Occupational and/or environmental exposure to some
types of asbestos fibers is related with both malignant and
non-malignant pulmonary diseases (1–3), of
which malignant mesothelioma (MM) and lung cancer are the most
typical ones (4). Instead,
non-malignant asbestos-related diseases include pleural plaques
(PPs), pleural effusions, diffuse pleural thickening and
parenchymal fibrosis (5–8).
In the 1990s, a greatly increased standardized rate
of mortality from MM was observed after epidemiological studies in
the municipality of Biancavilla (Sicily, Italy) (17,18).
Later studies spotted an asbestiform mineral fibre, called
fluoro-edenite (FE), in the lava rocks excavated from a local stone
quarry. The derived material had been used locally for about 50
years for building (17–19). The quarry was shut down in 1998
(20). Following some in
vitro, in vivo and epidemiological studies (21–25)
the IARC (Lyon, France) classified FE as carcinogenic to humans,
but only for MM (26).
The relationship between exposure to asbestos and
adverse health effects has been extensively studied (27). These asbestos-related diseases are
well documented and approximately 107,000 mortalities are
attributable to exposure to fiber worldwide, annually (28–30).
Little has been established with regard to FE-related diseases
(31–38); however, epidemiological studies
have shown that these fibers may cause chronic obstructive lung
disease (18,39), PPs and MMs (17,39).
These pathologies are the result of imbalanced
inflammatory processes that is the early response to inhaled
fibers. Historically, immune system cells have been regarded as the
main players in initiating acute or chronic inflammation. However,
recent studies have shown that epithelial cells of the respiratory
tract and mesothelial cells lining the body cavities are capable of
initiating inflammatory events after exposure to pathogenic fibers
in the absence of cells of the immune system (40). The aim of this study was to detect
FE immunotoxicity pathways in a group of occupationally exposed
construction workers (CW), to find any biological markers of its
effect.
The research protocol received the approval of the
Ethics Committee of Catania University Hospital (Catania, Italy)
and the written informed consent of all subjects was acquired
including them in the study.
Thirty-eight construction workers residing and
working in the area of Biancavilla (Sicily, Italy) were invited to
participate in this study. In the same period, 38 construction
workers living and working least 40 km away the area of Biancavilla
were recruited as control group. Exclusion criteria were thoracic
diseases (e.g. asthma, bronchopneumonia, and tuberculosis),
previous exposure to asbestos and involvement in construction work
in the Biancavilla area for <1 year (the latter only for exposed
subjects). A questionnaire was used to obtain information on
medical and occupational history, use of medications, smoking and
drinking habits. A free medical check, including spirometry and a
high-resolution computer tomography (HRCT) chest scan, was given to
all workers.
Respiratory function tests were carried out using a
bell spirometer (Biomedin, Padova, Italy). Equipment, calibration
and maneuvers were in conformity with the American Thoracic Society
(ATS) guidelines. Forced vital capacity, forced expiratory volume
in 1 sec, peak expiratory flow, maximal expiratory flow rate at
25–75% of the vital capacity, total lung capacity and TLCO were
assessed and expressed as a proportion of the European Coal and
Steel Community reference values, adjusted to individual
characteristics (age, weight and height) checked at the time of
testing.
Subjects underwent HRCT total chest scanning using
an Optima CT 580W (GE Healthcare, Fairfield, CT, USA) without
contrast medium. Interstitial and/or pleural abnormalities were
recorded. Pleural plaques (PPs) are circumscribed quadrangular
elevations with sharp borders and density comparable to tissue,
with/without signs of calcification and expressed in terms of
frequency and percentage. Parenchymal abnormalities (PA)
(subpleural dependent opacity, subpleural curvilinear opacities,
subpleaural perpendicular lines, parenchymal nodules, honeycombing
and ground glass opacities) were expressed in terms of frequency
and percentage.
Venous blood (10 ml) was collected in the morning,
following overnight fasting, to determine red blood cell count,
haematocrit, haemoglobin levels, white blood cell count,
erythrocyte sedimentation rate, C-reactive protein levels and liver
enzyme (aspartate aminotransferase and alanine aminotransferase)
levels. For cytokine detection, blood samples were drawn into
vacuum tubes with gel and clot activator (Vacuette, Greiner
Bio-One, Kremsmünster, Austria) before analysis. After collection,
the tubes were left in an upright position for at least 30 min at
room temperature but no more than 60 min. Samples were then
centrifuged at 3500 rpm for 10 min, then serum was separated and
stored at −20°C until analysis. Serum interleukin-1β (IL-1β), IL-6,
IL-8 and tumor necrosis factors-α (TNF-α) were measured using
highly sensitive quantitative sandwich assays (Quantikine ELISA
kit, R&D Systems, Minneapolis, MN, USA). The instruments were
adjusted and internal quality control was performed using the same
lot of the manufacturer's control and calibration material
throughout the study.
