUCUUACAUAAGAGGACUdTdT-3′ and reverse,
5′-AGTCCTCTTATGTAAGACCdTdT-3′; HBx-siRNA2 forward,
5′-CCGACCUUGAGGCAUACUUdTdT-3′ and reverse,
5′-AAGUAUGCCUCAAGGUCGGdTdT-3′; HBx-siRNA3 forward,
5′-GGACGUCCUUUGUUUACGUdTdT-3′ and reverse,
5′-ACGTAAACAAAGGACGTCCdTdT-3′. The miR-21 inhibitor, anti-miR-21
(sequence, 5-UCAACAUCAGUCUGAUAAGCUA-3), is a chemically modified
single strand RNA and a competitive inhibitor of miR-21. The
sequence of the miR-21 mimic used was:
5-UAGCUUAUCAGACUGAUGUUGAAACAUCAGUCUGAUAAGCUAUU-3. The miR-21 mimics
and miR-21 inhibitors were all obtained from Shanghai GenePharma
Co., Ltd. (Shanghai, China).
RT-qPCR for quantitative analysis of
miR-21
Total RNA was extracted from liver cancer cells
using TRIzol® (Takara Bio, Inc., Otsu, Japan) according
to the manufacturer's protocol. The cDNA samples of were
transcribed using the PrimeScript-RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China) in a 20 ml final reaction
volume according to the manufacturer's instructions. An RT-qPCR
assay was performed to evaluate miR-21 levels using SYBR Premix
ExTaq™ II (Takara Biotechnology Co., Ltd.) on an ABI 7500 system
according to the manufacturer's instructions, the thermocycling
conditions were 40 cycles of 12 sec at 95°C and 1 min at 58°C. Each
sample was analyzed in triplicate and U6 small nuclear RNA was used
for normalization. No template or reverse transcription were
included as negative controls. The sequence of primers for RT-qPCR
were as follows: MiR-21 RT:
5′-CGTCGCTACATCGAGTGTAGCATATGCGACGTCAACATC-3′; miR-21 forward,
5′-TAGCTTATCAGACTGATG-3′ and reverse, 5′-ACATCGAGTGTAGCATA-3′; U6
RT 5′-AACGCTTCACGAATTTGCGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3.
Antibodies
An anti-HBx antigen mouse monoclonal antibody
[3F6-G10] (cat. no. ab235) was purchased from Abcam (Cambridge,
UK). A phosphatase and tensin homolog (PTEN) rabbit monoclonal
antibody (138G6; cat. no. 9559S) was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). A phosphorylated (p)-protein
kinase B (Akt) 1/2/3 (Ser 473)-R antibody (a rabbit polyclonal IgG;
200 µg/ml; cat. no. sc-7985-R) and mouse monoclonal Akt antibody
(BDI111; 50 µg/0.5 ml; cat. no. sc-56878) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit polyclonal
anti-matrix metalloproteinase (MMP)-2 (cat. no. ab37150) and
anti-MMP-9 (cat. no. ab38898) antibodies were purchased from Abcam.
Mouse monoclonal anti-β-actin antibody (cat. no. TA310155) was
purchased from OriGene Technologies, Inc. (Beijing, China). All the
primary antibodies were diluted at 1:1,000. Goat anti-rabbit
IgG-horseradish peroxidase (HRP; cat. no. sc-2004) and goat
anti-mouse IgG-HRP (cat. no. sc-2005) secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc.
Western blot analysis
The liver cancer cells were seeded into 48-well
plates (5×105 cells) for 8 h, prior to treatment with
miR-21 inhibitors or miR-21 mimics for 24 or 48 h. The cell lysates
were prepared using radioimmunoprecipitation assay buffer (50
mmol/l Tris, 1% NP-40, 150 mmol/l NaCl, 1 mmol EDTA, 0.1% SDS,
0.25% sodium deoxycholate detergent). The lysates were separated by
10–12% SDS-PAGE and subsequently transferred onto a nitrocellulose
membrane (Beijing BioDee Biotechnology, Co., Ltd., Beijing, China)
at 400 mA for 1 h. The membrane was blocked with Tris-buffered
saline with 0.1% Tween-20 (TBST) supplemented with 5% bovine serum
albumin (BSA; Beyotime Institute of Biotechnology, Inc., Haimen,
China) for 30 min at room temperature prior to incubating the
membrane with specific antibodies in TBST containing 2% BSA at 4°C
overnight. The membrane was washed three times with TBST and
subsequently incubated with the corresponding secondary antibodies
for 1 h at room temperature. The bands were detected in a dark room
using chemiluminescence techniques. β-actin was used as an internal
reference. The experiments were repeated twice.
