Introduction
Chronic kidney disease (CKD), defined as a
progressive decline in renal function, has gradually become an
important problem of global public health during the past decades.
It occurs in developed and developing countries. There are
currently >1.4 million patients with CKD worldwide receiving
renal replacement therapy (1).
Renal fibrosis is the final common pathway of all
progressive renal disease. Various pathogenic factors stimulate the
cells of the kidney to continually produce inflammatory mediators
and cytokines, leading to chronic endothelial-mesenchymal
transition (EMT) and extracellular matrix (ECM) production that
eventually alters the normal structure and function of the nephron
(2). The Wnt/β-catenin signaling
pathway is important for the regulation of cell adhesion,
migration, EMT, embryonic development and homeostasis of tissues
and organs. Dysregulation of this important signaling pathway is
closely associated with the progression of renal fibrosis (3). Transforming growth factor (TGF)-β is
typically regarded as the most potent profibrogenic cytokine and a
central mediator of renal fibrosis (4). TGF-β is a primary ECM regulator,
acting as an inducer of ECM synthesis and an inhibitor of ECM
degradation, via the direct suppression of matrix
metalloproteinases and induction of tissue inhibitors of
metalloproteinases (5). In
addition, TGF-β may induce tubular EMT and directly contribute to
the myofibroblast pool responsible for interstitial matrix
production (6).
Previous studies have demonstrated various points of
cross-talk between the Wnt/β-catenin signaling pathway and TGF-β in
the development of tissue fibrosis and tumor progression.
Wnt/β-catenin signaling affects the TGF-β signaling pathway in a
multi-level synergistic manner in various settings of fibrosis, and
TGF-β signaling induces activation of the Wnt/β-catenin signaling
pathway, as the two pathways regulate ligand generation of the
other pathway (7,8).
Activated Wnt/β-catenin signaling has been
demonstrated to promote fibroblast activities in various organs,
including the kidneys, which indicates that this pathway may be
reactivated following tissue injury (9–12).
Strategies targeting the Wnt/β-catenin signaling pathway by Wnt
modulation in various animal models of fibrosis have proven to be
promising. Using a peptidomimetic small molecule, ICG-001, which
disrupts CREB-binding protein-catenin-mediated gene transcription,
suppressed expression of fibrosis-associated proteins and
ameliorated renal interstitial fibrotic injury in kidneys (13). However, whether inhibiting the
Wnt/β-catenin signaling pathway reverses fibrosis remains
controversial. Previous studies have provided evidence that
inhibition of Wnt/β-catenin signaling may ameliorate fibrosis and
injury (14–16). However, a separate study revealed
that activation of the β-catenin signaling pathway in alveolar
epithelial type 2 (AT2) cells contributes to epithelial repair
following injury, and depletion of β-catenin by Cre recombinase
promotes fibrosis (17).
Therefore, the aim of the present study was to
evaluate the role of Wnt/β-catenin signaling on kidney cell
apoptosis and the expression of markers of renal fibrosis.
Materials and methods
Animals and experimental design
All experimental protocols were approved by the
ethics committee of The People's Hospital of Guizhou Province
(Guiyang, China), and followed the guidelines of Care and Use of
Laboratory Animals formulated by the Ministry of Science and
Technology of China. Male Sprague-Dawley rats [n=20; weight,
180–220 g; certification number, SCXK (Yue) 2006–0015] were
purchased from the Experimental Animal Center of Southern Medical
University (Guangzhou, China) and were randomly divided into sham
and CKD groups (n=10 per group), and given free access to food and
water in a room with constant temperature (20±1°C) and a 12 h
light/dark cycle. Using full sterile technique, all rats underwent
sham surgery or 5/6 nephrectomy under anesthesia with 3% isoflurane
(RWD Life Science Co., Ltd, Shenzhen, China) in N2
O/O2 (2:1), as previously described (18). Briefly, the upper and lower poles
of the left kidney were removed using a retroperitoneal approach
and hemostatic absorbable collagen sponges were used to stop the
bleeding. The right kidney was removed one week later. In the
sham-operated rats, laparotomy was performed without 5/6
nephrectomy. All rats survived to the experimental endpoint of 12
weeks after the operation. Rats were anesthetized by administration
of 3% isoflurane in N2/O2 (2:1) prior to
sacrifice. Immediately after the rats were sacrificed, the kidneys
were dissected and rinsed in phosphate buffered saline. The 10%
formalin-fixed kidney and frozen sections were subsequently used
for histopathological examination and immunohistochemical
studies.
Culture of mesangial cells
The rat mesangial cell line HBZY-1 was obtained from
the Wuhan University Cell Bank (Wuhan, China), and cultured in
Dulbecco's modified Eagle's medium (PromoCell GmbH, Heidelberg,
Germany) supplemented with 5 mM glucose and 10% heat-inactivated
fetal calf serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
at 37°C in 5% CO2.
Histology and immunofluorescence
All samples were fixed in 4% buffered
paraformaldehyde, decalcified in 50 mM EDTA, embedded in paraffin
and sectioned at a thickness of 5 mm. The sections were processed
for periodic acid-Schiff (PAS), hematoxylin and eosin (H&E),
periodic acid silver methenamine (PASM), Masson's trichrome and
immunofluorescence staining.
For H&E staining, sections were incubated with
hematoxylin (Mayer's hematoxylin solution; VWR International,
Radnor, PA, USA) for 15 min, washed in water for 5 min, and stained
with eosin (VWR International) for 1 min.
For PAS Staining, sections were fixed in 95% alcohol
for 10 min prior to being washed, dried, stained with 1% periodic
acid for 20 min, and washed and dried again. Each section was
subsequently stained with Schiff's reagent for 60 min, washed and
dried, stained with hematoxylin for 5 min, and washed and
dried.
For PASM staining, sectionswere immersed in 0.005 M
periodic acid for 20 min at 22°C, rinsed twice in distilled water
and stained for 70 min at 50°C in freshly-made 20 ml
silver-methenamine solution (0.25% silver nitrate, 0.3 g
hexamethylenetetramine, 8 ml 5% sodium borate and 12 ml distilled
water). Following staining, sectionswere rinsed four times in
distilled water, treated with 0.5% sodium thiosulfate for 2 min at
22°C, washed twice in distilled water and dried on filter
paper.