Data were summarized as mean ± SD for continuous
variables and frequencies for categorical variables. Normality was
checked by Kolmogrov-Smirnov test and homogeneity of variance by
Levene's test. T-test was used for analyzed continuous variance,
Fischer's test for categorical variables. Statistical analyses were
performed by SPSS ver. 22 (IBM, Armonk, NY, USA) and GraphPad Prism
ver. 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Application of the exclusion criteria did not cause
any subject to be excluded from the sample. The main sample
characteristics are reported in Table
I. All 38 FE exposed CWs had been living in Biancavilla for
>35 years, and 74% (n=28) were born there. Besides, all had been
working almost exclusively in and around Biancavilla. As to their
occupational history, 21 (55%) of the participants revealed they
had personally handled and worked with gravel excavated from Mount
Calvario quarry until 1998. Furthermore, all had been involved in
restoring houses dating back to the 1950s, when the lava from the
quarry had been widely used as a building material.
Age, body mass index, smoking habits, alcohol
consumption and working age did not differ between exposed and
control. Blood examination tests were within the normal range in
all subjects. Functional respiratory tests were within the normal
range for all participants. A restrictive ventilatory defect was
detected in two subjects (5%) and an obstructive ventilatory defect
observed in one (3%) of the exposed group. Control subjects were
all in the normal range. TLCO was less in two additional
participants of exposed group (data not shown).
A number of observations show that altered immune
responses are also important in asbestos respiratory toxicity
(41). Some of the major cytokines
and growth factors involved in the pathogenesis of lung diseases
include IL-1, TNF-α, transforming growth factor-β (TGF-β), platelet
derived growth factor (PDGF) and IL-8. These agents augment
cellular injury and trigger fibroblast proliferation and collagen
deposition. Although alveolar macrophages (AM) are thought to be
the primary source of these proteins, a previous molecular study
suggests that pulmonary epithelial cells are also involved
(42). Further evidence suggests
that TNF-α is a key cytokine involved in asbestos induced lung
toxicity (43,44). TNF-α can increase the levels of
alveolar type II cell Macrophage Inflammatory Proteins-1α (MIP-1α)
mRNA, which suggests that TNF-α can promote pulmonary inflammation
through its effects on epithelial cells (45).
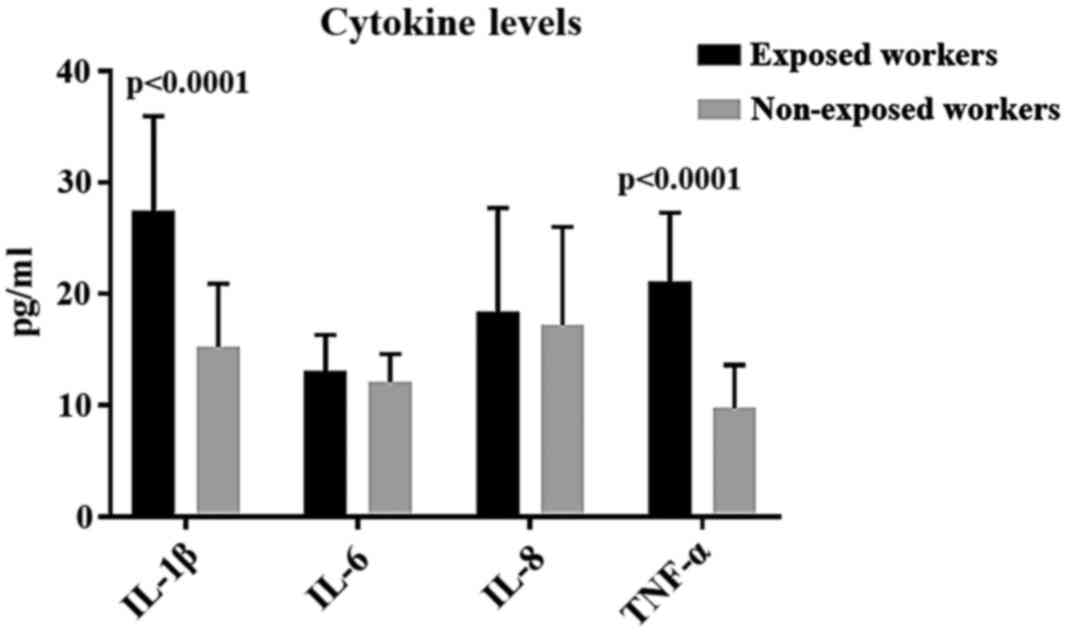
In our study, significantly higher levels of TNF-α
were detected in FE-exposed subjects, compared to the control ones.
Simeonova and colleagues (46,47)
found out that asbestos and H2O2 both
activate NF-κB and NF-IL-6 in A549 and normal human bronchial
epithelial cells which, in turn, stimulate IL-6 and IL-8 gene
expression and protein release. A function for reactive oxygen
species (ROS) was clarified by their observation that HO.
scavengers and N-acetylcysteine (NAC) each decreased asbestos or
H2O2 induced NF-κB and NF-IL-6 activity as
well as IL-8 and IL-6 protein expression. In vitro studies
conducted by Travaglione et al (48,49),
demonstrated that FE interfered with epithelial cell physiology, by
reducing the proliferation rate without perturbing the cell cycle
and increasing the release of pro-inflammatory cytokines IL-6 and
IL-8, from pulmonary epithelial cells.
In our study, FE-exposed subjects showed increased
levels of IL-6 and IL-8, although in a non-statistically relevant
way. Higher levels of IL-6, IL-8 and TNF-α, have been observed more
recurrently overexpressed in the microenvironment of mesothelial
cells during neoplastic transformation (50–52).