Transwell assay
A Transwell assay was used to detect the invasion
and metastasis of liver cancer cells. Corning®
Transwell® polycarbonate membrane cell culture inserts
(cat. no. CLS3421) were obtained from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). The BD Matrigel™ (BD Biosciences,
Franklin Lakes, NJ, USA) was stored at 4°C overnight to keep it in
a liquid state. Serum-free medium (300 µl) and the Matrigel™ (50
µl) were mixed and placed into the insert, which was incubated at
37°C for 4–5 h. The liver cancer cells (5×105) were
loaded into the insert and treated with the miR-21 mimics or miR-21
inhibitors for 48 h at 37°C. The insert was taken off and the cells
were washed twice with PBS buffer. The cells were subsequently
fixed with 5% glutaraldehyde and stained with crystal violet
(0.1%). The cells were washed twice with PBS, and counted under a
light microscope.
Statistical analysis
Statistical analysis was performed using analysis of
variance followed by Tukey's honest significant difference post hoc
test using SPSS 20.0 software (IBM SPSS, Armonk, NY, USA). The data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
HBx levels are positively associated
with the expression of miR-21
In order to detect whether the levels of HBx were
associated with the invasion of liver cancer cells, HBx levels were
detected in three liver cancer cell lines with different metastatic
potentials, including MHCC-97H (a highly metastatic human
hepatocellular carcinoma cell line), and MHCC-97L and SMMC-7721
(low metastatic human hepatocellular carcinoma cell lines). The L02
immortalized human liver cell line was used as the control. As
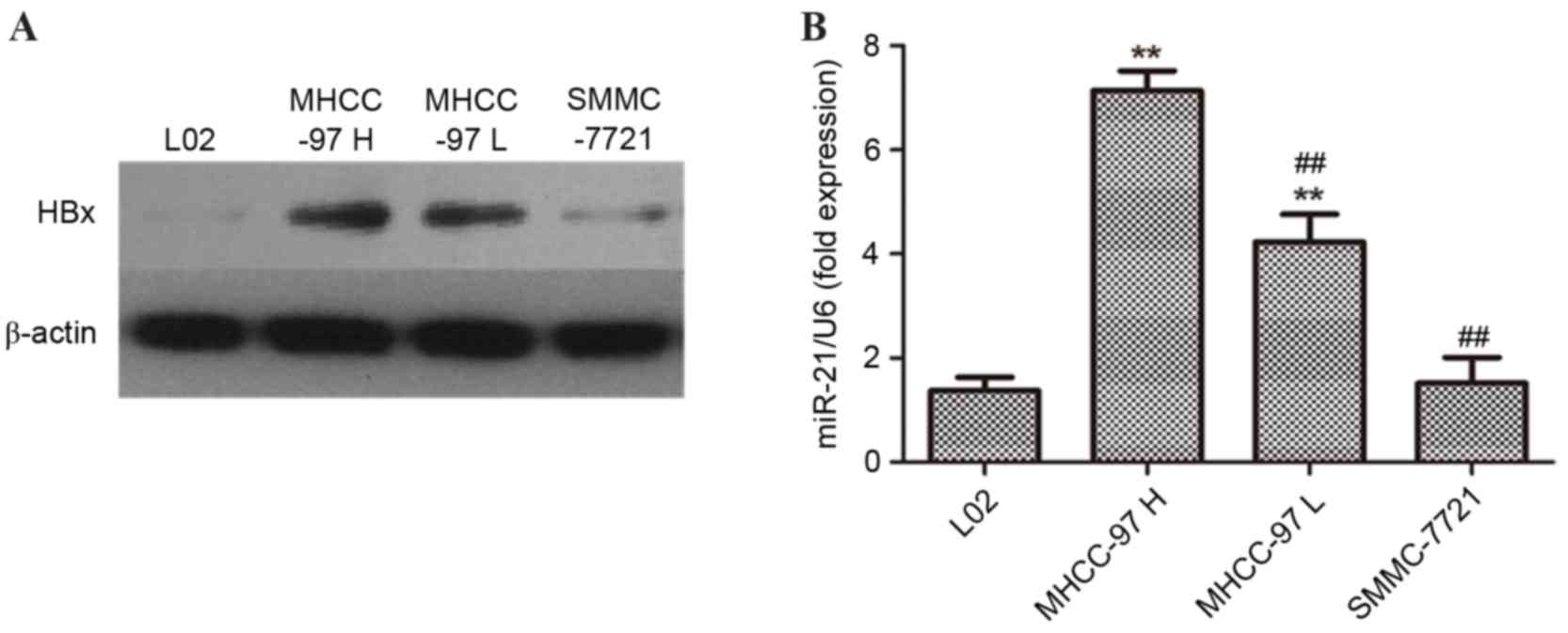
presented in Fig. 1A, the western
blotting results demonstrated that protein expression of HBx in
MHCC-97H cells was markedly increased compared with in MHCC-97L and
SMMC-7721 cells. miR-21 is the most commonly over-expressed miRNA
in cancer and it is a recognized oncogene. mRNA expression levels
of miR-21 in MHCC-97H, MCC-97L, SMMC-7721 and L02 cells were
additionally measured. As presented in Fig. 1B, the results demonstrated that the
expression levels of miR-21 were significantly increased in
MHCC-97H and MHCC-97L cells compared with L02 cells. In addition,
the miR-21 levels in MHCC-97H cells were significantly increased
than those observed in MHCC-97L and SMMC-7721 cells (##P<0.01).
