Introduction
As the incidence of cancer continues to rise
progressively, radiotherapy plays a vital role in the treatment of
tumor. However, survivors after radiotherapy were plagued by a
series of adverse side effects (1), including various degrees of
radioactive bone injuries (2),
such as radioactive osteoporosis, radioactive osteomyelitis,
radioactive fractures, osteoradionecrosis or radioactive bone
development disorders, which have been confirmed by animal
experiments and population epidemiological studies. It has been
reported that pelvic irradiation substantially increases the risk
of pelvic fractures in women (3,4), and
rib fracture is frequently seen on CT after stereotactic body
radiotherapy for lung cancer (5,6).
Additionally, even if patients show no obvious signs of symptoms,
they often face a higher risk of bone mineral density (BMD) decline
(7). Meanwhile, in a mouse model,
it has been proved that irradiation could induce acute cancellous
bone loss in the tibiae and lumbar vertebrae (8). Therefore, it is very important to
uncover the mechanism underlying the radioactive bone injury and
develop appropriate preventive and treatment measures.
Recently, evidence suggested a new hidden danger of
radioactive bone injury resulting from radiotherapy, which is in
addition to the already known dangers of direct irradiated bone
injury after radiotherapy, bone loss or injury known as indirect
effect (9–11) may arise at indirect irradiated bone
tissue (12). Radiation induced
indirect effects on bone refer to the responses detected in
unirradiated bone when neighboring or remote bone tissue is
irradiated. It has been reported that postmenopausal women with
uterine cervical cancer treated with concurrent chemoradiation had
a lower BMD and were confronted with a higher risk of osteoporosis
(13). Moreover, it has been
reported that bone formation activity and total-body BMD of rats
decreased after abdominal irradiation (11). In conclusion, it has been proved
that irradiation could cause systemic adverse effects on the
non-irradiated skeleton, however, the indirect effects and the
mechanism of bone injury after radiotherapy has not been
well-studied.
It is common knowledge that the interaction between
osteoblasts and osteoclasts keeps the bone remodeling balance,
which plays a key role in maintaining bone health (14). The osteoblasts are differentiated
from BMMSCs, which is a type of heterogeneous and radiosensitive
cell, with the ability to differentiate into osteoblasts, neural
cells, chondrocytes, adipocytes and myoblasts (15,16).
It has been confirmed that BMMSCs' proliferation and its osteogenic
differentiation abilities were inhibited after irradiation
(17,18), and the possibility of
radiation-induced bone injury by damaging bone marrow
microenvironment for stem cells (19) has also been put forward. However,
there were sufficient uncertainties about the indirect effects of
radioactive bone injuries. Thus, further investigations are needed
to characterize the potential hazard of radiation-induced bone
injury and their associations to the dysfunction of BMMSCs. In this
regard, we established a local irradiated rat model to evaluate the
effects of irradiation on the proliferation and osteogenic
potential of BMMSCs obtained from direct and indirect irradiated
bones of rats, in order to develop a more thorough understanding of
the complete mechanism of indirect radioactive bone injuries.
Materials and methods
Animals
Male Sprague-Dawley rats aged 6 weeks were obtained
from the Department of Experimental Animals of the Fudan University
(Shanghai, China). The rats were housed at 20–26°C with 16-h light
and 8-h dark cycle, and provided ad libitum food and water.
All the animal handling and experimental procedures were approved
by the Committee for Ethical Use of Experimental Animals at Fudan
University (Shanghai, China), and the ethical approval registration
number for animal study was 20150559A183.
Ionizing radiation procedure
The rats were locally irradiated with 2 Gy by an
Animal X-ray Irradiator (X-RAD320, PXi, USA) at a rate of 185.5
cGy/min after being anesthetized. The irradiation site was
restricted in the region between distal femur and proximal tibia,
which was sensitive to irradiation (20). In addition, the irradiation area is
limited with the collimators, only the left hind limbs of rats were
exposed to irradiation directly, and the remaining parts of the
rats (including opposite right hind limbs) were covered by a
shielding device. The Source-to-Surface-Distance of irradiation is
60 cm, and the irradiation field is 2.54 cm2. Control
groups of rats were anesthetized and placed in the irradiator but
no exposure to irradiation.
Isolation and culture of BMMSCs
The BMMSCs were obtained from three male
Sprague-Dawley rats in each group at 3, 7 and 14 days after
irradiation. The rats were sacrificed by cervical dislocation and
the left and right femur and tibia of the local irradiated rats and
control rats were dissected, and then bone marrow mesenchymal stem
cells (BMMSCs) were obtained by density gradient centrifugation.
The BMMSCs isolated at 3, 7 and 14 days post-irradiation were named
D3, D7 and D14, respectively. BMMSCs isolated from direct
irradiated bones (left hind limbs of local irradiated rats) and
indirect irradiated bones (right hind limbs of local irradiated
rats) were named direct irradiated group and indirect irradiated
group. BMMSCs obtained from the non-irradiated rats were named
control group. The cells were cultured in culture media (DMEM;
Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco/Invitrogen, Grand Island, NY, USA), 100 U/ml penicillin
and streptomycin (North China Pharmaceutical Co., Ltd.,
Shijiazhuang, China) at 37°C in 5% humidified CO2. As
soon as the cells reached 80% confluence, they were detached using
0.25% trypsin-EDTA and replated into cell culture flasks.