The continuous stimulation of mesothelial cells from FE fibers
could account for the increased values of these cytokines,
particularly the increase of TNF-α, that is involved in the
resistance against fiber toxicity (53). As a matter of fact, significantly
greater quantities of PPs were observable in exposed subjects than
in the control groups 25 (66%) vs. 2 (5%). PPs can arise even after
relatively low exposure to asbestiform fibers and are the most
common non-malignant effects (31–33,35,54–57).
Most research on the role of inflammation in
asbestos-related diseases has been centred on immune cellular
response, including the first cell type accumulating at sites of
initial deposition of inhaled asbestos fibres (42). When the injury occurs, mesothelial
cells can recruit neutrophils, monocytes and lymphocytes by
generating chemokines and cytokines which cause mesothelial cells
to release growth factors with paracrine functions (50–52,58–60).
New evidence on human mesothelial cells sustains a model where an
autocrine loop is perpetrated by fibre-induced inflammasone NLRP3
(NLR family pyrin domain containing 3) priming and activation, with
the subsequent augmented pro-inflammatory growth factors
transcription activity (61–64).
The inflammasome is a multiprotein complex that
links proIL-1β to its upstream activator. This complex is composed
of Pycard, NALP1, caspase-1 and likely caspase-5. It is expressed
in myeloid cells and is a component of the immune system (65). The IL-1 super-family of cytokines
encompasses at least 11 members, which include IL-1α, IL-1β, and
IL-18 (66). The inflammasome is
responsible for the activation of inflammatory processes (65) and has been shown to induce
caspase-1 dependent pyroptosis which is a form of cell death
characterized by both apoptosis and necrosis (67).
Procaspase-1 is recruited to the inflammasome
complex and processed to active caspase-1, which processes IL-1 and
IL-18 into their active forms (65,68).
This can account for the high serum levels of IL-1β detected in
FE-exposed workers compared to control ones. Besides, in a previous
study carried out on CWs in Biancavilla, high levels of IL-18 were
observed which well correlated with the presence of PPs and lung
parenchimal fibrosis (31). IL-1β
helps T-cell survival, B-cell proliferation and antibody
production, as well as mediating leukocyte transmigration (69,70).
IL-1β roles in carcinogenicity entails recruitment of
myeloid-derived suppressor cells (MDSCs) that prevent natural
killer (NK) cells from developing and functioning (71). IL-18 can work synergistically with
IL-12 to induce interferon-γ (IFNγ) production by activating T- and
NK-cells (69–70,72).
The pro-inflammatory cytokine IL-1β, which can be
inflammasome-dependent, is a critical mediator of
inflammation-promoting carcinogenesis (73–76)
and fibrosis (77,78). Cell death mechanisms such as
apoptosis and necrosis and release of chemokines/cytokines and
alarmines such as HMGB1 and TNF-α, may help cancer regress, resist
toxicity by fibers and cell growth (62,79),
in inflammasome-dependent and -independent pathways (80). The transcription factors, activator
protein-1 (AP-1) and NF-κB that may change inflammation-induced
tumor growth into cancer regression (79), are also signals inducing the
transcription or priming of pro-IL-1β.
The induction of caspase-1 dependent pyroptosis, a
type of cell death characterized by both apoptotic and necrotic
features, is another important function of inflammasomes (81). This progression features nuclear
DNA fragmentation, plasma membrane rupture and release of
inflammatory mediators such as IL-1α, IL-1β, IL-33 and HMGB1 which
play significant roles in inflammatory processes (72,81).
Consequently, the inflammasome and inflammation can promote
proliferation and maturation of target cells and immune suppression
in tumors (82). It is assembled
in response to a wide range of conserved exogenous molecules
including asbestos (83).
Multifaceted inflammasomes are critical in sensing and responding
to a variety of extracellular and intracellular stresses through
several pathways and may be important in both pulmonary defense and
promulgation of chronic inflammation leading to pathologies
(40).
In the lungs, highly inflammatory cytokines of the
IL-1 family, including IL-1β, are key factors to inflammation. For
instance, IL-6, a pleiotropic inflammatory cytokine frequently
released in tandem with IL-1β, is a key growth-promoting and
anti-apoptotic factor that is also involved in immune regulation
and inflammation (84,85). IL-6 is frequently expressed in
malignant respiratory epithelial cells, and high circulating levels
in serum are related to a poor prognosis in lung cancer patients
(86,87). Its potential use as a biomarker and
prognostic indicator is bolstered by the fact that it is not
detected in the serum of healthy individuals and patients with
benign lung pathologies (40).
Comar et al (88) observed
significant high levels of IL-6 in MM patients. This is in line
with what we observed with regard to the increased level of IL-6 in
the exposed group compared to the controls, although not
significantly; this may derive from mesothelial involvement,
although without cancer lesions.