Collectively, the results indicated that the levels of HBx may be
positively associated with the expression of miR-21, and that they
may contribute to the invasion of liver cancer cells.
Interference with endogenous HBx in
liver cancer cells is accompanied by a corresponding decrease in
miR-21 expression
To further investigate the association between HBx
and miR-21, three pairs of siRNAs specific to HBx were designed and
synthesized. The three pairs of HBx-siRNAs and the scrambled siRNA
were used for interference with endogenous HBx in human liver
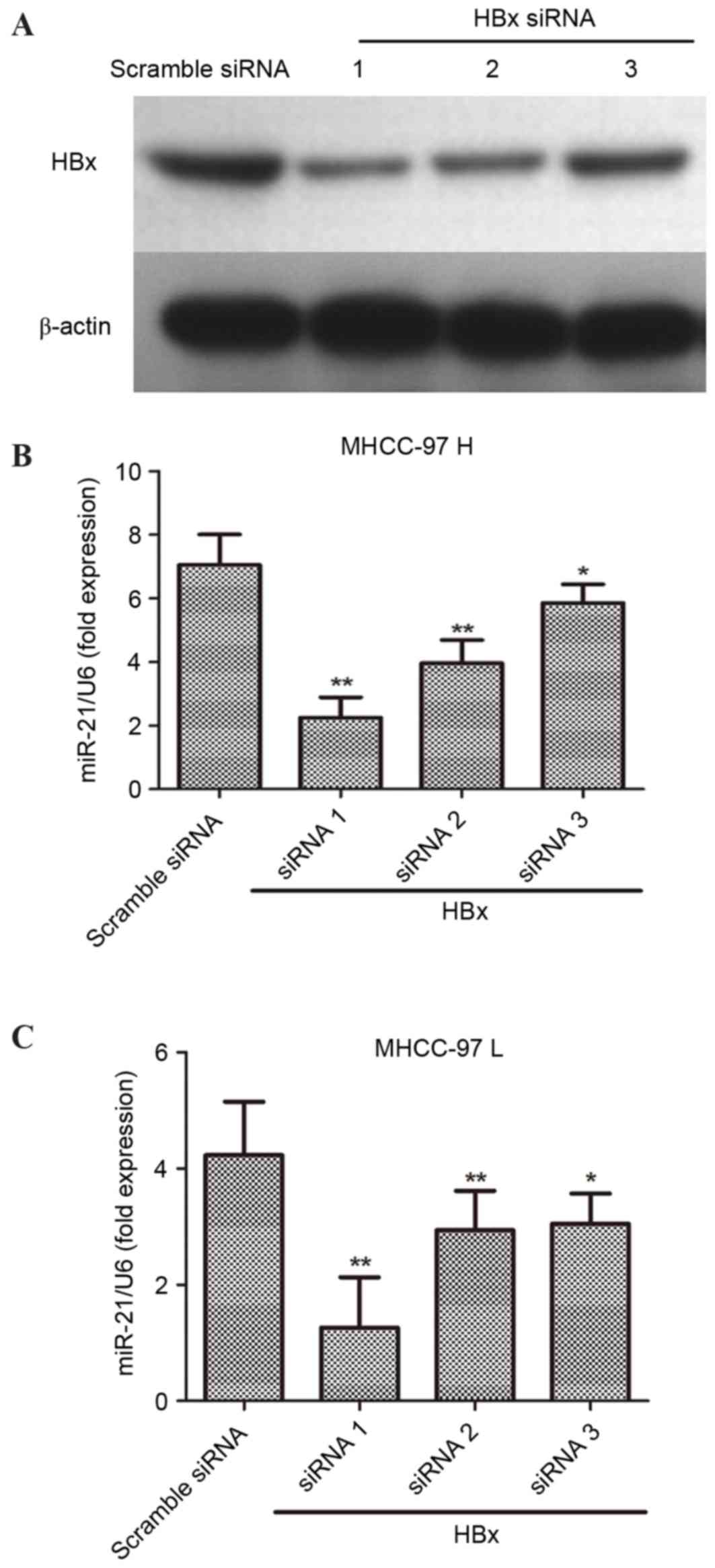
cancer cells. Following transfection with HBx-siRNA for 48 h,
HBx-siRNA1 was most effective at downregulating HBx in liver cancer
cells (Fig. 2A). The relative
expression of miR-21 was detected in MHCC-97H and MHCC-97L cells,
which were transfected with HBx-siRNA for 48 h. Notably, the
results demonstrated that interference with endogenous HBx
expression may significantly decreased the levels of miR-21 in
MHCC-97H (Fig. 2B) and MHCC-97L
(Fig. 2C).
