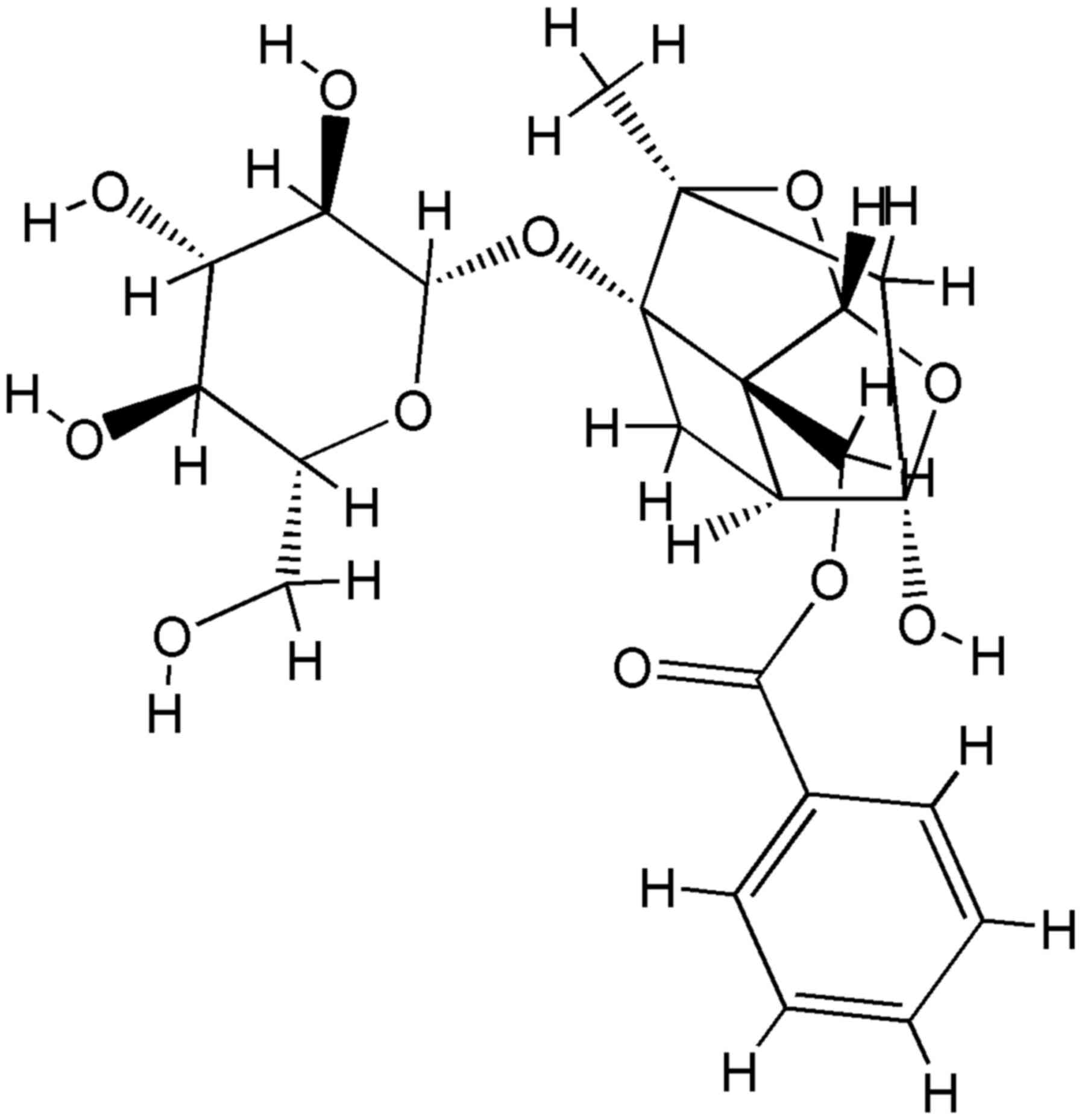
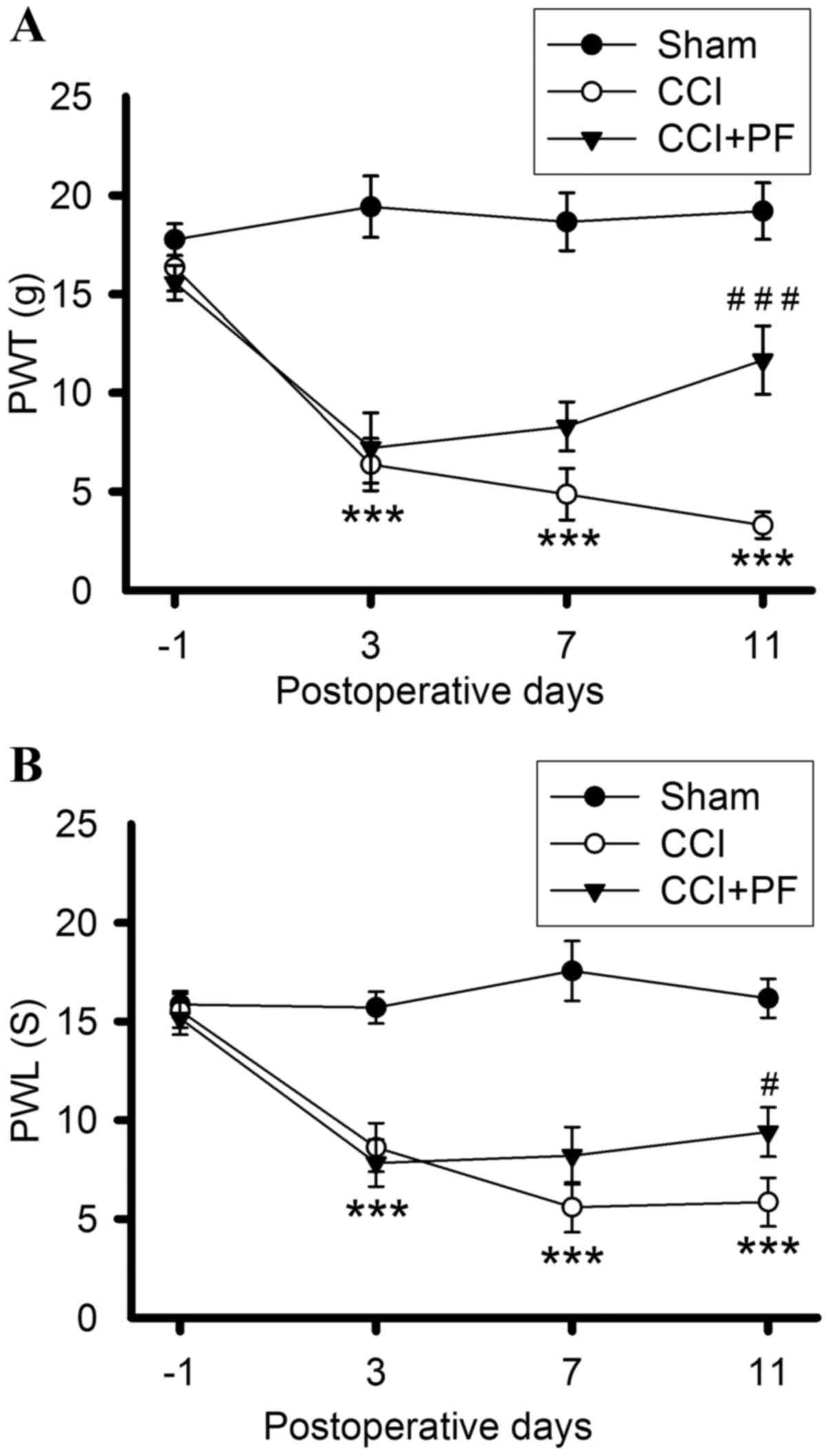
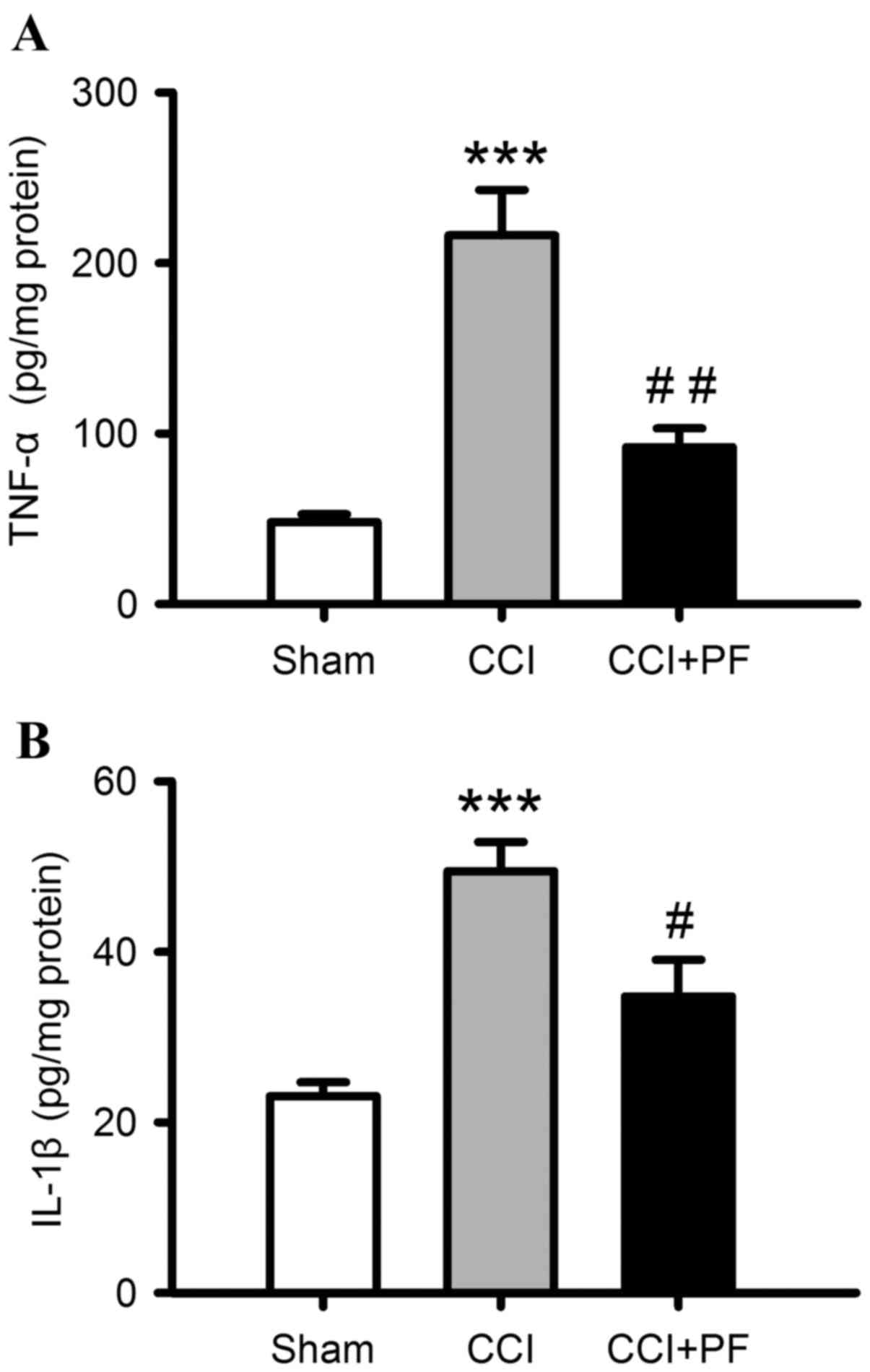
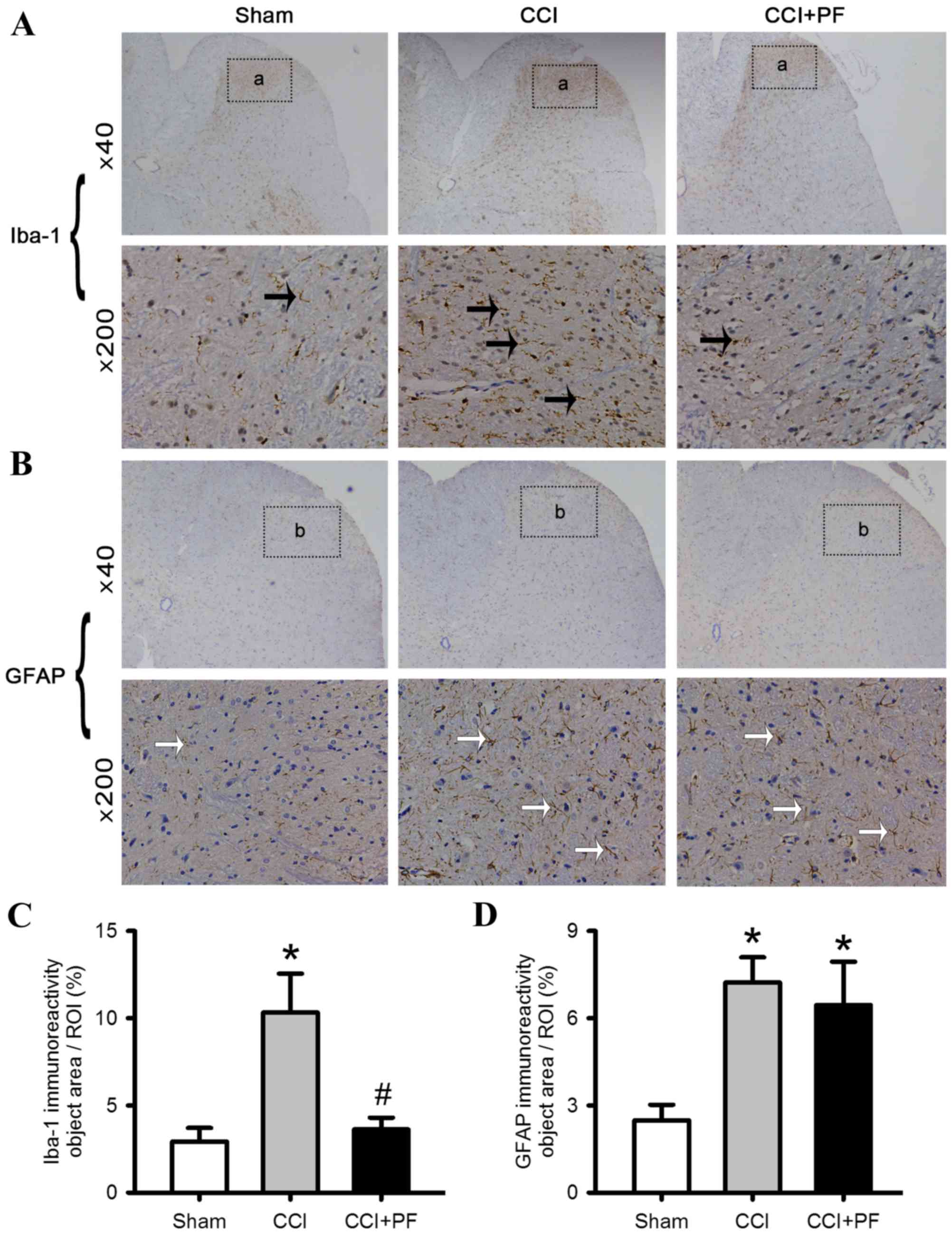
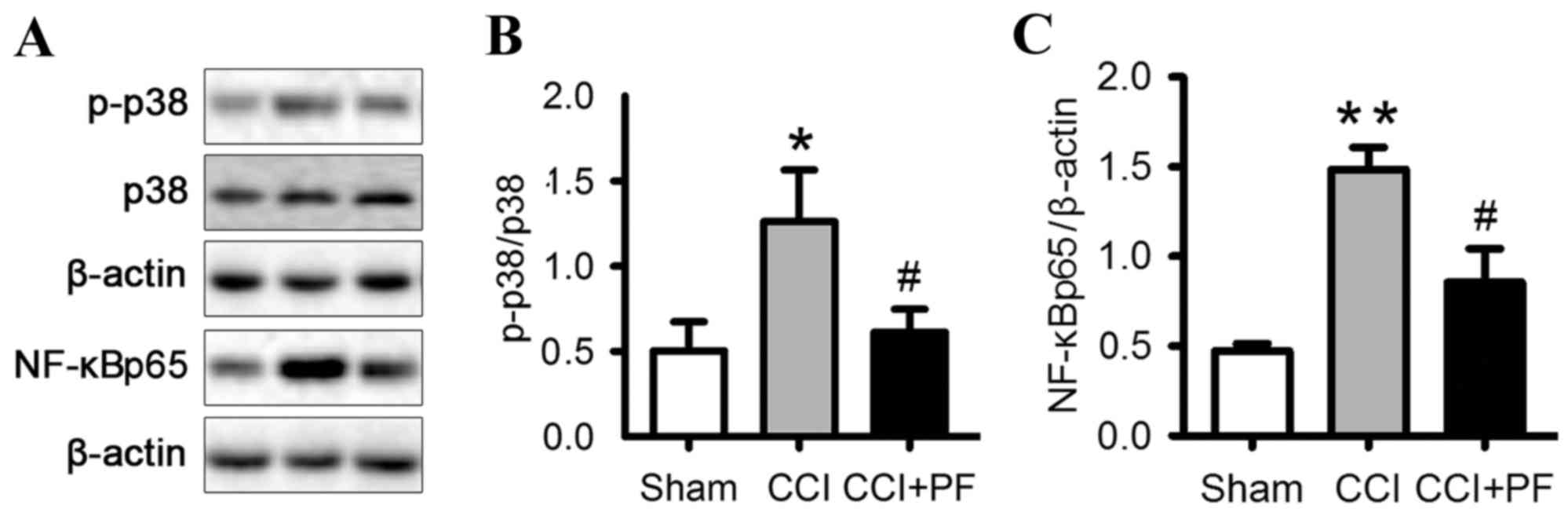
|
1
|
Gilron I, Watson CP, Cahill CM and Moulin
DE: Neuropathic pain: A practical guide for the clinician. CMAJ.
175:265–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cornelius VR, Sauzet O, Williams JE, Ayis
S, Farquhar-Smith P, Ross JR, Branford RA and Peacock JL: Adverse
event reporting in randomised controlled trials of neuropathic
pain: Considerations for future practice. Pain. 154:213–220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Streit WJ, Mrak RE and Griffin WS:
Microglia and neuroinflammation: A pathological perspective. J
Neuroinflammation. 1:142004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moalem G and Tracey DJ: Immune and
inflammatory mechanisms in neuropathic pain. Brain Res Rev.
51:240–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Myers RR, Campana WM and Shubayev VI: The
role of neuroinflammation in neuropathic pain: Mechanisms and
therapeutic targets. Drug Discov Today. 11:8–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wahba M and Waln O: Asterixis related to
gabapentin intake: A case report and review. Postgrad Med.
125:139–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marchand F, Perretti M and McMahon SB:
Role of the immune system in chronic pain. Nat Rev Neurosci.
6:521–532. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Milligan ED and Watkins LR: Pathological
and protective roles of glia in chronic pain. Nat Rev Neurosci.
10:23–36. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bradesi S: Role of spinal cord glia in the
central processing of peripheral pain perception.