Asbestiform fibers can trigger and activate the
NLRP3 inflammasome in mesothelial and epithelial cells, as well as
in cells of the immune system via multiple pathways. Inflammasome
priming and activation may play vital roles in both early lung and
pleural injury as well as in inflammation, tumor initiation and
promotion. TNF-α, a protein implicated in both proliferation of
mesothelial cells and prevention of asbestos-induced injury
(89,90), it may also stimulate MM cell
survival through the induction of genes encoding NF-κB-dependent
anti-apoptotic molecules (91,92)
and activate chemo-resistance. TNF-α receptors are regulated by
both TNF-α and IL-1α in human mesothelial cells (58) that synthesize and release both
IL-1β (93) and IL-1α after injury
(67,68). The fact that the chemotactic and
autocrine growth factor IL-8 (94–97)
is generated by human mesothelial cells in response to TNF-α and
IL-1 released by macrophages or after exposure to asbestos
(98), supports the concept that
inflammasome-mediated mature 1L-1β release is essential to the
production of other cytokines and chemokines critical to the
development of MMs (40).
In our study, the observation of significantly high
levels of TNF-α in exposed subjects could be accounted for with the
resistance action against the stimulus deriving from inhaling FE
fibers. As described for asbestos, it is conceivable that FE may
promote the NLRP3 inflammasome in macrophages, monocytes and lung
epithelial cells, leading to IL-1β secretion (91,92).
Increased production of TGF-β, a hallmark of inflammation and the
fibrotic process, triggers the activation, proliferation and
trans-differentiation of epithelial cells and resident
fibroblasts into collagen-producing myofibroblasts. This has been
put down to inflammasome-induced secretion of mature IL-1β
(98). IL-1β also triggers the
secretion of neutrophil-attracting CXC chemokines, resulting in a
further influx of neutrophils that amplify cell damage and oxidant
release (99). Unlike macrophages
and monocytes, NADPH oxidase-derived ROS are neither required for
inflammasome priming nor activation by human neutrophils, but are
necessary for exporting mature IL-1β from the cell (100). IL-1β secretion by AMs is enhanced
by fibrogenic agents that also raise production of PDGF, a
growth-promoting cytokine and chemo-tactic factor (101).
A recent immunohistochemical study, conducted in
lung tissue of sheep exposed to FE fibers showed a significant
activation of AM and mast cells (102). An in vitro study on
mesothelial Met-5A and monocyte-macrophage J774 cells exposed to FE
caused induction of the heat shock protein 70 (Hsp70), stimulated
formation of ROS and NO. (measured as nitrite). Exposure of cells
to FE induced lactate dehydrogenase activity and decreased cell
viability (103). It is
conceivable that FE may trigger inflammatory processes that, like
in the case of asbestos, may lead to the activation of
inflammosome.
In this study, as well as in a previous one
(31) on IL-18, where high IL-1β serum levels have been
detected, both cytokines are primarily involved in the inflammosome
activation process. Our results suggest that it is necessary to go
on with research on immune-modulators involved in the pathogenic
mechanisms responsible for FE related diseases.
|
1
|
Alberg AJ and Samet JM: Epidemiology of
lung cancer. Chest. 123:(Suppl). 21S–49S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
LaDou J: The asbestos cancer epidemic.
Environ Health Perspect. 112:285–290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujimoto N, Gemba K, Aoe K, Kato K,
Yokoyama T, Usami I, Onishi K, Mizuhashi K, Yusa T and Kishimoto T:
Clinical investigation of benign asbestos pleural effusion. Pulm
Med. 2015:4161792015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prazakova S, Thomas PS, Sandrini A and
Yates DH: Asbestos and the lung in the 21st century: An update.
Clin Respir J. 8:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hillerdal G: Non-malignant asbestos
pleural disease. Thorax. 36:669–675. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guidotti TL, Miller A, Christiani D,
Wagner G, Balmes J, Harber P, Brodkin CA, Rom W, Hillerdal G,
Harbut M, et al: American Thoracic Society: Diagnosis and initial
management of nonmalignant diseases related to asbestos. Am J
Respir Crit Care Med. 170:691–715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cugell DW and Kamp DW: Asbestos and the
pleura: A review. Chest. 125:1103–1117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gevenois PA, de Maertelaer V, Madani A,
Winant C, Sergent G and De Vuyst P: Asbestosis, pleural plaques and
diffuse pleural thickening: Three distinct benign responses to
asbestos exposure. Eur Respir J. 11:1021–1027. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bayram M, Dongel I, Bakan ND, Yalçin H,
Cevit R, Dumortier P and Nemery B: High risk of malignant
mesothelioma and pleural plaques in subjects born close to
ophiolites. Chest. 143:164–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanarek MS: Mesothelioma from chrysotile
asbestos: Update. Ann Epidemiol. 21:688–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen J, de Klerk NH, Eccles JL, Musk AW
and Hobbs MS: Malignant mesothelioma after environmental exposure
to blue asbestos. Int J Cancer. 54:578–581. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Constantopoulos SH: Environmental
mesothelioma associated with tremolite asbestos: Lessons from the
experiences of Turkey, Greece, Corsica, New Caledonia and Cyprus.
Regul Toxicol Pharmacol. 52:(Suppl 1). S110–S115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voisin C, Marin I, Brochard P and Pairon
JC: Environmental airborne tremolite asbestos pollution and pleural
plaques in Afghanistan. Chest. 106:974–976. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schüz J, Schonfeld SJ, Kromhout H, Straif
K, Kashanskiy SV, Kovalevskiy EV, Bukhtiyarov IV and McCormack V: A
retrospective cohort study of cancer mortality in employees of a
Russian chrysotile asbestos mine and mills: Study rationale and key
features. Cancer Epidemiol. 37:440–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sullivan PA: Vermiculite, respiratory
disease, and asbestos exposure in Libby, Montana: Update of a
cohort mortality study. Environ Health Perspect. 115:579–585. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szychlinska MA, Parenti R, Loreto C,
Salvatorelli L, Spadola S, Trovato FM, Pirri C, Rapisarda V, Pace
MM, Magro G, et al: Fluoro edenite-associated pathogenesis in
pleural malignant mesothelioma. Acta Med Mediter. 30:981–989.