For Masson's trichrome staining, a Masson-Goldner
Staining kit (Merck KGaA, Darmstadt, Germany) was used, according
to the manufacturer's protocol. Nuclei were stained with
hematoxylin for 15 min using Weigert's Iron Hematoxylin kit (VWR
International), followed by azophloxin staining for 15 min. Samples
were washed with acetic acid (1%) and placed in acid Orange G
solution. Samples were rinsed with 1% acetic acid for 30 sec,
stained with Light Green for 5 min and rinsed again in 1% acetic
acid for 5 min.
For immunofluorescence staining, sections were
blocked with 5% normal sheep serum (Sigma-Aldrich; Merck KGaA), in
PBS for 45 min at room temperature and incubated with a monoclonal
mouse anti-rat β-catenin antibody (RM-2101-RQ;1:200; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 4°C overnight. The reaction
was visualized under a fluorescence microscope following incubation
with a chicken anti-mouse Texas Red-conjugated IgG secondary
antibody (sc-2954; 1:100; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). A substitution of primary antibody with 10% non-immune
goat serum (Invitrogen; Thermo Fisher Scientific, Inc.) was used as
a negative control.
Terminal dUTP nick end labelling
analysis
DNA strand breaks were assessed by fluorescent
labeling of terminal dUTP nick end labeling (TUNEL), using the One
Step TUNEL apoptosis assay kit (Beyotime Institute of
Biotechnology, Haimen, China) as previously described (18). Images were captured with a Nikon
DXM 1200C camera using Nikon ACT-1C software (Nikon Corporation,
Tokyo, Japan). Within the delineated areas (measured and assisted
with Stereo Investigator software v.10.5; MBF Bioscience,
Williston, VT, USA), TUNEL-positive cells were counted, and their
densities were calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the renal cortex using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
dissolved in diethylpyrocarbonate-treated water. Total RNA was
reverse transcribed to cDNA using 50 ng of total RNA in a 20 µl
reaction mixture using a RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.), each reaction contained 200 U
RevertAidM-MuLV reverse transcriptase. Quantitative PCR was
performed using the SYBR-Green method in atotal reaction mixture of
20 µl containing 0.2 U of JumpStart Taq polymerase (Sigma-Aldrich;
Merck KGaA), 10 pmol of each forward and reverse primer, and 1 µl
cDNA in a MyiQ real-time PCR detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) in triplicate. The
thermocycling conditions were as follows: Initial melting, 98°C for
30 sec; 35 cycles of 98°C for 10 sec, 62°C for 30 sec, 72°C for 2
min; final extension, 72°C for 10 min; hold, 4–10°C. Sequences of
specific primers were as follows: collagen I, forward,
5′-ATCCTGCCGATGTCGCTAT-3′ and reverse, 5′-CCACAAGCGTGCTGTAGGT-3′;
collagen III, forward, 5′-CTGGTCCTGTTGGTCCATCT-3′ and reverse,
5′-ACCTTTGTCACCTCGTGGAC-3′; collagen IV, forward,
5′-GCCCTACGTTAGCAGATGTACC-3′ and reverse,
5′-TATAAATGGACTGGCTCGGAAT-3′; β-catenin, forward,
5′-ATTTGATGGAGTTGGACATGGC-3′ and reverse,
5′-GAGGAAGAGGATGTGGATACCTCC-3′; fibronectin, forward
5′-GTGATCTACGAGGGACAGC-3′, and reverse 5′-GCTGGTGGTGAAGTCAAAG-3′;
TGF-β, forward, 5′-GCCAGATCCTGTCCAAACTAA-3′, and reverse,
5′-TTGTTGCGGTCCACCATTA-3′. GAPDH, forward,
5′-ATGCTGGTGCTGAGTATGTC-3′ and reverse, 5′-AGTTGTCATATTTCTCGTGG-3.
Detected levels of target mRNAs were calculated using the ΔΔCq
method (19) and normalized to
GAPDH in arbitrary units.
Western blot analysis
Kidney tissue (~40 mg) was snap-frozen and ground in
a mortar, thawed and homogenized in lysis buffer (#9803, Cell
Signaling Technology, Inc., Danvers, MA, USA) supplemented with 8
µl protease inhibitor cocktail (Calbiochem; Merck KGaA) and
phosphatase inhibitor cocktail (Sigma-Aldrich; Merck KGaA), and
centrifuged at 15,000 × g for 30 min at 4°C. Protein concentrations
were determined by a modified Bradford assay using a Bio-Rad
microtiter plate (Bio-Rad Laboratories, Inc.). Equal amounts of
protein (50 µg) were loaded onto 10% sodium dodecyl
sulfate-polyacrylamide gels for separation, transferred to
polyvinylidene difluoride membranes and blocked at room temperature
for 1 h in 5% non-fat milk in Tris-buffered saline (100 mM NaCl, 50
mM Tris pH 7.5) containing 0.1% Tween-20. Membraneswere incubated
with primary antibodies targeting β-catenin (rabbit monoclonal
antibody (mAb); #8480; 1,1000), cleaved caspase-3 (rabbit mAb;
#9654; 1:1,000), B-cell lymphoma 2 (Bcl-2) -associated X protein
(Bax) (rabbit mAb; #14796; 1,1000) (all from Cell Signaling
Technology, Inc.), Bcl-2 (rabbit mAb; sc-509; 1,200; Santa Cruz
Biotechnology) and GAPDH (mousemAb; 60004-1-Ig, 1:1,000,
ProteinTech Group, Inc., Chicago, IL, USA) overnight at 4°C.
Membranes were subsequently incubated with sheep anti-mouse
immunoglobulin (Ig)-G(RPN4201; GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA) and goat anti-rabbit IgG (RPN4301, GE
Healthcare Bio-Sciences) horseradish peroxidase-conjugated
secondary antibodies, diluted 1:10,000, for 2 h at room
temperature. Protein signals were detected using an Enhanced
Chemiluminescence reagent (GE Healthcare Bio-Sciences) and images
were captured using Image Station 2000MM (Kodak, Rochester, NY,
USA). Densitometry of the blot images was performed using Molecular
Imaging Software version 4.0 (Kodak).
