Introduction
Varicella-zoster virus (VZV), belonging to the
α-subgroup of herpesviruses, contains a linear, double-stranded DNA
(dsDNA) genome encoding ~71 open reading frames (ORF) (1). The VZV virion particle is composed of
an icosahedral nucleocapsid containing the viral genome, a layer of
tegument proteins that surrounds the nucleocapsid and a plasma
membrane acquired from cellular membranes with the viral
glycoproteins (1,2).
Similar to other herpesviruses, VZV is proposed to
enter host cells via binding to receptors or fusion of the viral
envelope with cellular plasma membrane followed by endocytosis
(1). After entry, the nucleocapsid
is transported into the nucleus, and viral gene expression is
induced by VZV tegument proteins encoded by ORF4, 10 and 62
(1). Among the viral tegument
proteins, the immediate-early (IE) protein 62 (IE62) encoded by
ORF62, is a major component and transactivator essential for viral
lytic gene expression and replication (2). During lytic replication, VZV gene
expression occurs in a temporal cascade involving three classes of
genes: IE, early (E), and late (L). IE genes regulate the
expression of early and late genes. The E gene subset encodes
proteins for viral DNA replication while L genes encode structural
constituents of the virion, including glycoproteins and
nucleocapsid proteins (3,4). VZV replication utilizes a rolling
circle mechanism and is highly cell-associated (1,5).
VZV is widely distributed worldwide and affects
almost the entire human population owing to the high morbidity of
infection (1). Primary VZV
infection causes chickenpox (also known as varicella), a normal
childhood illness concurrent with fever and pruritic vesicular rash
(5). During primary infection, VZV
inoculates respiratory mucosal epithelium, is transported to the
skin by cell-associated virus infection, causes dermal lesions and
infects sensory ganglia, where it establishes latent infection
(6). Herpes zoster (shingles),
caused by reactivation of VZV, provokes severe pain in the area of
latently infected ganglia (7).
Several antiviral agents, such as acyclovir,
famciclovir, valacyclovir and foscarnet, are currently used to
treat VZV-associated diseases (5,7,8).
These licensed drugs inhibit viral DNA polymerase and are used in
combination with corticosteroids for inflammation and narcotics for
pain (1,9). However, the efficacy of these agents
to treat chronic VZV infections in immunocompromised individuals is
limited because of their adverse effects and emergence of resistant
VZV strains (7,9). In addition to antiviral drugs, a
FDA-approved live-attenuated VZV vaccine is available for infant
immunization (10). However,
significant concerns still exist with regard to efficacy of
vaccination and prevalence of unvaccinated individuals carrying
latent VZV (9). Development of
novel antiviral drugs to treat VZV-associated diseases with high
efficacy and fewer side-effects therefore remains an urgent unmet
medical need. In the present study, we showed that the
Lysimachia mauritiana extract exerts antiviral activity
against VZV lytic gene expression and replication. Our findings
support the potential utility of LME as a therapeutic agent for
active VZV infection.
Materials and methods
Cells, viruses and plant
materials
MRC-5 cells were obtained from the Korean Cell Line
Bank (KCLB no. 10171). The recombinant laboratory pOka strain of
VZV (VZV-pOka) expressing green fluorescent protein (VZV-pOka-GFP)
and clinical YC01 strain of VZV (VZV-YC01) were kindly provided by
Dr Chan-Hee Lee (Chungbuk National University, Cheongju, Korea)
(11). Plant material
(Lysimachia mauritiana Lam.) and the 70% ethanol extract
used in the present study were collected from Jeju Island in Korea
and provided by the Jeju Biodiversity Research Institute (Jeju,
Korea; specimen no. JBR-324).
Fluorescence microscopy
Fluorescence was examined and images analyzed using
an inverted Nikon TS100-F fluorescence microscope (Tokyo, Japan)
equipped with a digital camera and Nikon NIS-Elements microscope
imaging software.