2014.
|
|
17
|
Paoletti L, Batisti D, Bruno C, Di Paola
M, Gianfagna A, Mastrantonio M, Nesti M and Comba P: Unusually high
incidence of malignant pleural mesothelioma in a town of eastern
Sicily: An epidemiological and environmental study. Arch Environ
Health. 55:392–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Paola M, Mastrantonio M and Carboni M:
Mortality from malignant pleural neoplasms in Italy in the years
1988–1992ISTISAN. 96. Rome: pp. 1–30. 1996
|
|
19
|
Comba P, Gianfagna A and Paoletti L:
Pleural mesothelioma cases in Biancavilla are related to a new
fluoro-edenite fibrous amphibole. Arch Environ Health. 58:229–232.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miozzi E, Rapisarda V, Marconi A, Costa C,
Polito I, Spandidos DA, Libra M and Fenga C: Fluoro-edenite and
carbon nanotubes: The health impact of ‘asbestos-like’ fibres. Exp
Ther Med. 11:21–27. 2016.PubMed/NCBI
|
|
21
|
Ballan G, Del Brocco A, Loizzo S, Fabbri
A, Maroccia Z, Fiorentini C and Travaglione S: Mode of action of
fibrous amphiboles: The case of Biancavilla (Sicily, Italy). Ann
Ist Super Sanita. 50:133–138. 2014.PubMed/NCBI
|
|
22
|
DeNardo P, Bruni B, Paoletti L, Pasetto R
and Sirianni B: Pulmonary fibre burden in sheep living in the
Biancavilla area (Sicily): Preliminary results. Sci Total Environ.
325:51–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loreto C, Rapisarda V, Carnazza ML,
Musumeci G, Valentino M, Fenga C and Martinez G: Fluoro-edenite
fibres induce lung cell apoptosis: An in vivo study. Histol
Histopathol. 23:319–326. 2008.PubMed/NCBI
|
|
24
|
Martinez G, Loreto C, Rapisarda V,
Masumeci G, Valentino M and Carnazza ML: Effects of exposure to
fluoro-edenite fibre pollution on the respiratory system: An in
vivo model. Histol Histopathol. 21:595–601. 2006.PubMed/NCBI
|
|
25
|
Soffritti M, Minardi F, Bua L, Esposti
Degli D and Belpoggi F: First experimental evidence of peritoneal
and pleural mesotheliomas induced by fluoro-edenite fibres present
in etnean volcanic material from Biancavilla (Sicily, Italy). Eur J
Oncol. 9:169–175. 2004.
|
|
26
|
Grosse Y, Loomis D, Guyton KZ,
Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L,
Guha N, Scoccianti C, Mattock H, et al: International Agency for
Research on Cancer Monograph Working Group: Carcinogenicity of
fluoro-edenite, silicon carbide fibres and whiskers, and carbon
nanotubes. Lancet Oncol. 15:1427–1428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bernstein D, Dunnigan J, Hesterberg T,
Brown R, Velasco JAL, Barrera R, Hoskins J and Gibbs A: Health risk
of chrysotile revisited. Crit Rev Toxicol. 43:154–183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robinson BWS and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
World Health Organization (WHO): Asbestos:
elimination of asbestos-related diseases. http://www.who.int/mediacentre/factsheets/fs343/en/Accessed.
January 21–2017.
|
|
30
|
Ledda C and Rapisarda V: Malignant pleural
mesothelioma: The need to move from research to clinical practice.
Arch Med Res. 47:4072016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ledda C, Loreto C, Matera S, Massimino N,
Cannizzaro E, Musumeci A, Migliore M, Fenga C, Pomara C and
Rapisarda V: Early effects of fluoro-edenite: Correlation between
IL-18 serum levels and pleural and parenchymal abnormalities.
Future Oncol. 12:59–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ledda C, Loreto C, Bracci M, Mangano D,
Migliore M, Ricceri V, Musumeci A, Costa C, Pomara C and Rapisarda
V: High risk of pleural plaques and parenchymal abnormalities in
women living in Biancavilla (Italy). Future Oncol. 12:63–65. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ledda C, Pomara C, Bracci M, Mangano D,
Ricceri V, Musumeci A, Ferrante M, Musumeci G, Loreto C, Fenga C,
et al: Natural carcinogenic fiber and pleural plaques assessment in
a general population: A cross-sectional study. Environ Res.
150:23–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ledda C, Loreto C, Pomara C, Rapisarda G,
Fiore M, Ferrante M, Bracci M, Santarelli L, Fenga C and Rapisarda
V: Sheep lymph-nodes as a biological indicator of environmental
exposure to fluoro-edenite. Environ Res. 147:97–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rapisarda V, Ledda C, Migliore M, Salemi
R, Musumeci A, Bracci M, Marconi A, Loreto C and Libra M: FBLN-3 as
a biomarker of pleural plaques in workers occupationally exposed to
carcinogenic fibers: A pilot study. Future Oncol. 11:(Suppl).