Interference with the expression of
HBx inhibits the proliferation of MHCC-97H cells
The present study revealed that interference with
the levels of HBx decreased the expression of miR-21 in MHCC-97H
and MHCC-97L cells. Thus, it was of interest to investigate whether
knockdown of endogenous HBx would affect the proliferation of
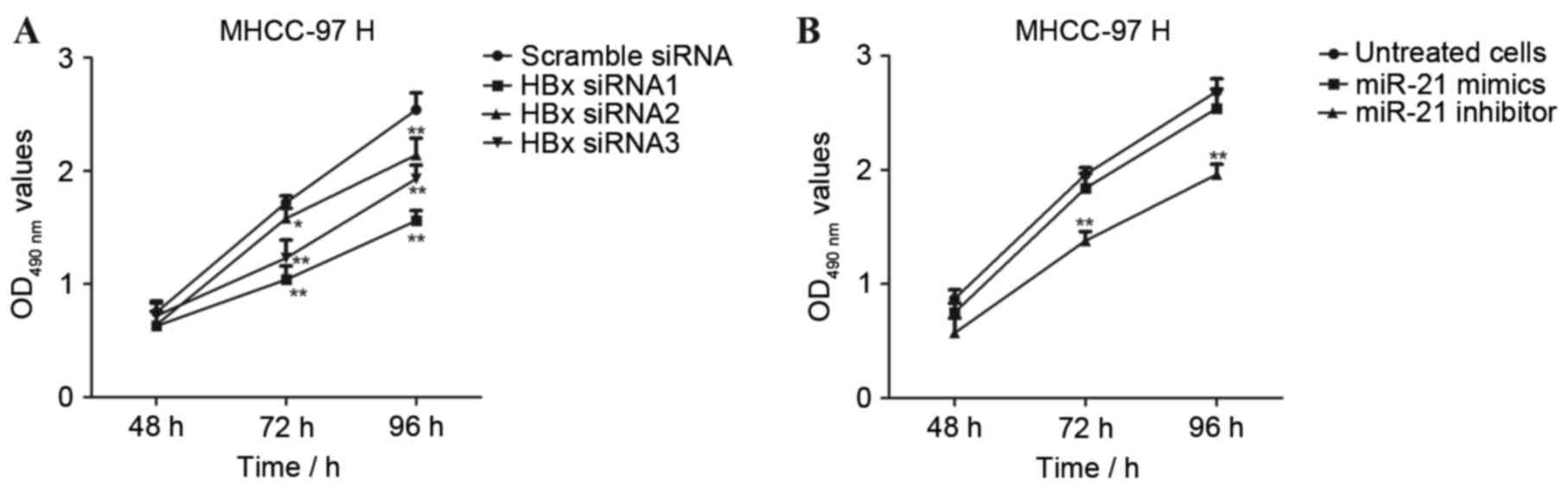
MHCC-97H cells. The MHCC-97H cells were transfected with
HBx-siRNA1, HBx-siRNA2 and HBx-siRNA3 for 48 h and cell viability
was determined by MTT assay. As presented in Fig. 3A, proliferation of MHCC-97H cells
was significantly suppressed following transfection with siRNA
specific to HBx when compared with scrambled siRNA (*P<0.05 and
**P<0.01), indicating that knockdown of HBx levels in MHCC-97H
cells may inhibit liver cancer cell proliferation.
Transfection with miR-21 inhibitor or
mimics regulates the cell proliferation of MHCC-97H cells
The results so far revealed that HBx expression
regulated the levels of miR-21 in liver cancer cells. To further
confirm the role of miR-21 in the proliferation of MHCC-97H cells,
the miR-21 mimics and miR-21 inhibitor were transfected into
MHCC-97H cells and cell viability was determined by MTT assay. As
presented in Fig. 3B, transfection
with miR-21 mimics had no marked effects on the proliferation of
MHCC-97H cells. However, in MHCC-97H cells transfected with miR-21
inhibitor for 48, 72 and 96 h, cell viability was significantly
decreased compared with miR-21 mimics-transfected cells
(**P<0.01).