Quantification of VZV DNA
replication
Total DNA was isolated using an Accuprep Genomic DNA
Extraction kit (Bioneer, Daejeon, Korea), and VZV DNA was amplified
and quantified in a StepOnePlus Real-Time PCR system (Applied
Biosystem, Foster City, CA, USA) using HOT FIREPol®
EvaGreen quantitative PCR (qPCR) mix Plus (Solis BioDyne, Tartu,
Estonia). The following primers were used for qPCR: VZV ORF62
forward, 5′-TCTTGTCGAGGAGGCTTCTG-3′ and reverse,
5′-TGTGTGTCCACCGGATGAT-3′; GAPDH forward,
5′-CATGAGAAGTATGACAACAGCCT-3′ and reverse,
5′-AGTCCTTCCACGATACCAAAGT-3′.
Plaque reduction assay
MRC-5 cells were inoculated with serially diluted
VZV-YC01-infected MRC-5 cells and treated with either DMSO or LME
at concentrations of 5, 10, 25, 50 µg/ml. At 6 days after
infection, cells were fixed with 10% formaldehyde at room
temperature for 10 min and stained with 0.3% crystal violet. After
5 h, the number of plaques was counted, and the virus titer
expressed as plaque-forming units (pfu/ml).
Quantification of VZV transcript
expression
VZV transcript levels were quantified using
quantitative reverse transcription PCR (qRT-PCR). Total RNA was
isolated using a HiGene™ Total RNA Prep kit (BIOFACT, Daejeon,
Korea) and reverse-transcribed into complementary DNA (cDNA) with a
QuantiTECT® reverse transcription (RT) kit, according to
the manufacturer's instructions (Qiagen, Hilden, Germany). cDNAs
were amplified and quantified in a StepOnePlus Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA) using HOT
FIREPol® EvaGreen qPCR mix Plus (Solis BioDyne, Tartu,
Estonia). The following primers were used for qRT-PCR: VZV ORF62
(immediate early, IE) forward, 5′-TCTTGTCGAGGAGGCTTCTG-3′ and
reverse, 5′-TGTGTGTCCACCGGATGAT-3′; ORF28 (E) forward,
5′-CGAACACGTTCCCCATCAA-3′ and reverse, 5′-CCCGGCTTTGTTAGTTTTGG-3′;
gB (L) forward, 5′-GATGGTGCATACAGAGAACATTCC-3′ and reverse,
5′-CCGTTAAATGAGGCGTGACTAA-3′; GAPDH forward,
5′-CATGAGAAGTATGACAACAGCCT-3′ and reverse,
5′-AGTCCTTCCACGATACCAAAGT-3′.
Western blot analysis
Cells were collected, fractionated and transferred
onto nitrocellulose membranes, as described previously (12). Antibodies specific for VZV IE62 and
tubulin were purchased from Abcam (Cambridge, UK) and Sigma-Aldrich
(St. Louis, MO, USA), respectively. Enhanced chemiluminescence
detection reagent (Pierce, Rockford, IL, USA) and secondary
peroxidase-labeled anti-mouse immunoglobulin G antibodies (Amersham
Biosciences, Piscataway, NJ, USA) were used according to the
manufacturer's specifications.
Cell viability assays
CellTiter-Glo luminescent cell viability assay
(Promega, Madison, WI, USA) was performed in keeping with the
manufacturer's protocol.