35–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fazzo L, Minelli G, De Santis M, Bruno C,
Zona A, Marinaccio A, Conti S, Pirastu R and Comba P: Mesothelioma
mortality surveillance and asbestos exposure tracking in Italy. Ann
Ist Super Sanita. 48:300–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Loreto C, Carnazza ML, Cardile V, Libra M,
Lombardo L, Malaponte G, Martinez G, Musumeci G, Papa V and Cocco
L: Mineral fiber-mediated activation of phosphoinositide-specific
phospholipase c in human bronchoalveolar carcinoma-derived alveolar
epithelial A549 cells. Int J Oncol. 34:371–376. 2009.PubMed/NCBI
|
|
38
|
Musumeci G, Loreto C, Cardile V, Carnazza
ML and Martinez G: Immunohistochemical expression of retinoblastoma
and phospho-retinoblastoma protein in sheep lung exposed to
fluoro-edenite fibers. Anat Sci Int. 85:74–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Biggeri A, Pasetto R, Belli S, Bruno C, Di
Maria G, Mastrantonio M, Trinca S, Uccelli R and Comba P: Mortality
from chronic obstructive pulmonary disease and pleural mesothelioma
in an area contaminated by natural fiber (fluoro-edenite). Scand J
Work Environ Health. 30:249–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sayan M and Mossman BT: The NLRP3
inflammasome in pathogenic particle and fibre-associated lung
inflammation and diseases. Part Fibre Toxicol. 13:512016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Churg A, Sun J and Zay K: Cigarette smoke
increases amosite asbestos fiber binding to the surface of tracheal
epithelial cells. Am J Physiol. 275:L502–508. 1998.PubMed/NCBI
|
|
42
|
Kamp DW and Weitzman SA: The molecular
basis of asbestos induced lung injury. Thorax. 54:638–652. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Irani K, Xia Y, Zweier JL, Sollott SJ, Der
CJ, Fearon ER, Sundaresan M, Finkel T and Goldschmidt-Clermont PJ:
Mitogenic signaling mediated by oxidants in Ras-transformed
fibroblasts. Science. 275:1649–1652. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Manna SK, Zhang HJ, Yan T, Oberley LW and
Aggarwal BB: Overexpression of manganese superoxide dismutase
suppresses tumor necrosis factor-induced apoptosis and activation
of nuclear transcription factor-kappaB and activated protein-1. J
Biol Chem. 273:13245–13254. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Byrnes RW: Evidence for involvement of
multiple iron species in DNA single-strand scission by H2O2 in
HL-60 cells. Free Radic Biol Med. 20:399–406. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Simeonova PP and Luster MI: Iron and
reactive oxygen species in the asbestos-induced tumor necrosis
factor-alpha response from alveolar macrophages. Am J Respir Cell
Mol Biol. 12:676–683. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Simeonova PP, Toriumi W, Kommineni C,
Erkan M, Munson AE, Rom WN and Luster MI: Molecular regulation of
IL-6 activation by asbestos in lung epithelial cells: Role of
reactive oxygen species. J Immunol. 159:3921–3928. 1997.PubMed/NCBI
|
|
48
|
Travaglione S, Bruni BM, Falzano L,
Filippini P, Fabbri A, Paoletti L and Fiorentini C: Multinucleation
and pro-inflammatory cytokine release promoted by fibrous
fluoro-edenite in lung epithelial A549 cells. Toxicol In Vitro.
20:841–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Travaglione S, Bruni B, Falzano L,
Paoletti L and Fiorentini C: Effects of the new-identified
amphibole fluoro-edenite in lung epithelial cells. Toxicol In
Vitro. 17:547–552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adachi Y, Aoki C, Yoshio-Hoshino N,
Takayama K, Curiel DT and Nishimoto N: Interleukin-6 induces both
cell growth and VEGF production in malignant mesotheliomas. Int J
Cancer. 119:1303–1311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Galffy G, Mohammed KA, Nasreen N, Ward MJ
and Antony VB: Inhibition of interleukin-8 reduces human malignant
pleural mesothelioma propagation in nude mouse model. Oncol Res.
11:187–194. 1999.PubMed/NCBI
|
|
52
|
Galffy G, Mohammed KA, Dowling PA, Nasreen
N, Ward MJ and Antony VB: Interleukin 8: An autocrine growth factor
for malignant mesothelioma. Cancer Res. 59:367–371. 1999.PubMed/NCBI
|
|
53
|
Luo JL, Maeda S, Hsu LC, Yagita H and
Karin M: Inhibition of NF-kappaB in cancer cells converts
inflammation-induced tumor growth mediated by TNFalpha to
TRAIL-mediated tumor regression. Cancer Cell. 6:297–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Clin B, Paris C, Ameille J, Brochard P,
Conso F, Gislard A, Laurent F, Letourneux M, Luc A, Schorle E, et
al: Do asbestos-related pleural plaques on HRCT scans cause
restrictive impairment in the absence of pulmonary fibrosis?