Invasion and metastasis of MHCC-97H
cells is regulated by miR-21 and HBx
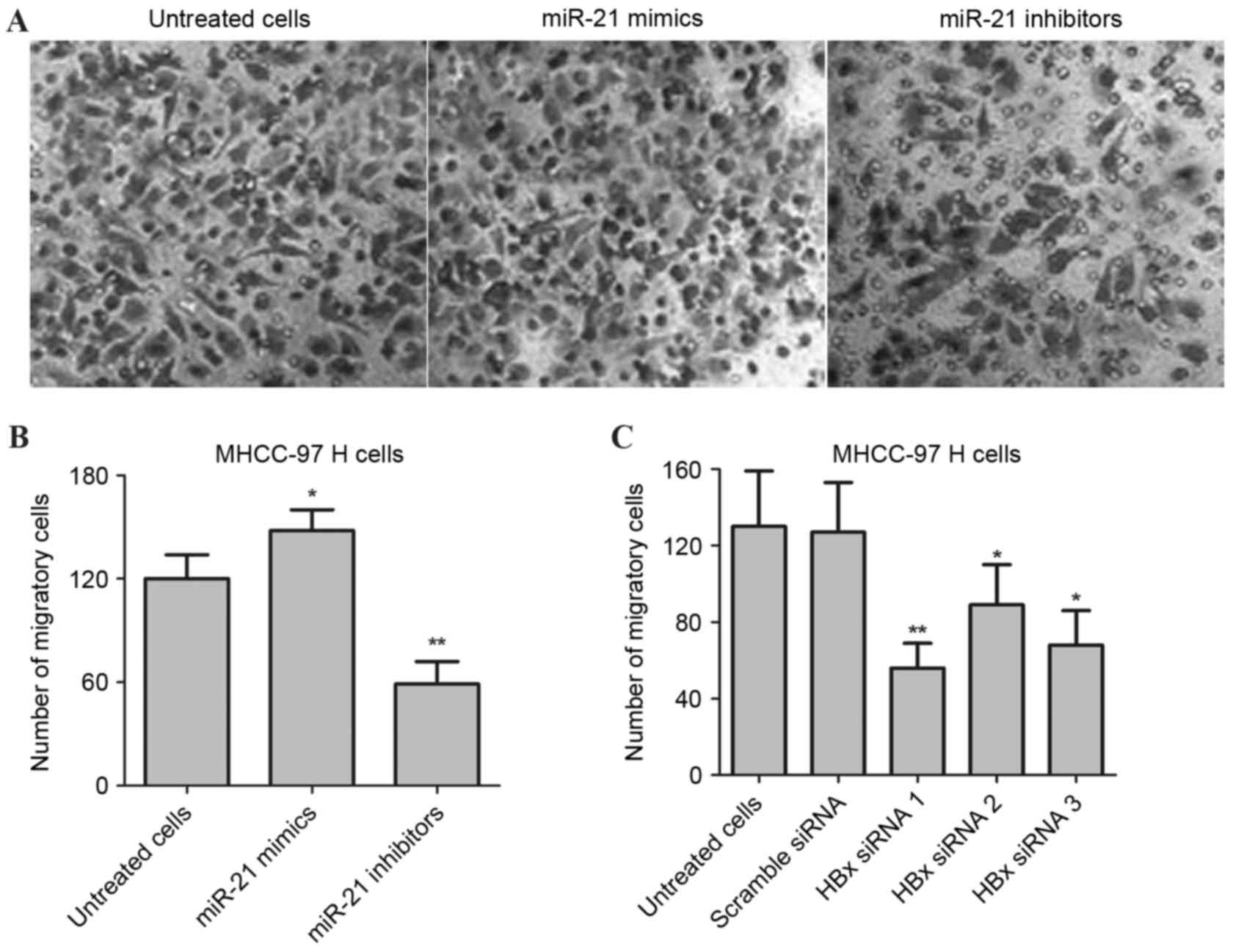
To further determine the effects of miR-21 on the
invasion and metastasis of MHCC-97H cells, a Transwell assay was
performed by transfecting with miR-21 mimics and miR-21 inhibitors.
As presented in Fig. 4A and B,
following transfection with inhibitors, the number of migratory
cells were significantly decreased in MHCC-97H cells compared with
the untreated group (**P<0.01). In addition, miR-21 mimics were
used to transfect the MHCC-97H cells for 48 h, and the results
demonstrated that the number of migratory cells significantly
increased when compared with untreated cells (*P<0.05). The role
of HBx in the invasion and metastasis of MHCC-97H cells was
additionally investigated. Briefly, the MHCC-97H cells were
transfected with three pairs of siRNA specific to HBx for 48 h, and
a Transwell assay was performed. As presented in Fig. 4C, the migration and invasion of
MHCC-97H cells was significantly suppressed in HBx-knockdown cells
compared with scrambled siRNA-transfected cells (*P<0.05 and
**P<0.01). Collectively, these results revealed that
downregulation of HBx and miR-21 inhibitors may contribute to the
inhibition of invasion and metastasis in human liver cancer
cells.
HBx and miR-21 regulate the
PTEN/PI3K/Akt signaling pathway
It has been reported that PTEN may be a direct
target of miR-21 and that overexpression of miR-21 may lead to
increased p-Akt signaling by directly targeting PTEN (26). The present study investigated
whether HBx regulates miR-21 via the PTEN/PI3K/Akt signaling
pathway. The miR-21 inhibitors were used to transfect MHCC-97H
cells for 24 h. The level of PTEN was upregulated, which was
accompanied by a decrease in the levels of PI3K and p-Akt, as well
as downregulation of MMP-2 levels (Fig. 5A). Notably, the expression of MMP-9
was not altered in miR-21 inhibitor-treated cells. Western blot
analysis further confirmed that transfection with HBx-siRNA led to
an upregulation of PTEN; however, the levels of p-Akt and MMP-2
were reduced when compared with cells transfected with scramble
siRNA (Fig. 5B). Collectively,
these results indicated that downregulation of miR-21 and HBx may
suppress the activity of MMP-2 via the PTEN/PI3K/Akt signaling
pathway.