Neurogastroenterol Motil. 22:499–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sweitzer SM, Schubert P and DeLeo JA:
Propentofylline, a glial modulating agent, exhibits antiallodynic
properties in a rat model of neuropathic pain. J Pharmacol Exp
Ther. 297:1210–1217. 2001.PubMed/NCBI
|
|
11
|
Jean YH, Chen WF, Sung CS, Duh CY, Huang
SY, Lin CS, Tai MH, Tzeng SF and Wen ZH: Capnellene, a natural
marine compound derived from soft coral, attenuates chronic
constriction injury-induced neuropathic pain in rats. Br J
Pharmacol. 158:713–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin YC, Huang SY, Jean YH, Chen WF, Sung
CS, Kao ES, Wang HM, Chakraborty C, Duh CY and Wen ZH: Intrathecal
lemnalol, a natural marine compound obtained from Formosan soft
coral, attenuates nociceptive responses and the activity of spinal
glial cells in neuropathic rats. Behav Pharmacol. 22:739–750. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YS, Park HJ, Kim TK, Moon DE and Lee
HJ: The effects of Ginkgo biloba extract EGb 761 on mechanical and
cold allodynia in a rat model of neuropathic pain. Anesth Analg.
108:1958–1963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao T, Hao J, Wiesenfeld-Hallin Z, Wang DQ
and Xu XJ: Analgesic effect of sinomenine in rodents after
inflammation and nerve injury. Eur J Pharmacol. 721:5–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Cheng H, Xu D, Yin Q, Cheng L,
Wang L, Song S and Zhang M: Attenuation of neuropathic pain by
saikosaponin a in a rat model of chronic constriction injury.
Neurochem Res. 39:2136–2142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu SH, Wu DG and Chen YW: Chemical
constituents and bioactivities of plants from the genus
Paeonia. Chem Biodivers. 7:90–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong SZ, Ge QH, Li Q, Qu R and Ma SP:
Peoniflorin attentuates Abeta(1–42)-mediated neurotoxicity by
regulating calcium homeostasis and ameliorating oxidative stress in
hippocampus of rats. J Neurol Sci. 280:71–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nam KN, Yae CG, Hong JW, Cho DH, Lee JH
and Lee EH: Paeoniflorin, a monoterpene glycoside, attenuates
lipopolysaccharide-induced neuronal injury and brain microglial
inflammatory response. Biotechnol Lett. 35:1183–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu YM, Jin R, Yang L, Zhang J, Yang Q, Guo
YY, Li XB, Liu SB, Luo XX and Zhao MG: Phosphatidylinositol 3
kinase/protein kinase B is responsible for the protection of
paeoniflorin upon H2O2-induced neural
progenitor cell injury. Neuroscience. 240:54–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu HQ, Zhang WY, Luo XT, Ye Y and Zhu XZ:
Paeoniflorin attenuates neuroinflammation and dopaminergic
neurodegeneration in the MPTP model of Parkinson's disease by
activation of adenosine A1 receptor. Br J Pharmacol. 148:314–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang HR, Peng JH, Cheng XB, Shi BZ, Zhang
MY and Xu RX: Paeoniflorin atttenuates amyloidogenesis and the
inflammatory responses in a transgenic mouse model of Alzheimer's
disease. Neurochem Res. 40:1583–1592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong H, Li R, Yu C, Xu T, Zhang X and Dong
M: Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis
in PC12 cells via suppressing reactive oxygen species-mediated
PKCδ/NF-κB pathway. Neuroscience. 285:70–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Wang J, Wang J, Wang P and Xue Y:
Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis
of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling
pathways. Brain Res. 1618:149–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen F, Lu HT and Jiang Y: Purification of
paeoniflorin from Paeonia lactiflora Pall. By high-speed
counter-current chromatography. J Chromatogr A. 1040:205–208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vierck CJ, Acosta-Rua AJ and Johnson RD:
Bilateral chronic constriction of the sciatic nerve: A model of
long-term cold hyperalgesia. J Pain. 6:507–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaggi AS, Jain V and Singh N: Animal
models of neuropathic pain. Fundam Clin Pharmacol. 25:1–28. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu LW, Chen JY, Yu KL, Cheng KI, Wu PC
and Wu BN: Neuroprotective and anti-inflammatory activities of
atorvastatin in a rat chronic constriction injury model. Int J
Immunopathol Pharmacol. 25:219–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LX and Wang ZJ: Animal and cellular
models of chronic pain. Adv Drug Deliv Rev. 55:949–965. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Y, Qiu HQ, Liu H, Liu M, Huang ZY, Yang
J, Su YP and Yu CX: Effects of koumine, an alkaloid of Gelsemium
elegans Benth., on inflammatory and neuropathic pain models and
possible mechanism with allopregnanolone. Pharmacol Biochem Behav.