Results
VZV replication is inhibited by
LME
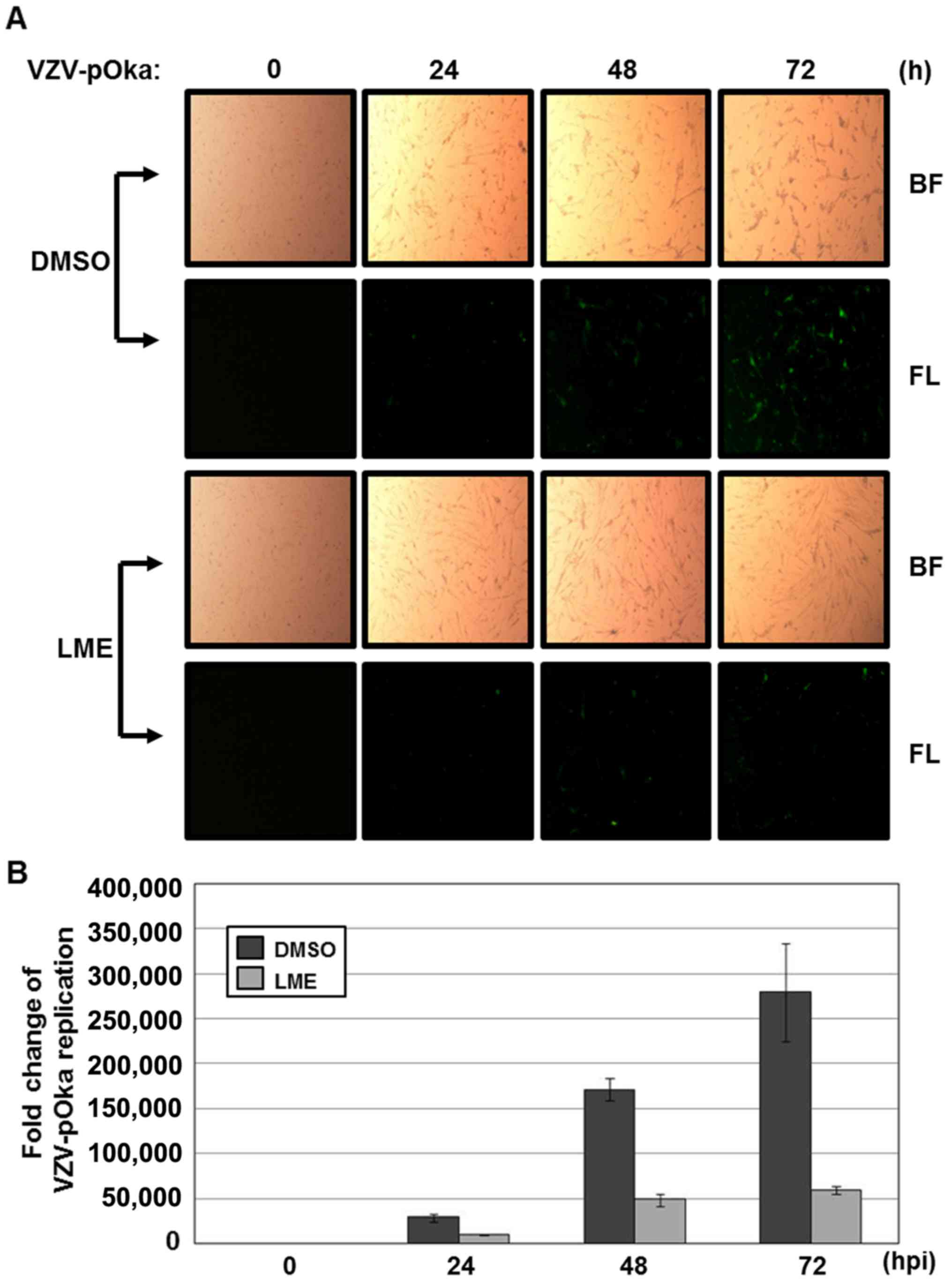
To examine the antiviral effects of the 70% ethanol
extract of Lysimachia mauritiana (LME) against VZV,
uninfected MRC-5 cells were inoculated with cells infected with
VZV-pOka-GFP at an MOI of 0.1, and treated with either 50 µg/ml
DMSO or LME. At 0, 24, 48 or 72 h after infection, GFP fluorescence
was measured under a fluorescence microscope. At 72 h after
infection, significantly reduced VZV-pOka-GFP fluorescence was
observed in the LME-treated cells (Fig. 1A). For quantitative analysis of
VZV-pOka-GFP replication, the relative amounts of viral DNA were
measured using qPCR with primers specific for ORF62. Compared to 0
h after infection, VZV DNA was amplified 170,000- and 280,000-fold
at 48 and 72 h, respectively (Fig.