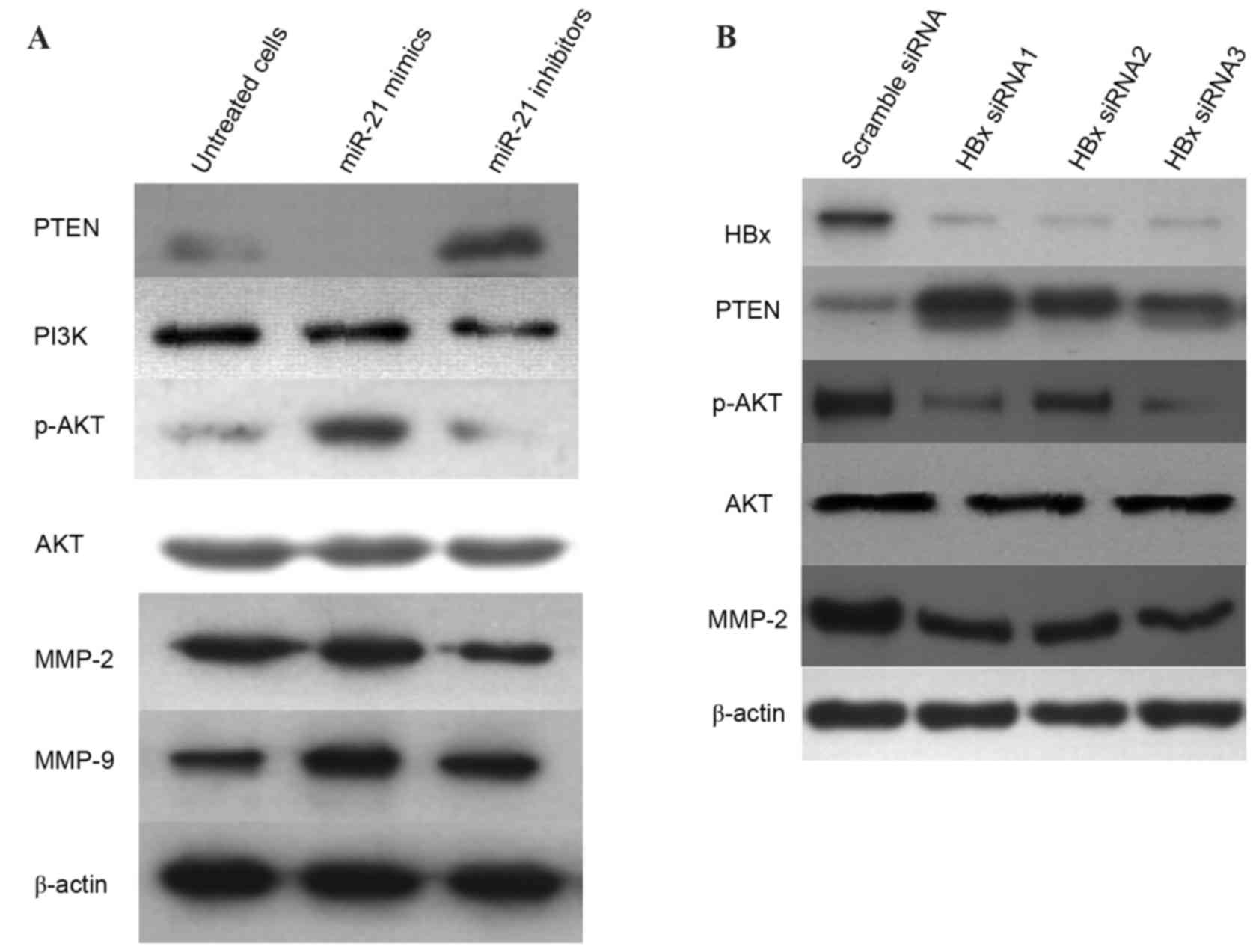 | Figure 5.HBx and miR-21 regulate the
PTEN/PI3K/Akt signaling pathway. (A) MHCC-97H cells were
transfected with miR-21 mimics and miR-21 inhibitors for 24 h, and
western blotting was performed to determine protein expression
levels of PTEN, PI3K, p-Akt, Akt, MMP-2 and MMP-9. (B) MHCC-97H
cells were transfected with HBx-siRNA1, HBx-siRNA2 or HBx-siRNA3
for 48 h. Protein expression levels of HBx, PTEN, p-Akt, Akt and
MMP-2 were determined by western blot analysis. β-actin served as
an internal control. HBx, hepatitis B virus X protein; miR,
microRNA; PTEN, phosphatase and tensin homolog; PI3K,
phosphoinositide 3-kinase; p, phosphorylated; Akt, protein kinase
B; MMP, matrix metalloproteinase; siRNA, small interfering RNA. |
Discussion
In China, the HBV infection is one of the primary
causes of HCC. In the HBV genome, the association between the
X-gene and HCC has been a key area of interest. A recent study
using HBx-expressing transgenic mice under authentic promoter
control demonstrated a high rate of HCC development (86%) (27). The HBV gene integrates into the
genome of the host cell, which may interfere with normal cell cycle
and apoptosis, and thus may be an important factor in the induction
of malignant transformation (28).