Thorax. 66:985–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Laurent F, Paris C, Ferretti GR, Beigelman
C, Montaudon M, Latrabe V, Jankowski A, Badachi Y, Clin B, Gislard
A, et al: Inter-reader agreement in HRCT detection of pleural
plaques and asbestosis in participants with previous occupational
exposure to asbestos. Occup Environ Med. 71:865–870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rapisarda V, Ledda C, Ricceri V, Arena F,
Musumeci A, Marconi A, Fago L, Bracci M, Santarelli L and Ferrante
M: Detection of pleural plaques in workers exposed to inhalation of
natural fluoro-edenite fibres. Oncol Lett. 9:2046–2052.
2015.PubMed/NCBI
|
|
57
|
Maxim LD, Niebo R and Utell MJ: Are
pleural plaques an appropriate endpoint for risk analyses? Inhal
Toxicol. 27:321–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang Y, Faux SP, Hallden G, Kirn DH,
Houghton CE, Lemoine NR and Patrick G: Interleukin-1β and tumour
necrosis factor-α promote the transformation of human immortalised
mesothelial cells by erionite. Int J Oncol. 25:173–178.
2004.PubMed/NCBI
|
|
59
|
Zanella CL, Posada J, Tritton TR and
Mossman BT: Asbestos causes stimulation of the extracellular
signal-regulated kinase 1 mitogen-activated protein kinase cascade
after phosphorylation of the epidermal growth factor receptor.
Cancer Res. 56:5334–5338. 1996.PubMed/NCBI
|
|
60
|
Mantovani A, Allavena P, Sozzani S, Vecchi
A, Locati M and Sica A: Chemokines in the recruitment and shaping
of the leukocyte infiltrate of tumors. Semin Cancer Biol.
14:155–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Eisenbarth SC and Flavell RA: Innate
instruction of adaptive immunity revisited: The inflammasome. EMBO
Mol Med. 1:92–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dostert C, Pétrilli V, Van Bruggen R,
Steele C, Mossman BT and Tschopp J: Innate immune activation
through Nalp3 inflammasome sensing of asbestos and silica. Science.
320:674–677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hillegass JM, Miller JM, MacPherson MB,
Westbom CM, Sayan M, Thompson JK, Macura SL, Perkins TN, Beuschel
SL, Alexeeva V, et al: Asbestos and erionite prime and activate the
NLRP3 inflammasome that stimulates autocrine cytokine release in
human mesothelial cells. Part Fibre Toxicol. 10:392013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fox SA, Loh SS, Mahendran SK and Garlepp
MJ: Regulated chemokine gene expression in mouse mesothelioma and
mesothelial cells: TNF-α upregulates both CC and CXC chemokine
genes. Oncol Rep. 28:707–713. 2012.PubMed/NCBI
|
|
65
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-β. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fink SL and Cookson BT: Apoptosis,
pyroptosis, and necrosis: Mechanistic description of dead and dying
eukaryotic cells. Infect Immun. 73:1907–1916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Agostini L, Martinon F, Burns K, McDermott
MF, Hawkins PN and Tschopp J: NALP3 forms an IL-1β-processing
inflammasome with increased activity in Muckle-Wells
autoinflammatory disorder. Immunity. 20:319–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dinarello CA: Interleukin-1 in the
pathogenesis and treatment of inflammatory diseases. Blood.
117:3720–3732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sims JE and Smith DE: The IL-1 family:
Regulators of immunity. Nat Rev Immunol. 10:89–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Colotta F, Re F, Muzio M, Bertini R,
Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE and Mantovani
A: Interleukin-1 type II receptor: A decoy target for IL-1 that is
regulated by IL-4. Science. 261:472–475. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim SH, Eisenstein M, Reznikov L, Fantuzzi
G, Novick D, Rubinstein M and Dinarello CA: Structural requirements
of six naturally occurring isoforms of the IL-18 binding protein to
inhibit IL-18. Proc Natl Acad Sci USA. 97:1190–1195. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tschopp J, Martinon F and Burns K: NALPs:
A novel protein family involved in inflammation. Nat Rev Mol Cell
Biol. 4:95–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bank U and Ansorge S: More than
destructive: Neutrophil-derived serine proteases in cytokine
bioactivity control. J Leukoc Biol. 69:197–206. 2001.PubMed/NCBI
|
|
75
|
Martinon F, Agostini L, Meylan E and
Tschopp J: Identification of bacterial muramyl dipeptide as
activator of the NALP3/cryopyrin inflammasome. Curr Biol.
14:1929–1934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kanneganti TD, Ozören N, Body-Malapel M,
Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M,
Vandenabeele P, et al: Bacterial RNA and small antiviral compounds
activate caspase-1 through cryopyrin/Nalp3. Nature. 440:233–236.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mariathasan S, Weiss DS, Newton K, McBride
J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM and
Dixit VM: Cryopyrin activates the inflammasome in response to
toxins and ATP. Nature. 440:228–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Eisenbarth SC, Colegio OR, O'Connor W Jr,
Sutterwala FS and Flavell RA: Crucial role for the Nalp3
inflammasome in the immunostimulatory properties of aluminium
adjuvants. Nature. 453:1122–1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Krakowski M and Owens T: Interferon-γ
confers resistance to experimental allergic encephalomyelitis. Eur
J Immunol. 26:1641–1646. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Vermeire K, Heremans H, Vandeputte M,
Huang S, Billiau A and Matthys P: Accelerated collagen-induced
arthritis in IFN-γ receptor-deficient mice. J Immunol.