1B; compare 0 h with 48 and 72 h). Relative to DMSO-treated
cells, VZV replication was downregulated 3.8- and 4.7-fold in
LME-treated cells at 48 and 72 h after infection, respectively
(Fig. 1B), clearly supporting an
inhibitory effect of LME on VZV replication.
LME-mediated inhibition of replication
of the VZV clinical isolate is dose-dependent
The plaque reduction assay was performed to further
investigate the inhibitory activity of LME against the clinical
isolate of VZV. Uninfected MRC-5 cells were inoculated with
serially diluted VZV-YC01-infected MRC-5 cells and treated with
either DMSO or LME at concentrations of 5, 10, 25 or 50 µg/ml.
Cells were re-treated with DMSO or LME at 3 days after infection.
At 6 days post-infection, cells were fixed and stained, and the
plaque forming units (pfu) per ml were determined via counting.
Interestingly, LME reduced the number of plaques by 2.3- and
17.3-fold at concentrations of 25 and 50 µg/ml, respectively,
compared to cells treated with DMSO (0 µg/ml) (Fig. 2). The 50% inhibitory concentration
(IC50) of LME for VZV at which the number of plaques was
reduced by 50% was calculated as 26.09 µg/ml.
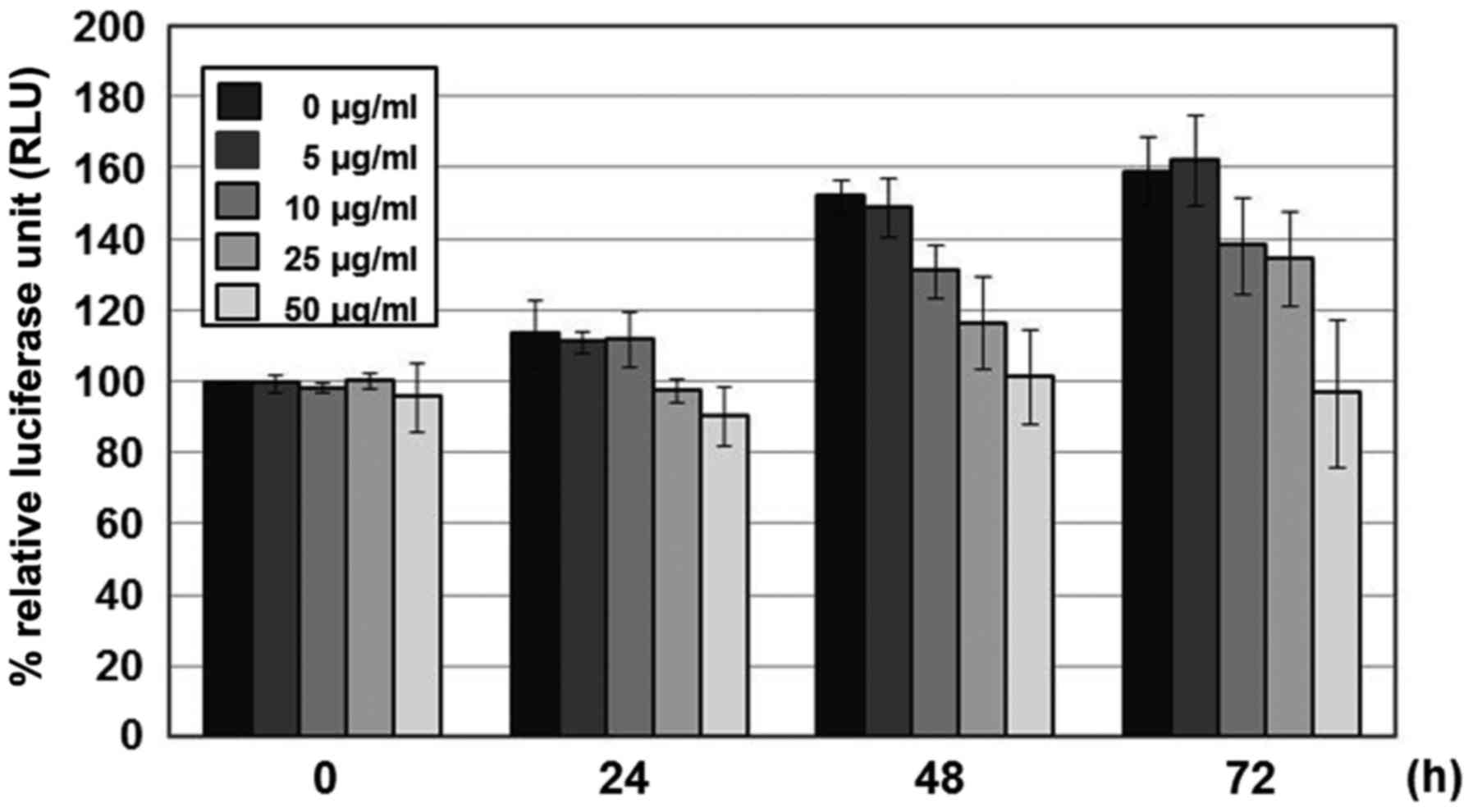
To ascertain whether the inhibitory activity of LME
on VZV replication was due to effects on cell viability, MRC-5
cells were treated with different concentrations of LME, and the
levels of cellular ATP representing the presence of metabolically
active cells were measured at 24, 48 or 72 h after treatment using
the CellTiter-Glo assay. With prolongation of incubation time,
cellular ATP levels were increased, possibly owing to cell
proliferation. Interestingly, at 72 h post-treatment, the viability
of MRC-5 cells treated with 25 µg/ml LME was similar to that of
DMSO-treated cells (Fig. 3).
Treatment with 50 µg/ml LME slightly reduced MRC-5 cell viability
by 33 and 39% at 48 and 72 h, respectively (Fig. 3). Since LME exerted no significant
adverse effects on MRC5 cell viability at the IC50