Transfection
According to the sequence in the study of Verma
et al (20), small
interfering RNA (siRNA) targeting β-catenin (CTNNB1, NM001904) was
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. The sense
was 5′-CCAUGCUGUAUACUCCACAGGAAAU-3′, which extends between amino
acids 79 and 85 of β-catenin. A scrambled siRNA without homology to
any mammalian gene sequence served as a negative control. The sense
was: 5′-GUCAUUGACUUAUCGAUGGdTdT-3′. Mesangial cells in the
logarithmic phase were harvested by trypsinization and seeded at
1×106 cells/well into 6-well plates to yield 80–90%
confluence. Transfections were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) with 100 nM β-catenin or scramble control siRNA
(silencer FAM-labeled scramble negative control), according to the
manufacturer's protocol. Transfected cells were incubated overnight
under normal growth conditions, the medium was subsequently
replaced and cells were stimulated with 2.5 ng/ml TNF-α (R&D
Systems, Inc., Minneapolis, MN, USA) or vehicle control (Sterile
PBS containing 0.1% bovine serum albumin (Tocris Bioscience,
Bristol, UK)) for 24 h. Morphological alterations indicating
apoptosis were observed by Hoechst 33258 staining (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) under a fluorescence microscope,
and quantified using Image J software v1.32 (National Institutes of
Health, Bethesda, MD, USA) (21).
Statistical analysis
Data are presented as the mean ± standard error.
Statistical analysis was performed using SPSS software version 19.0
(IBM SPSS, Armonk, NY, USA). For data that were normally
distributed, one-way analysis of variance (ANOVA) was performed
followed by pair-wise comparison using the least-significant
difference post hoc test. For data that were unequal and
nonparametric, one-way ANOVA followed by Dunn's method for
pair-wise comparison was used. P<0.05 was considered to indicate
a statistically significant difference.
Results
Histological and protein expression
alterations in kidney tissues from CKD rats
As demonstrated in Fig.
1A, renal histology in sham-operated rats (control group) was
normal. However, histological examination of kidney tissues from