However, the exact mechanisms underlying HBV carcinogenesis have
not been fully elucidated. Previous studies have revealed that the
molecular mechanism of HBx in the carcinogenesis of HCC is complex
and may involve multiple mechanisms (29).
In the present study, three human HCC cell lines
with different metastatic potentials were selected. MHCC-97H is a
human liver cancer cell line with high metastatic potential,
whereas MHCC-97L and SMMC-7721 are human liver cell lines with low
metastatic potentials. In order to detect the role of HBx in the
progression and metastasis of human HCC cells, protein expression
levels of HBx in MHCC-97H, MHCC-97L and SMMC-7721 cells were
detected by western blot analysis. The L02 immortalized liver cell
line was used as negative control. The results demonstrated that
MHCC-97H cells had increased levels of HBx compared with MHCC-97L
and SMMC-7721 cells, indicating that HBx may be involved in the
metastasis of liver cancer cells.
In addition, increased levels of HBx were associated
with increased expression of miR-21 in the highly metastatic
MHCC-97H liver cancer cell line. However, interference with HBx
decreased the levels of endogenous HBx, which was accompanied by a
corresponding downregulation of miR-21. MHCC-97H cells were treated
with miR-21 inhibitors, which decreased the migration and invasion
of MHCC-97H cells compared with untreated cells. This was
consistent with the results of MHCC-97H cells with knocking down of
HBx by HBx-siRNA.
PTEN is considered the target gene of miR-21 and
exerts its biological function as a tumor-suppressor (30,31).
In the present study, interference with endogenous HBx was
demonstrated to contribute to upregulation and activation of PTEN,
which led to a decrease in p-Akt and downregulation of MMP-2. This
was consistent with the results of cells treated with miR-21
inhibitors. Thus, interference with endogenous HBx may suppress the
activity of MMP-2 via the PTEN/PI3K/Akt signaling pathway to
inhibit the invasion and metastasis of MHCC-97H cells, mediated by
downregulation of miR-21 levels. The present study provided novel
information, and potentially an effective target, for future HCC
therapies.
Acknowledgements
The present study was supported by the National
Science Foundation of Hunan (grant no. 11JJ6084), the Hunan
Provincial Natural Science Foundation (grant no. 10JJ5034) and the
Hunan Provincial Science and Technology Program (grant nos.
2010SK3093 and 2013SK3025).
References
|
1
|
Waller LP, Deshpande V and Pyrsopoulos N:
Hepatocellular carcinoma: A comprehensive review. World J Hepatol.
7:2648–2663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wogan GN, Kensler TW and Groopman JD:
Present and future directions of translational research on
aflatoxin and hepatocellular carcinoma. A review. Food Addit Contam
Part A Chem Anal Control Expo Risk Assess. 29:249–257. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin HJ, Kao ST, Siao YM and Yeh CC: The
Chinese medicine Sini-San inhibits HBx-induced migration and
invasiveness of human hepatocellular carcinoma cells. BMC
Complement Altern Med. 15:3482015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka M, Katayama F, Kato H, Tanaka H,
Wang J, Qiao YL and Inoue M: Hepatitis B and C virus infection and
hepatocellular carcinoma in China: A review of epidemiology and
control measures. J Epidemiol. 21:401–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Li L, Xiang X, Wang H, Cai W, Xie J,
Han Y, Bao S and Xie Q: Three common functional polymorphisms in
microRNA encoding genes in the susceptibility to hepatocellular
carcinoma: A systematic review and meta-analysis. Gene.
527:584–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pollicino T and Saitta C: Occult hepatitis
B virus and hepatocellular carcinoma. World J Gastroenterol.
20:5951–5961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali A, Abdel-Hafiz H, Suhail M, Al-Mars A,
Zakaria MK, Fatima K, Ahmad S, Azhar E, Chaudhary A and Qadri I:
Hepatitis B virus, HBx mutants and their role in hepatocellular
carcinoma. World J Gastroenterol. 20:10238–10248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin L, Yin X, Hu X, Wang Q and Zheng L:
The impact of hepatitis B virus×protein and microRNAs in
hepatocellular carcinoma: A comprehensive analysis. Tumour Biol.
35:11695–11700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motavaf M, Safari S, Jourshari Saffari M
and Alavian SM: Hepatitis B virus-induced hepatocellular carcinoma:
The role of the virus×protein. Acta Virol. 57:389–396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kouwaki T, Okamoto T, Ito A, Sugiyama Y,
Yamashita K, Suzuki T, Kusakabe S, Hirano J, Fukuhara T, Yamashita
A, et al: Hepatocyte factor JMJD5 regulates hepatitis B virus
replication through interaction with HBx. J Virol. 90:3530–3542.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carmona S, Ely A, Crowther C, Moolla N,
Salazar FH, Marion PL, Ferry N, Weinberg MS and Arbuthnot P:
Effective inhibition of HBV replication in vivo by anti-HBx short
hairpin RNAs. Mol Ther. 13:411–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim CM, Koike K, Saito I, Miyamura T and
Jay G: HBx gene of hepatitis B virus induces liver cancer in
transgenic mice. Nature. 351:317–320. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng H, Zhang J, Li X and Chen WN:
HBX-mediated migration of HBV-replicating HepG2 cells: Insights on
development of hepatocellular carcinoma. J Biomed Biotechnol.