value, it is unlikely that its anti-VZV activity of LME is related
to cytotoxicity.
VZV lytic gene expression is
downregulated by LME
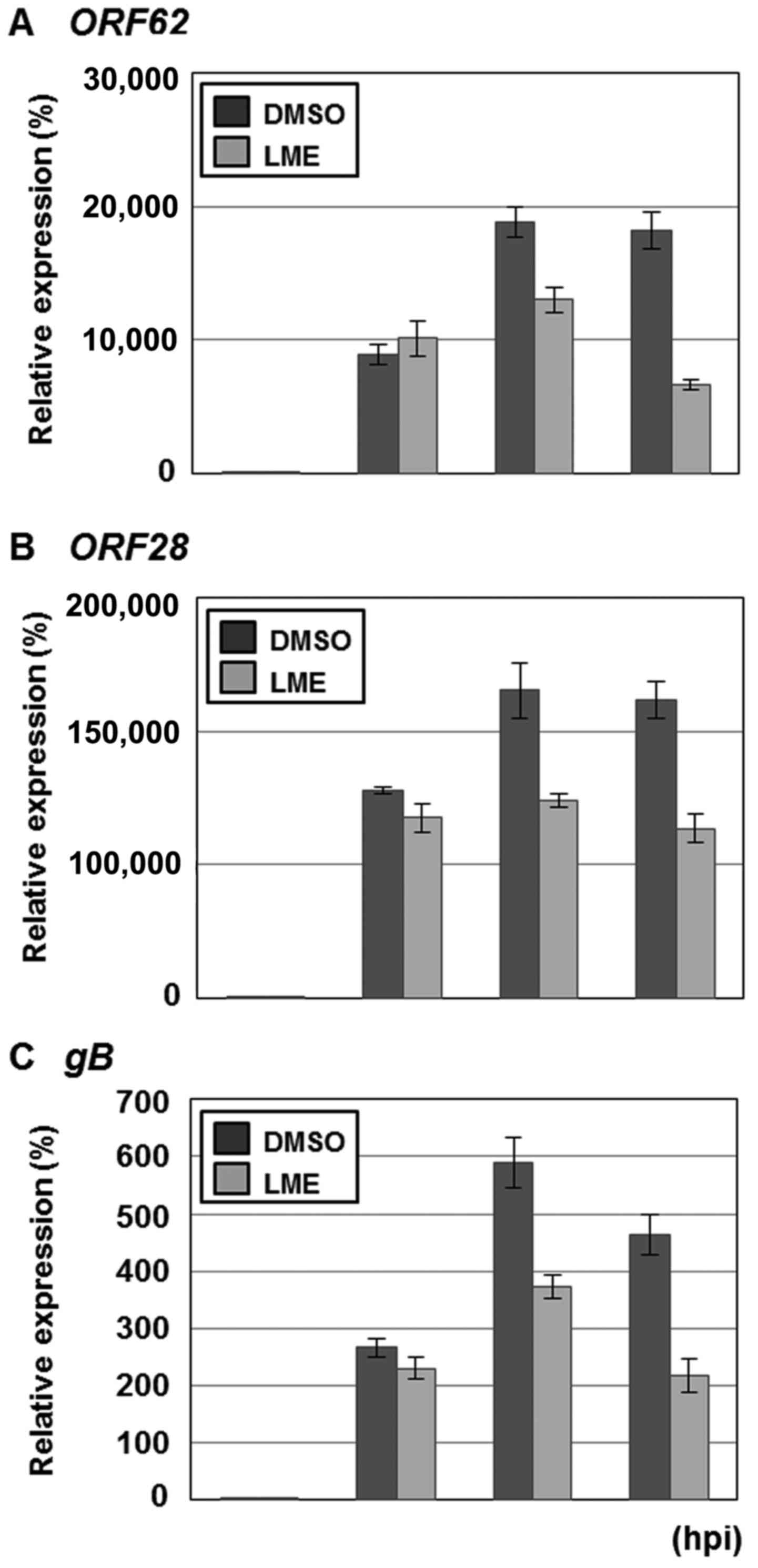
The effects of LME on VZV lytic gene expression were
determined via qRT-PCR analysis of IE, E or L transcript levels.
MRC-5 cells were inoculated with VZV-YC01-infected MRC-5 cells at
an MOI of 0.1 and treated with either DMSO or LME at a
concentration of 25 µg/ml. At 0, 24, 48 and 72 h after infection,
the relative amounts of transcripts for ORF62 encoding IE62 (IE),
ORF28 encoding a subunit of viral DNA polymerase (E) and
glycoprotein B (gB) (L) were determined (Fig. 4). In DMSO-treated cells, ORF62,
ORF28 and gB genes were induced at 24 h and higher levels expressed
at 48 and 72 h after infection (Fig.
4). LME had no effect on VZV lytic gene expression at 24 h
after infection (Fig. 4). However,
at 48 and 72 h, ORF62, ORF28 and gB transcript levels were
significantly reduced in the LME-treated cells (Fig. 4).