101:504–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Costa B, Trovato AE, Colleoni M, Giagnoni
G, Zarini E and Croci T: Effect of the cannabinoid CB1 receptor
antagonist, SR141716, on nociceptive response and nerve
demyelination in rodents with chronic constriction injury of the
sciatic nerve. Pain. 116:52–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kiguchi N, Kobayashi Y and Kishioka S:
Chemokines and cytokines in neuroinflammation leading to
neuropathic pain. Curr Opin Pharmacol. 12:55–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kawasaki Y, Zhang L, Cheng JK and Ji RR:
Cytokine mechanisms of central sensitization: Distinct and
overlapping role of interleukin-1beta, interleukin-6, and tumor
necrosis factor-alpha in regulating synaptic and neuronal activity
in the superficial spinal cord. J Neurosci. 28:5189–5194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao H and Zhang YQ: Spinal glial
activation contributes to pathological pain states. Neurosci
Biobehav Rev. 32:972–983. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Obata K, Yamanaka H, Kobayashi K, Dai Y,
Mizushima T, Katsura H, Fukuoka T, Tokunaga A and Noguchi K: Role
of mitogen-activated protein kinase activation in injured and
intact primary afferent neurons for mechanical and heat
hypersensitivity after spinal nerve ligation. J Neurosci.
24:10211–10222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu JT, Xin WJ, Wei XH, Wu CY, Ge YX, Liu
YL, Zang Y, Zhang T, Li YY and Liu XG: p38 activation in uninjured
primary afferent neurons and in spinal microglia contributes to the
development of neuropathic pain induced by selective motor fiber
injury. Exp Neurol. 204:355–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu L, Huang Y, Yu X, Yue J, Yang N and Zuo
P: The influence of p38 mitogen-activated protein kinase inhibitor
on synthesis of inflammatory cytokine tumor necrosis factor alpha
in spinal cord of rats with chronic constriction injury. Anesth
Analg. 105:1838–1844, table of contents. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji RR and Suter MR: p38 MAPK, microglial
signaling, and neuropathic pain. Mol Pain. 3:332007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rojewska E, Popiolek-Barczyk K, Jurga AM,
Makuch W, Przewlocka B and Mika J: Involvement of pro- and
antinociceptive factors in minocycline analgesia in rat neuropathic
pain model. J Neuroimmunol. 277:57–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM and
Yao SL: Alleviation of neuropathic pain by intrathecal injection of
antisense oligonucleotides to p65 subunit of NF-kappaB. Br J
Anaesth. 97:553–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Makarov SS: NF-kappaB as a therapeutic
target in chronic inflammation: Recent advances. Mol Med Today.
6:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Niederberger E and Geisslinger G: The
IKK-NF-kappaB pathway: A source for novel molecular drug targets in
pain therapy. FASEB J. 22:3432–3442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Laughlin TM, Bethea JR, Yezierski RP and
Wilcox GL: Cytokine involvement in dynorphin-induced allodynia.
Pain. 84:159–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wei XH, Yang T, Wu Q, Xin WJ, Wu JL, Wang
YQ, Zang Y, Wang J, Li YY and Liu XG: Peri-sciatic administration
of recombinant rat IL-1β induces mechanical allodynia by activation
of src-family kinases in spinal microglia in rats. Exp Neurol.
234:389–397. 2012. View Article : Google Scholar : PubMed/NCBI
|