158:5507–5513. 1997.PubMed/NCBI
|
|
81
|
Chang JT, Segal BM, Nakanishi K, Okamura H
and Shevach EM: The costimulatory effect of IL-18 on the induction
of antigen-specific IFN-γ production by resting T cells is IL-12
dependent and is mediated by up-regulation of the IL-12 receptor β2
subunit. Eur J Immunol. 30:1113–1119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cua DJ, Sherlock J, Chen Y, Murphy CA,
Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al:
Interleukin-23 rather than interleukin-12 is the critical cytokine
for autoimmune inflammation of the brain. Nature. 421:744–748.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mills KHG, Dungan LS, Jones SA and Harris
J: The role of inflammasome-derived IL-1 in driving IL-17
responses. J Leukoc Biol. 93:489–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mangan PR, Harrington LE, O'Quinn DB,
Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR and
Weaver CT: Transforming growth factor-β induces development of the
T(H)17 lineage. Nature. 441:231–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zielinski CE, Mele F, Aschenbrenner D,
Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A and
Sallusto F: Pathogen-induced human TH17 cells produce IFN-γ or
IL-10 and are regulated by IL-1β. Nature. 484:514–518. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Comar M, Zanotta N, Bonotti A, Tognon M,
Negro C, Cristaudo A and Bovenzi M: Increased levels of C-C
chemokine RANTES in asbestos exposed workers and in malignant
mesothelioma patients from an hyperendemic area. PLoS One.
9:e1048482014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Davis GS, Pfeiffer LM and Hemenway DR:
Persistent overexpression of interleukin-1β and tumor necrosis
factor-α in murine silicosis. J Environ Pathol Toxicol Oncol.
17:99–114. 1998.PubMed/NCBI
|
|
90
|
Pan LH, Ohtani H, Yamauchi K and Nagura H:
Co-expression of TNFα and IL-1β in human acute pulmonary fibrotic
diseases: An immunohistochemical analysis. Pathol Int. 46:91–99.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Beamer CA and Holian A: Scavenger receptor
class A type I/II (CD204) null mice fail to develop fibrosis
following silica exposure. Am J Physiol Lung Cell Mol Physiol.
289:L186–L195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Holley JA, Janssen YMW, Mossman BT and
Taatjes DJ: Increased manganese superoxide dismutase protein in
type II epithelial cells of rat lungs after inhalation of
crocidolite asbestos or cristobalite silica. Am J Pathol.
141:475–485. 1992.PubMed/NCBI
|
|
93
|
Porter DW, Hubbs AF, Chen BT, McKinney W,
Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, et
al: Acute pulmonary dose-responses to inhaled multi-walled carbon
nanotubes. Nanotoxicology. 7:1179–1194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu RM: Oxidative stress, plasminogen
activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal.
10:303–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Carbone M and Yang H: Molecular pathways:
Targeting mechanisms of asbestos and erionite carcinogenesis in
mesothelioma. Clin Cancer Res. 18:598–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Donaldson K, Poland CA, Murphy FA,
MacFarlane M, Chernova T and Schinwald A: Pulmonary toxicity of
carbon nanotubes and asbestos - similarities and differences. Adv
Drug Deliv Rev. 65:2078–2086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Goldberg JL, Zanella CL, Janssen YMW,
Timblin CR, Jimenez LA, Vacek P, Taatjes DJ and Mossman BT: Novel
cell imaging techniques show induction of apoptosis and
proliferation in mesothelial cells by asbestos. Am J Respir Cell
Mol Biol. 17:265–271. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sekido Y: Molecular pathogenesis of
malignant mesothelioma. Carcinogenesis. 34:1413–1419. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kitasato Y, Hoshino T, Okamoto M, Kato S,
Koda Y, Nagata N, Kinoshita M, Koga H, Yoon DY, Asao H, et al:
Enhanced expression of interleukin-18 and its receptor in
idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol.
31:619–625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lindroos PM, Coin PG, Osornio-Vargas AR
and Bonner JC: Interleukin 1 beta (IL-1 beta) and the IL-1
beta-alpha 2-macroglobulin complex upregulate the platelet-derived
growth factor alpha-receptor on rat pulmonary fibroblasts. Am J
Respir Cell Mol Biol. 13:455–465. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sabo-Attwood T, Ramos-Nino M, Bond J,
Butnor KJ, Heintz N, Gruber AD, Steele C, Taatjes DJ, Vacek P and
Mossman BT: Gene expression profiles reveal increased mClca3 (Gob5)
expression and mucin production in a murine model of
asbestos-induced fibrogenesis. Am J Pathol. 167:1243–1256. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Musumeci G, Loreto C, Giunta S, Rapisarda
V, Szychlinska MA, Imbesi R, Castorina A, Annese T, Castorina S,
Castrogiovanni P, et al: Angiogenesis correlates with macrophage
and mast cell infiltration in lung tissue of animals exposed to
fluoro-edenite fibers. Exp Cell Res. 346:91–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cardile V, Lombardo L, Belluso E, Panico
A, Renis M, Gianfagna A and Balazy M: Fluoro-edenite fibers induce
expression of Hsp70 and inflammatory response. Int J Environ Res
Public Health. 4:195–202. 2007. View Article : Google Scholar : PubMed/NCBI
|