5/6-nephrectomized rats (CKD group) revealed a moderate glomerular
mesangial cell proliferation and glomerular ECM accumulation,
localized or diffuse. Segmental sclerosis of capillary vessels,
capsular synechia and tubular atrophy were additionally observed.
Masson's trichrome staining demonstrated prominent interstitial
fibrosis and extensive inflammatory cell invasion in the CKD group
compared with the control group. The mRNA expression levels of
markers of mesangial expansion and tubulointerstitial fibrosis were
examined by RT-qPCR. TGF-β, fibronectin, and collagen I, III and IV
mRNA expression levels were significantly upregulated, by 3.2-,
5.3-, 2.2-, 1.8- and 5.6-fold (P<0.05), respectively, in CKD rat
kidney tissues compared with the control group (Fig. 1B).
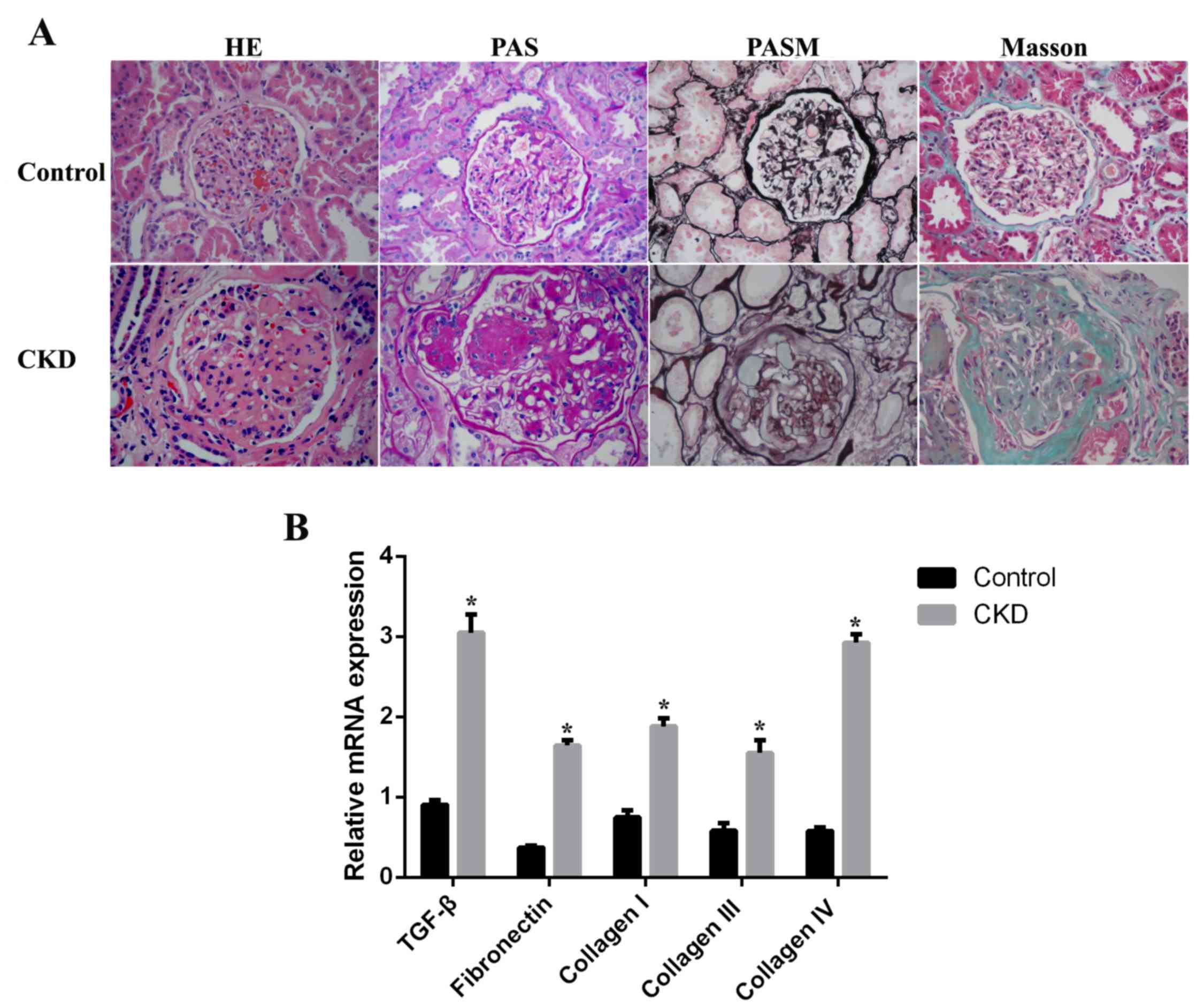 | Figure 1.Histological assessment of kidneys
from CKD rats. (A) Paraffin sections of kidney tissues from control
(sham-operated) and CKD (5/6-nephrectomized) rats were stained with
H&E, PAS, PASM or Masson's trichrome. Original magnification,
×400. (B) mRNA expression levels of TGF-β, fibronectin, and
collagen I, III and IV in control and CKD kidney tissues. Data are
expressed as the mean ± standard error (n=8 per group). *P<0.05
vs. control. CKD, chronic kidney disease; H&E, hematoxylin and
eosin; PAS, periodic acid-Schiff; PASM, periodic acid silver
methenamine; TGF-β, transforming growth factor-β. |
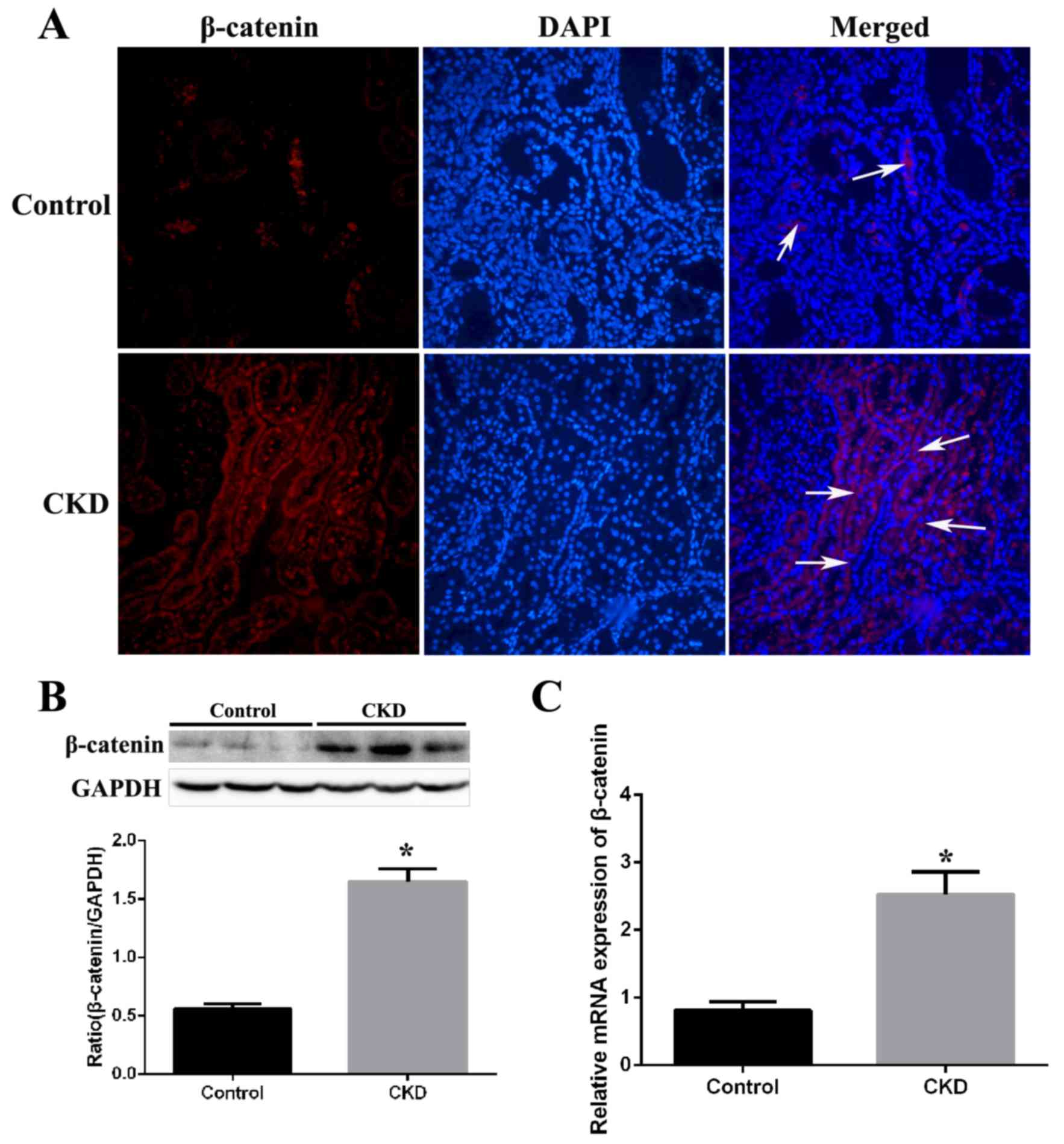
Expression of β-catenin in kidney
tissues from CKD rats
Expression of β-catenin was examined by
immunofluorescence staining in kidney tissue sections from CKD and
control rats. β-catenin staining was barely observed in the kidney
tissues of control rats, but was markedly increased in the kidneys
of CKD rats (Fig. 2A). Western
blotting revealed that protein expression levels of β-catenin were
significantly increased by 2.8-fold (P=0.002) in CKD group kidney
tissues compared with the control group (Fig. 2B). Assessment of mRNA expression
levels by RT-qPCR revealed a significant 3.1-fold (P<0.001)
increase in β-catenin mRNA expression levels in the CKD compared
with the control group (Fig.
2C).