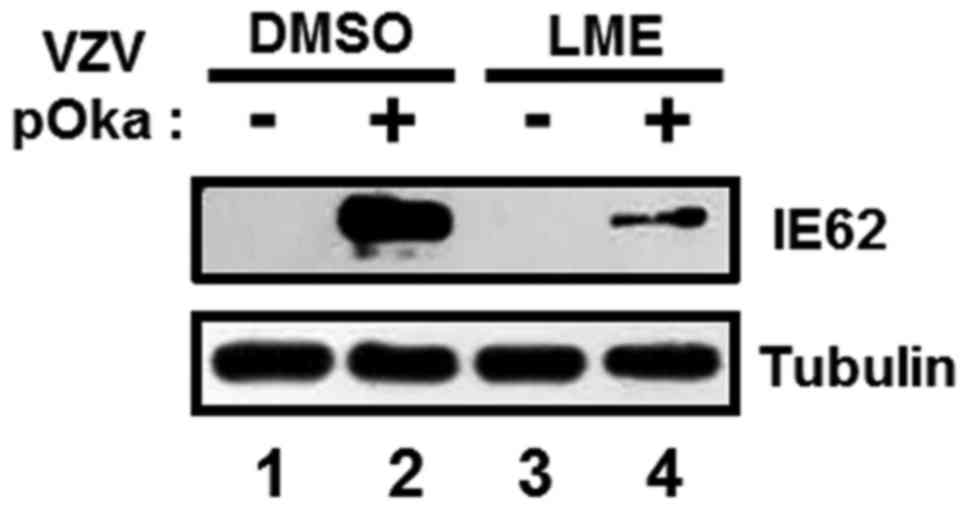
The protein level of IE62 encoded by ORF62, an
essential transactivator of VZV lytic gene expression, was further
determined. MRC-5 cells were inoculated with VZV-pOka-infected
MRC-5 cells at an MOI of 0.1 and treated with either DMSO or LME at
a concentration of 25 µg/ml. At 72 h after infection, IE62 protein
expression was assessed by western blot analysis with a specific
anti-IE62 antibody. IE62 protein was strongly induced in
DMSO-treated cells (Fig. 5;
compare lanes 2 and 1), and expression was significantly reduced by
LME (Fig. 5; compare lane 4 with
2). Based on the collective results, we propose that LME
downregulates IE62 protein expression and, in turn, negatively
affecting VZV replication in vitro.
Discussion
Varicella-zoster virus infection causes severe
disease in immunocompromised individuals, especially those with
impaired cell-mediated immune responses. VZV infection can develop
to disseminated disease, such as widespread skin lesion, pneumonia,
hepatitis, or encephalitis (3).
Owing to side effects and emergence of resistant VZV
strains, limited antiviral drugs are currently available to
VZV-associated diseases (7,9).
Plant extracts have been utilized extensively to develop novel
therapeutic agents that induce fewer side effects than synthetic
drugs (4). The main objective of
the present study was to investigate the antiviral activities of
plant extracts against VZV.
In LME-treated MRC-5 cells, replication of both
laboratory and clinical strains of VZV was significantly inhibited.
Since LME did not exert significant adverse effects on MRC5 cell
viability, its anti-VZV activity does not appear to be attributable
to cytotoxicity. Lysimachia mauritiana is a biennial herb
distributed worldwide, mainly in the temperate and subtropical
climate regions of both hemispheres, along coastal regions in East
Asia, the Philippines, Micronesia, Polynesia and the Indian Ocean
islands (13). The chemical
constituents and biological activities of Lysimachia
mauritiana have not been investigated to date.
Our data indicate that LME reduces VZV lytic gene
expression and inhibits VZV replication. LME induced significant
downregulation of IE62 protein, an important transactivation of IE,
E and L genes (2,3), in turn, leading to suppression of E
and L genes and inhibition of VZV replication.
The mechanisms underlying LME-mediated
downregulation of VZV IE62 protein expression remain to be
elucidated. Interestingly, LME suppressed the expression of lytic
genes at later stages of VZV infection to a significant extent, but
had almost no effect on lytic gene expression at the earlier time
points (Fig. 4; compare 24 with 48
and 72 hpi). At the earlier stages of infection, pre-generated IE62
protein in tegument may induce VZV lytic genes. LME may reduce
lytic gene expression by downregulating IE62 protein expression,
consequently leading to suppression of lytic gene expression at
subsequent time-points of VZV infection. LME may either directly
inhibit the activities of transcription factors or indirectly
interfere with a signal transduction pathway(s) to activate
transcription factors for IE62 expression. In addition to
downregulation of IE62 protein expression, it is possible that LME
affects other phases of the VZV life cycle.
To our knowledge, this is the first study to
demonstrate that LME exhibits antiviral activity against VZV. LME
may provide an effective source for further development and
tailoring of novel therapeutic agents to treat VZV-associated
diseases.