2009:9302682009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong J, He XX and Tian A: Emerging role of
microRNA in hepatocellular carcinoma (Review). Oncol Lett.
9:1027–1033. 2015.PubMed/NCBI
|
|
15
|
Yin J, Bai Z, Song J, Yang Y, Wang J, Han
W, Zhang J, Meng H, Ma X, Yang Y, et al: Differential expression of
serum miR-126, miR-141 and miR-21 as novel biomarkers for early
detection of liver metastasis in colorectal cancer. Chin J Cancer
Res. 26:95–103. 2014.PubMed/NCBI
|
|
16
|
Liu Z, Wang J, Guo C and Fan X:
MicroRNA-21 mediates epithelial-mesenchymal transition of human
hepatocytes via PTEN/Akt pathway. Biomed Pharmacother. 69:24–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Xiao Z, Lai D, Sun J, He C, Chu
Z, Ye H, Chen S and Wang J: MiR-21, miR-17 and miR-19a induced by
phosphatase of regenerating liver-3 promote the proliferation and
metastasis of colon cancer. Br J Cancer. 107:352–359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang CH, Yue J, Pfeffer SR, Handorf CR and
Pfeffer LM: MicroRNA miR-21 regulates the metastatic behavior of
B16 melanoma cells. J Biol Chem. 286:39172–39178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Wang X, Shen H, Deng R and Xue K:
Combination of miR-21 with circulating tumor cells markers improve
diagnostic specificity of metastatic breast cancer. Cell Biochem
Biophys. 73:87–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soria-Valles C, Gutiérrez-Fernández A,
Guiu M, Mari B, Fueyo A, Gomis RR and López-Otín C: The
anti-metastatic activity of collagenase-2 in breast cancer cells is
mediated by a signaling pathway involving decorin and miR-21.
Oncogene. 33:3054–3063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu X, Luo F, Liu Y, Zhang A, Li J, Wang B,
Xu W, Shi L, Liu X, Lu L and Liu Q: The IL-6/STAT3 pathway via
miR-21 is involved in the neoplastic and metastatic properties of
arsenite-transformed human keratinocytes. Toxicol Lett.
237:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferraro A, Kontos CK, Boni T, Bantounas I,
Siakouli D, Kosmidou V, Vlassi M, Spyridakis Y, Tsipras I, Zografos
G and Pintzas A: Epigenetic regulation of miR-21 in colorectal
cancer: ITGB4 as a novel miR-21 target and a three-gene network
(miR-21-ITGBeta4-PDCD4) as predictor of metastatic tumor potential.
Epigenetics. 9:129–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Connolly EC, Van Doorslaer K, Rogler LE
and Rogler CE: Overexpression of miR-21 promotes an in vitro
metastatic phenotype by targeting the tumor suppressor RHOB. Mol
Cancer Res. 8:691–700. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spinner DM: MTT growth assays in ovarian
cancer. Methods Mol Med. 39:175–177. 2001.PubMed/NCBI
|
|
25
|
Sargent J, Elgie A, Taylor CG, Wilson J,
Alton P and Hill JG: The identification of drug resistance in
ovarian cancer and breast cancer: Application of the MTT assay.
Contrib Gynecol Obstet. 19:64–75. 1994.PubMed/NCBI
|
|
26
|
Liu H, Huang X, Liu X, Xiao S, Zhang Y,
Xiang T, Shen X, Wang G and Sheng B: MiR-21 promotes human nucleus
pulposus cell proliferation through PTEN/AKT signaling. Int J Mol
Sci. 15:4007–4018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koike K, Moriya K, Iino S, Yotsuyanagi H,
Endo Y, Miyamura T and Kurokawa K: High-level expression of
hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic
mice. Hepatology. 19:810–819. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Benhenda S, Cougot D, Neuveut C and
Buendia MA: Liver cell transformation in chronic HBV infection.
Viruses. 1:630–646. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blumberg BS: Primary and secondary
prevention of liver cancer caused by HBV. Front Biosci (Schol Ed).
2:756–763. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong B, Cheng Y, Ma L and Zhang C: MiR-21
regulates biological behavior through the PTEN/PI-3K/Akt signaling
pathway in human colorectal cancer cells. Int J Oncol. 42:219–228.
2013.PubMed/NCBI
|
|
31
|
Xu J, Zhang W, Lv Q and Zhu D:
Overexpression of miR-21 promotes the proliferation and migration
of cervical cancer cells via the inhibition of PTEN. Oncol Rep.
33:3108–3116. 2015.PubMed/NCBI
|