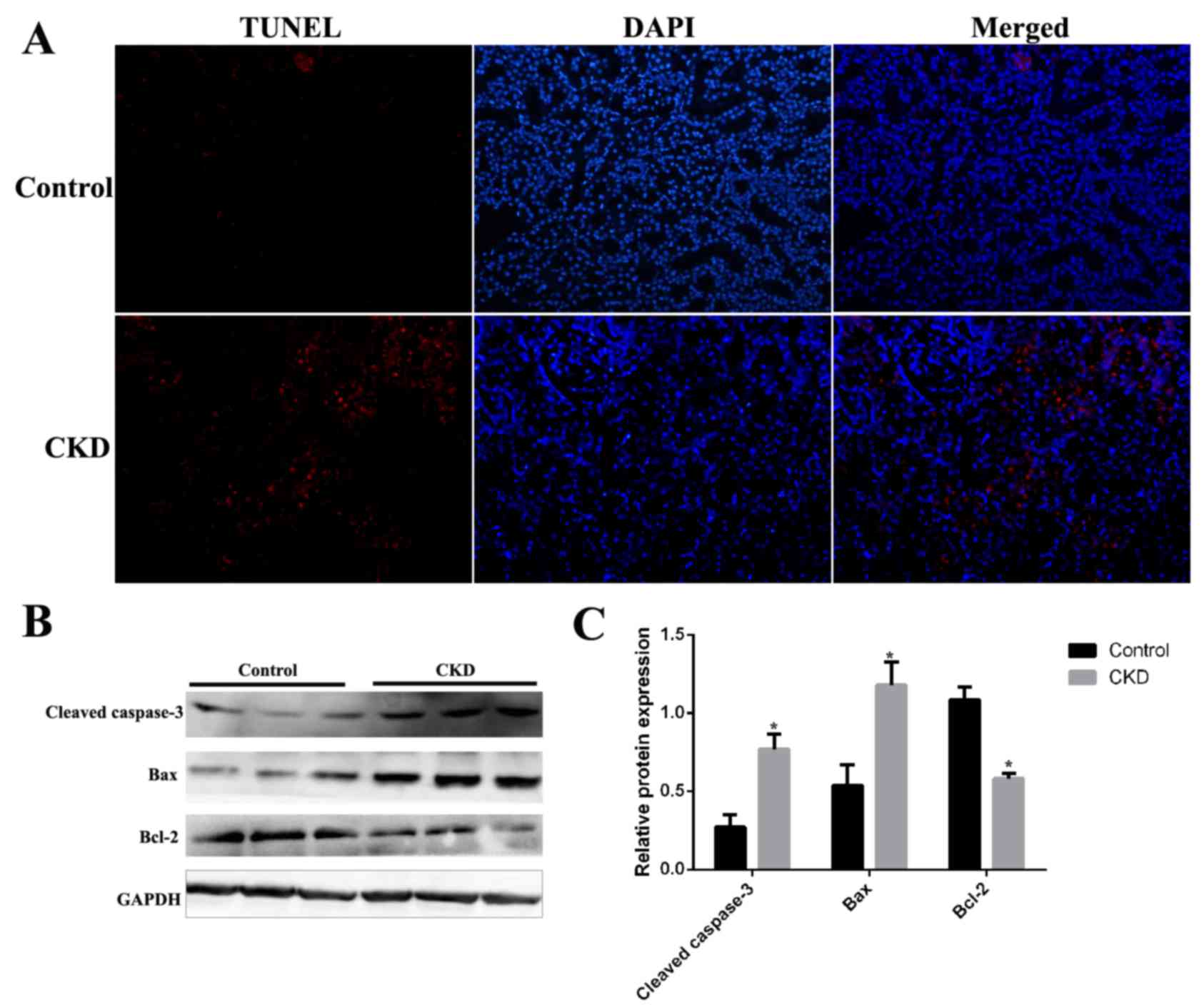
Renal cell apoptosis in CKD rats
Renal cell apoptosis was examined in kidney tissues
from CKD and control rats by terminal deoxynucleotidyl transferase
UTP nick-end labeling (TUNEL) assay. As demonstrated in Fig. 3, although TUNEL staining was almost
absent in kidney sections from the control group, it was present in
those from CKD rats (Fig. 4A). The
expression levels of the apoptosis-associated proteins cleaved
caspase-3, Bax and Bcl-2 were examined by western blotting in
kidney tissues from CKD and control rats. Protein expression levels
of cleaved caspase-3 and Bax were significantly increased and
levels of Bcl-2 were significantly decreased in CKD rat kidney
tissues compared with the control group (Fig. 3B).
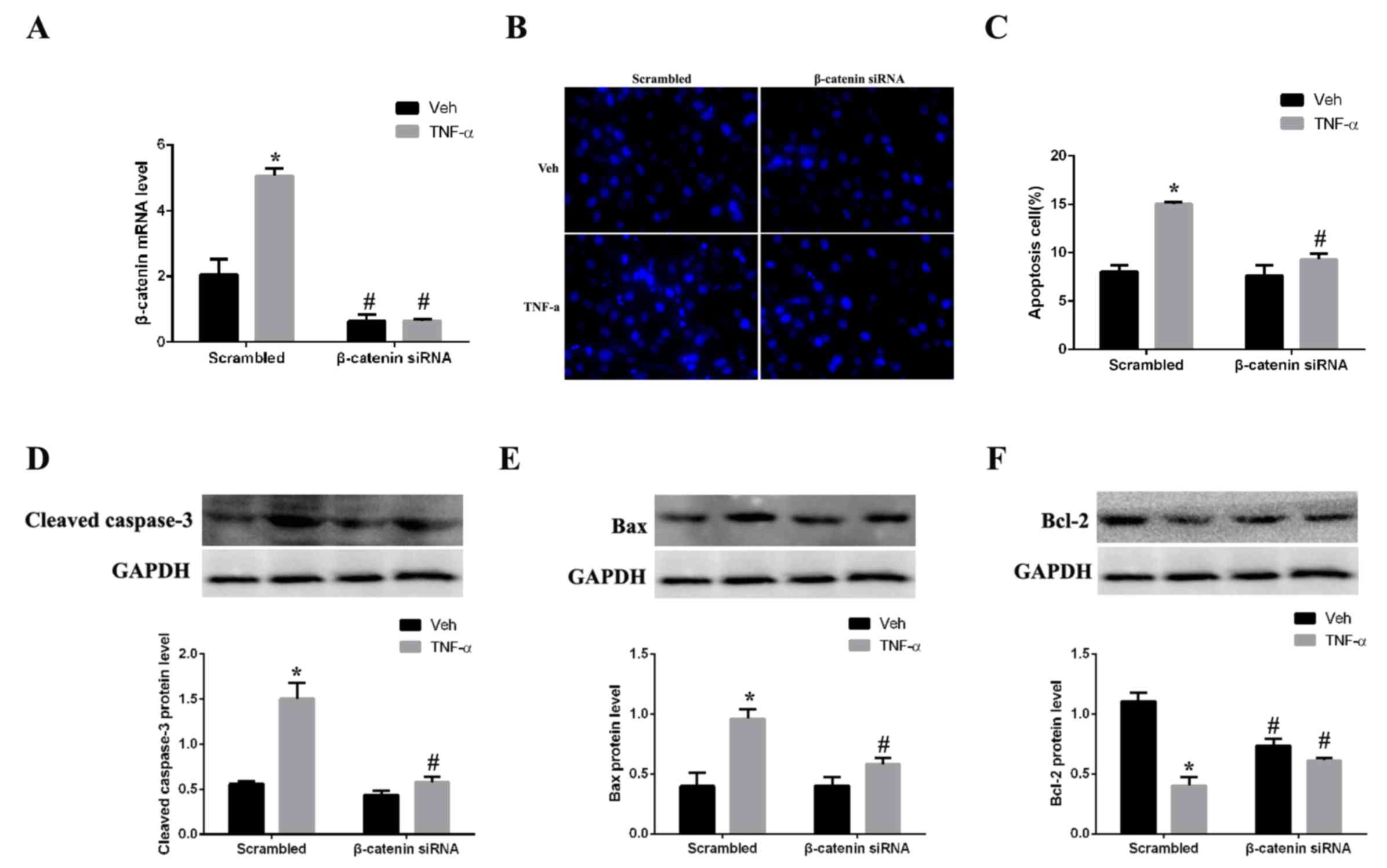
Silencing of β-catenin attenuates
apoptosis
As CKD rat kidney tissues exhibited increased
expression levels of β-catenin and apoptosis-associated proteins,
the hypothesis that β-catenin has an active role in regulating
apoptosis in renal cells was further tested. Expression of
β-catenin was silenced by siRNA in mesangial cells in vitro.