Acknowledgements
We thank Dr Chan-Hee Lee (Chungbuk National
University, Cheongju, Korea) for kindly providing the pOka and YC01
strains of VZV. We are grateful to Dr Jin-Hyun Ahn (Sungkyunkwan
University, Suwon, Korea) for helpful discussions. This study was
supported by the Bio-industry Technology Development Program,
Ministry of Agriculture, Food and Rural Affairs (no. 311063-5) and
the Gachon University Research Fund of 2014 (GCU-2014-0198).
Glossary
Abbreviations
Abbreviations:
|
VZV
|
Varicella-Zoster virus
|
|
IE
|
immediate-early
|
|
LME
|
ethanol extract of Lysimachia
mauritiana
|
|
MOI
|
multiplicity of infection
|
|
PFU
|
plaque forming unit
|
|
ORF
|
open reading frame
|
References
|
1
|
Arvin AM and Gilden D: Varicella-zoster
virusFields Virology. Knipe DM and Howley PM: 6th. Lippincott
Williams & Wilkins; Philadelphia, PA: pp. 2015–2057. 2013
|
|
2
|
Zerboni L, Sen N, Oliver SL and Arvin AM:
Molecular mechanisms of varicella zoster virus pathogenesis. Nat
Rev Microbiol. 12:197–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen JI, Brunell PA, Straus SE and Krause
PR: Recent advances in varicella-zoster virus infection. Ann Intern
Med. 130:922–932. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
To KP, Kang SC and Song YJ: The extract of
Elaeocarpussylvestris inhibits human cytomegalovirus immediate
early gene expression and replication in vitro. Mol Med Rep.
9:744–748. 2014.PubMed/NCBI
|
|
5
|
Arvin AM: Varicella-zoster virus. Clin
Microbiol Rev. 9:361–381. 1996.PubMed/NCBI
|
|
6
|
Zerboni L, Ku CC, Jones CD, Zehnder JL and
Arvin AM: Varicella-zoster virus infection of human dorsal root
ganglia in vivo. Proc Natl Acad Sci USA. 102:pp. 6490–6495. 2005;
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasivimolphan P, Lipipun V,
Likhitwitayawuid K, Takemoto M, Pramyothin P, Hattori M and Shiraki
K: Inhibitory activity of oxyresveratrol on wild-type and
drug-resistant varicella-zoster virus replication in vitro.
Antiviral Res. 84:95–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Docherty JJ, Sweet TJ, Bailey E, Faith SA
and Booth T: Resveratrol inhibition of varicella-zoster virus
replication in vitro. Antiviral Res. 72:171–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi EJ, Lee CH, Kim YC and Shin OS:
Wogonin inhibits Varicella-Zoster (shingles) virus replication via
modulation of type I interferon signaling and adenosine
monophosphate-activated protein kinase activity. J Funct Foods.
17:399–409. 2015. View Article : Google Scholar
|
|
10
|
Gilden D, Mahalingam R, Nagel MA,
Pugazhenthi S and Cohrs RJ: Review: The neurobiology of varicella
zoster virus infection. Neuropathol Appl Neurobiol. 37:441–463.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Won YH, Kim JI, Kim YY and Lee CH:
Characterization of the repeat sequences of varicella-zoster virus.
J Bacteriol Virol. 44:326–335. 2014. View Article : Google Scholar
|
|
12
|
Kim SY, Kim JE, Won J and Song YJ:
Characterization of the rapamycin-inducible EBV LMP1 activation
system. J Microbiol. 53:732–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kono Y, Chung KF, Chen CH, Hoshi Y,
Setoguchi H, Chou CH, Oginuma K and Peng CI: Intraspecific
karyotypic polymorphism is highly concordant with allozyme
variation in Lysimachia mauritiana (Primulaceae: Myrsinoideae) in
Taiwan: implications for the colonization history and dispersal
patterns of coastal plants. Ann Bot. 110:1119–1135. 2012.
View Article : Google Scholar : PubMed/NCBI
|