The mRNA expression levels of β-catenin in mesangial cells were
significantly decreased following β-catenin siRNA transfection
compared with scrambled control-transfected cells (Fig. 4A). Mesangial cells were
subsequently stimulated with TNF-α, an inducer of apoptosis, for 24
h. Expression of β-catenin was successfully silenced by siRNA in
the TNF-α-stimulated cells compared with the scramble
control-transfected cells (Fig.
4A). Assessment of apoptosis by Hoechst nuclear staining
demonstrated that TNF-α-treated cells exhibited concentrated cell
nuclei and numerous dead cells with collapsed plasmalemma and
solved karyotheca were observed, compared with vehicle-treated
cells (Fig. 4B). Quantification of
the staining revealed that the apoptosis rate was significantly
greater in the TNF-α group compared with the vehicle group
(Fig. 4C). However, β-catenin
silencing significantly inhibited TNF-α-induced apoptosis in
mesangial cells, compared with scrambled control-transfected cells,
suggesting that β-catenin may be important in regulating apoptosis.
Western blotting revealed that the expression levels of the
pro-apoptotic proteins cleaved caspase-3 (Fig. 4D) and Bax (Fig. 4E) were decreased in the β-catenin
siRNA + TNF-α group compared with the scrambled control + TNF-α
group, 2.1 and 1.5-fold (P<0.05), respectively. By contrast,
expression levels of the anti-apoptotic protein Bcl-2 were
increased 1.6-fold in the β-catenin siRNA+ TNF-α group compared
with the scrambled control + TNF-α group (Fig. 4F).
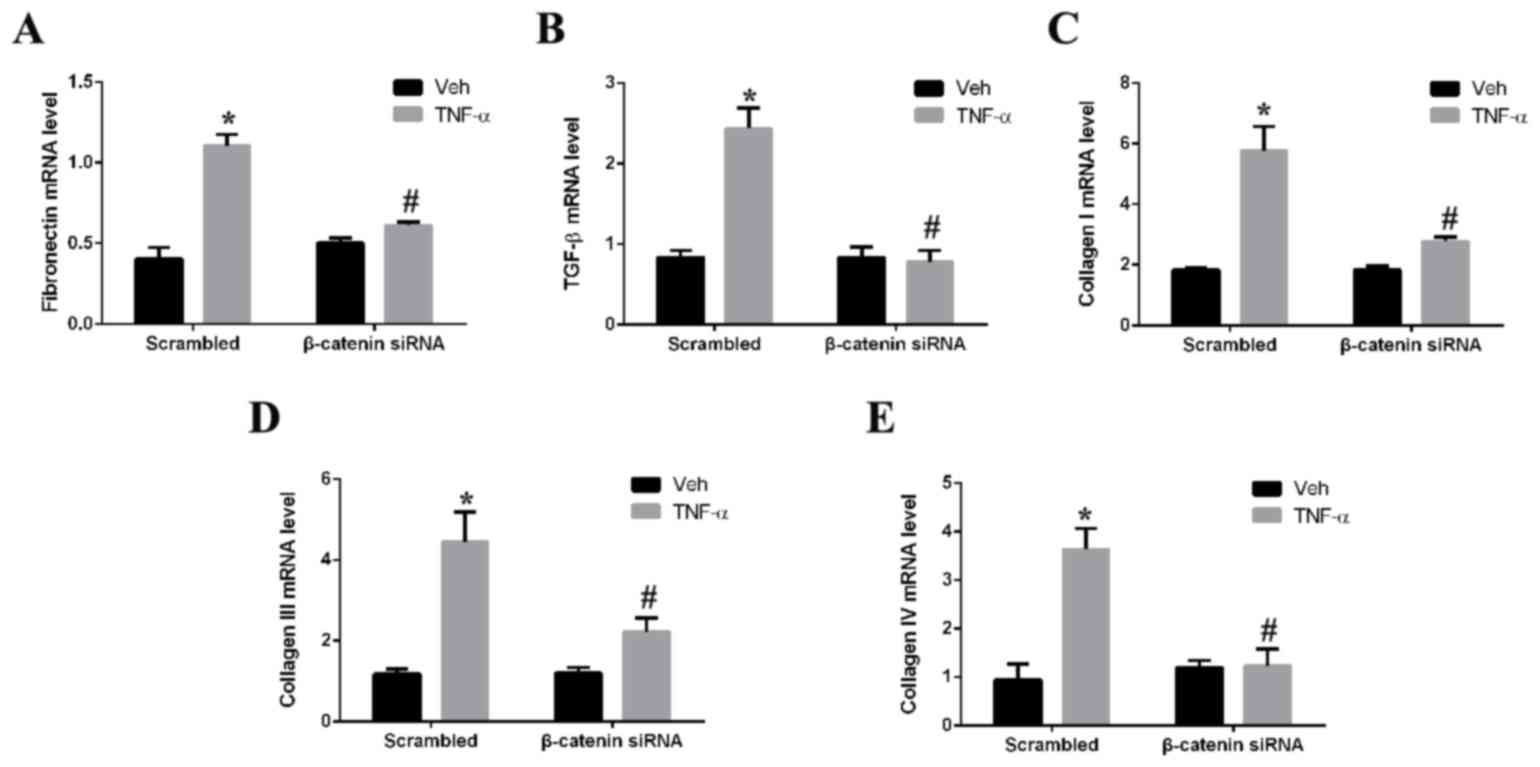
Silencing of β-catenin alleviates
fibrosis
The role of β-catenin in renal fibrosis was assessed
by determining the effect of β-catenin silencing on TNF-α-induced
expression of fibrotic markers in mesangial cells in vitro.
Treatment of mesangial cells with TNF-α significantly upregulated
mRNA expression levels of fibronectin (Fig. 5A), TGF-β (Fig. 5B), and collagen I (Fig. 5C), III (Fig. 5D) and IV (Fig. 5E) compared with vehicle-treated
cells. Silencing of β-catenin by siRNA prior to TNF-α treatment,
however, significantly decreased the mRNA expression levels of all
fibrotic markers assessed. The mRNA expression levels of
fibronectin, TGF-β, and collagen I, III and IV decreased by 2,
2.9,2.4, 2.1 and 3.1-fold (P<0.05), respectively, in the
β-catenin siRNA + TNF-α group compared to the scrambled control +
TNF-α group.
Discussion
The evolutionarily conserved Wnt/β-catenin signaling
pathway is a complex and critical developmental pathway that has
crucial roles in maintaining tissue homeostasis and initiating
organ repair following injury (22). Previous studies have demonstrated a
protective role for Wnt/β-catenin signaling in healing and repair
following acute kidney injury (23,24);
however, excessive and continuous Wnt/β-catenin signaling may
result in renal fibrosis and CKD progression. Chronic and
progressive upregulation of β-catenin is a final pathway common to
a wide variety of fibrotic CKDs (25,26),
including diabetic nephropathy, obstructive nephropathy, adriamycin
nephropathy, chronic allograft nephropathy, polycystic kidney
disease and, as demonstrated in the present study, remnant
5/6-nephrectomy. A recent study has indicated that sustained
activation of Wnt/β-catenin signaling induces renal interstitial
fibroblast activation and promotes fibronectin expression,
resulting in the progression from acute kidney injury to CKD in
ischemia/reperfusion injury mice (27).
The present results indicated that expression of
β-catenin, and the apoptosis-associated proteins cleaved caspase-3
and Bax, were significantly increased in the kidneys of CKD rats
compared with controls. By contrast, expression of the
anti-apoptotic protein Bcl-2 was decreased in the kidneys of CKD
rats compared with controls. This is consistent with a previous
study, which demonstrated that the Wnt/β-catenin signaling pathway
is closely associated with mesangial cell apoptosis (28). The present study suggested that
renal tissue from CKD rats exhibited increased Wnt/β-catenin
signaling and increased apoptosis compared with control rats.
Previous studies have revealed cross-talk between
the Wnt/β-catenin signaling pathway and other fibrotic pathways,
including TGF-β signaling, and confirmed that certain
differentially expressed microRNAs targeting these pathways lead to
tissue injury and fibrosis (29,30).
These studies suggested that the Wnt/β-catenin signaling pathway is
important in renal fibrogenesis (3). In the tubulointerstitium and
glomeruli, hyperactive Wnt/β-catenin signaling exerts its effects
via induction of target fibrosis-associated genes, including snail
family transcriptional repressor 1, fibronectin, plasminogen
activator inhibitor-1, twist family bHLH transcription factor 1 and
matrix metalloproteinase-7 (22).
Of these fibrosis-associated genes, the role of fibronectin in
fibrosis has long been established. In the present study, mRNA
expression levels of fibronectin and various collagen genes were
demonstrated to be significantly increased in kidney tissues from
CKD rats compared with control rats.
Previous studies have confirmed that a variety of
strategies to block Wnt/β-catenin signaling, including at the
cellular, cytoplasmic and nuclear levels, protect against renal
fibrosis (12,13,31).
The present study demonstrated the role of the Wnt/β-catenin
signaling pathway in inducing apoptosis and renal fibrosis in
5/6-nephrectomized rats. Silencing of β-catenin in mesangial cells
in vitro using siRNA decreased the mRNA expression levels of
the pro-apoptotic proteins cleaved caspase-3 and Bax, and of the
fibrosis-associated proteins fibronectin, TGF-β, and collagen I,
III and IV. In addition, β-catenin silencing attenuated
TNF-α-induced apoptosis in mesangial cells. The present results
suggested that inhibition of Wnt/β-catenin signaling by β-catenin
silencing may be a promising strategy for therapeutic intervention
in renal fibrotic disorders. However, various limitations of the
present study should be considered. First, the in vivo
experiments consisted of static observations and therefore did not
fully describe the fibrosis process. Second, Wnt/β-catenin
inhibition was evaluated only in vitro in mesangial cells
and not in the rat CKD model. Further studies are required to fully
assess the potential of Wnt/β-catenin signaling inhibition as a
potential method of ameliorating renal fibrosis and CKD in
vivo.
In conclusion, the present study demonstrated that
β-catenin silencing by siRNA attenuated apoptosis and expression of
fibrosis-associated markers in mesangial cells. Thus, Wnt/β-catenin
signaling inhibition may be a potential intervention strategy for
renal fibrotic disorders in the future.
Acknowledgements
The present study was funded by the Science and
Technology Fund of Guizhou Health and Family Planning Commission
(grant no. gzwkj2014-2-133), National Natural Science Foundation of
China (grant no. 81503398), China Postdoctoral Science Foundation
(grant no. 2015M582372), Science and Technology Planning Project of
Guangdong Province (grant no. 2016A020226032), Natural Science
Foundation of Guangxi Province (grant nos. 2015GXNSFBA139171 and
2016GXNSFAA380005), Shenzhen Science and Technology Project (grant
nos. JCYJ20160428175036148 and JSGG20141017103353178), and Health
and Family Planning Commission of Shenzhen Municipality (grant no.
201,605,013).
References
|
1
|
Kazancioğlu R: Risk factors for chronic
kidney disease: An update. Kidney Int Suppl (2011). 3:368–371.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falke LL, Gholizadeh S, Goldschmeding R,
Kok RJ and Nguyen TQ: Diverse origins of the
myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol.
11:233–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo Y, Xiao L, Sun L and Liu F:
Wnt/beta-catenin signaling: A promising new target for fibrosis
diseases. Physiol Res. 61:337–346. 2012.PubMed/NCBI
|
|
4
|
Sutariya B, Jhonsa D and M N Saraf: TGF-β:
The connecting link between nephropathy and fibrosis.
Immunopharmacol Immunotoxicol. 38:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wynn TA: Common and unique mechanisms
regulate fibrosis in various fibroproliferative diseases. J Clin
Invest. 117:524–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kriz W, Kaissling B and Le Hir M:
Epithelial-mesenchymal transition (EMT) in kidney fibrosis: Fact or
fantasy? J Clin Invest. 121:468–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eger A, Stockinger A, Park J, Langkopf E,
Mikula M, Gotzmann J, Mikulits W, Beug H and Foisner R:
Beta-catenin and TGFbeta signalling cooperate to maintain a
mesenchymal phenotype after FosER-induced epithelial to mesenchymal
transition. Oncogene. 23:2672–2680. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato M: Upregulation of the
Wnt/beta-catenin pathway induced by transforming growth factor-beta
in hypertrophic scars and keloids. Acta Derm Venereol. 86:300–307.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng JH, She H, Han YP, Wang J, Xiong S,
Asahina K and Tsukamoto H: Wnt antagonism inhibits hepatic stellate
cell activation and liver fibrosis. Am J Physiol Gastrointest Liver
Physiol. 294:G39–G49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei J, Melichian D, Komura K, Hinchcliff
M, Lam AP, Lafyatis R, Gottardi CJ, MacDougald OA and Varga J:
Canonical Wnt signaling induces skin fibrosis and subcutaneous
lipoatrophy: A novel mouse model for scleroderma? Arthritis Rheum.
63:1707–1717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou T, He X, Cheng R, Zhang B, Zhang RR,
Chen Y, Takahashi Y, Murray AR, Lee K, Gao G and Ma JX: Implication
of dysregulation of the canonical wingless-type MMTV integration
site (WNT) pathway in diabetic nephropathy. Diabetologia.
55:255–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He W, Dai C, Li Y, Zeng G, Monga SP and
Liu Y: Wnt/beta-catenin signaling promotes renal interstitial
fibrosis. J Am Soc Nephrol. 20:765–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao S, He W, Li Y, Ding H, Hou Y, Nie J,
Hou FF, Kahn M and Liu Y: Targeted inhibition of β-catenin/CBP
signaling ameliorates renal interstitial fibrosis. J Am Soc
Nephrol. 22:1642–1653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akhmetshina A, Palumbo K, Dees C, Bergmann
C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, et al:
Activation of canonical Wnt signalling is required for
TGF-β-mediated fibrosis. Nat Commun. 3:7352012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arend RC, Londoño-Joshi AI, Samant RS, Li
Y, Conner M, Hidalgo B, Alvarez RD, Landen CN, Straughn JM and
Buchsbaum DJ: Inhibition of Wnt/β-catenin pathway by niclosamide: A
therapeutic target for ovarian cancer. Gynecol Oncol. 134:112–120.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Zhu H, Sun Z, Xiang Z, Ge Y, Ni C,
Luo Z, Qian W and Han X: Inhibition of Wnt/β-catenin signaling
promotes epithelial differentiation of mesenchymal stem cells and
repairs bleomycin-induced lung injury. Am J Physiol Cell Physiol.
307:C234–C244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanjore H, Degryse AL, Crossno PF, Xu XC,
McConaha ME, Jones BR, Polosukhin VV, Bryant AJ, Cheng DS, Newcomb
DC, et al: β-Catenin in the alveolar epithelium protects from lung
fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med.
187:630–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang DT, Huang RH, Cheng X, Zhang ZH, Yang
YJ and Lin X: Tanshinone IIA attenuates renal fibrosis and
inflammation via altering expression of TGF-β/Smad and NF-κB
signaling pathway in 5/6 nephrectomized rats. Int Immunopharmacol.
26:4–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verma UN, Surabhi RM, Schmaltieg A,
Becerra C and Gaynor RB: Small interfering RNAs directed against
β-catenin inhibit the in vitro and in vivo growth of colon cancer
cells. Clin Cancer Res. 9:1291–1300. 2003.PubMed/NCBI
|
|
21
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan RJ, Zhou D, Zhou L and Liu Y:
Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl
(2011). 4:84–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou D, Tan RJ, Fu H and Liu Y:
Wnt/β-catenin signaling in kidney injury and repair: A double-edged
sword. Lab Invest. 96:156–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng J and Dong Z: Role changes of
β-catenin in kidney injury and repair. Kidney Int. 82:509–511.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai C, Stolz DB, Kiss LP, Monga SP,
Holzman LB and Liu Y: Wnt/beta-catenin signaling promotes podocyte
dysfunction and albuminuria. J Am Soc Nephrol. 20:1997–2008. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
von Toerne C, Schmidt C, Adams J, Kiss E,
Bedke J, Porubsky S, Gretz N, Lindenmeyer MT, Cohen CD, Gröne HJ
and Nelson PJ: Wnt pathway regulation in chronic renal allograft
damage. Am J Transplant. 9:2223–2239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou
FF and Liu Y: Sustained activation of Wnt/β-Catenin signaling
drives AKI to CKD progression. J Am Soc Nephrol. 27:1724–1740.
2016. View Article : Google Scholar
|
|
28
|
Lin CL, Cheng H, Tung CW, Huang WJ, Chang
PJ, Yang JT and Wang JY: Simvastatin reverses high glucose-induced
apoptosis of mesangial cells via modulation of Wnt Signaling
pathway. Am J Nephrol. 28:290–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haramis AP, Begthel H, van den Born M, Van
Es J, Jonkheer S, Offerhaus GJ and Clevers H: De novo crypt
formation and juvenile polyposis on BMP inhibition in mouse
intestine. Science. 30:1684–1686. 2004. View Article : Google Scholar
|
|
30
|
Polakis P: The many ways of Wnt in cancer.
CurrOpin Genet Dev. 17:45–51. 2007. View Article : Google Scholar
|
|
31
|
Zhou L, Li Y, Zhou D, Tan RJ and Liu Y:
Loss of Klotho contributes to kidney injury by derepression of
Wnt/β-Catenin signaling. J Am Soc Nephrol. 24:771–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|