Proliferation assay
The proliferation ability of BMMSCs was evaluated by
3-(4,5-dimethylithiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) assay. The cells isolated from direct, indirect irradiated
bones as well as the control group at 3, 7 and 14 days
post-irradiation were seeded in 96-well plates with 3,000 cells per
well. When the cells were cultured for 2, 3, 5, 7, 9 and 11 days,
the MTT assay were performed as described below. The culture medium
was removed and 100 µl of fresh culture medium containing 10 µl of
MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each
well (21,22). The cells were then incubated at
37°C for 4 h. The color was extracted with 100 µl 10% sodium
dodecyl sulfate (China Pharmaceutical Shanghai Chemical Reagent
Co., Ltd., Shanghai, China) at 37°C for 2 h. OD values, which were
directly related to the viable cell numbers, were determined at
room temperature using a microplate reader (Epoch 2 Microplate
Spectrophotometer; BioTek, Milan, Italy) and cell growth curves
were plotted.
Osteogenic induction of BMMSCs
When the third passage of BMMSCs isolated from
direct, indirect irradiated bones at 3, 7 and 14 days
post-irradiation were cultured for 48 h, we changed the medium into
osteogenic inductive medium. Specifically, the medium was prepared
by supplementing DMEM with 15% FBS, 50 mg/l ascorbic acid and
10−8 M dexamethasone (both from Sigma-Aldrich), 10 mM
β-glycerol phosphate (China Pharmaceutical Shanghai Chemical
Reagent Co., Ltd.) (17,23) and 100 U/ml penicillin and
streptomycin (North China Pharmaceutical Co., Ltd.). The medium was
changed every 3 days.
Alkaline phosphatase activity and
staining
The BMMSCs isolated from direct, indirect irradiated
bones as well as the control group at 3, 7 and 14 days
post-irradiation were seeded in 96-well plates with 3,000 cells per
well for ALP activity assay. On the 7th day after osteogenic
induction, ALP activity assay was performed as described below.
After being rinsed twice with phosphate-buffered saline (PBS), the
BMMSCs were lysed with 0.1% Triton X-100 (Sigma-Aldrich) at 4°C for
2 h. Then the cells were lysed three times by ultrasonication
(VCX130PB Serial; Sonics & Materials, Inc., Newtown, CT, USA)
for 10 sec at 20 kHz on ice. The cell supernatant was collected.
Next, 2-amino-2-methyl-1-propanol buffer containing p-nitrophenyl
phosphate (Fluka, Co., Milwaukee, WI, USA) were subsequently added
to the supernatant and the mixture was incubated for 30 min at
37°C. The reaction was stopped by adding 0.1 M NaOH. Finally, the
ALP activity was determined at the wavelength of 405 nm. Protein
content was measured using a BCA assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacturer's
instructions. All results were normalized in relation to protein
content (24). The ALP staining
assay was also performed on the 7th day after osteogenic induction,
and the BMMSCs isolated from direct, indirect irradiated bones and
unirradiated bones at 3, 7 and 14 days post-irradiation were seeded
in 48-well plate with 6,000 cells per well. The staining procedure
was washed the cells twice with PBS and fixed them in 2.5%
glutaraldehyde solution for 5 min, then, a BCIP/NBT Alkaline
Phosphatase Color Development kit (Beyotime Institute of
Biotechnology) was performed according to the manufacturer's
instructions.
Mineralization assay
The BMMSCs from direct and indirect irradiated
groups as well as the control group in D3, D7 and D14 were cultured
in 48-well plates (5×104 cells/well) with osteogenic
inductive medium for 3 weeks. Subsequently, the differentiated
cells were stained with Alizarin Red for osteogenic mineralized
nodules on the 21th day after osteogenic induction. Cells were
first washed with PBS and fixed in cold 95% (v/v) ethanol for an
hour, then the fixed BMMSCs were incubated with staining solution
(Alizarin Red-Tris-HCl, pH 8.3) at room temperature for 10 min. The
areas of positively stained mineral nodules were measured and
analyzed (25) with an optical
microscope and Simple PCI 5.2.1. imaging software.
RNA isolation, cDNA synthesis and gene
expression
The BMMSCs isolated from direct, indirect irradiated
bones as well as the control group at 3, 7 and 14 days
post-irradiation were cultured in 6-well plates (5×105
cells/well) with osteogenic inductive medium for a week. Then the
total RNA was harvested from cells using RNAprep Pure Cell/Bacteria
kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturer's protocols. The RNA was then used to synthesize
complementary DNA (cDNA) by using a Quantscript RT kit (Tiangen
Biotech Co., Ltd.). According to the manufacturer's instructions,
RT was performed in a 20-µl reaction mixture. Then cDNA was
analyzed on Real Time PCR Amplifier (Light Cycler 2.0; Roche
Diagnostics GmbH, Mannheim, Germany) with SYBR Premix Ex Taq Mix
(Takara Bio Inc., Otsu, Japan) for the expression of RUNX2 (also
called Cbfa1, initially expressed in osteoprogenitor cells) and
osteocalcin (OCN, also called Bglap, a late period marker in matrix
maturation). The PCR primers were designed by Primer 5. The gene
sequences were searched in MEDLINE. Rat RUNX2 forward,
5′-TGCCACCTCTGACTTCTGC-3′ and reverse, 3′-GATGAAATGCCTGGGAACTG-5′;
Rat OCN forward, 5′-GAACAGACAAGTCCCACAC-3′ and reverse,
3′-GAGCTCACACACCTCCCTG-5′; Rat β-actin forward,
5′-CACCCGCGAGTACAACCTTC-3′ and reverse, 3′-CCCATACCCACCATCACACC-5′.
All detections were in triplicate for each sample and data were
normalized to β-actin levels (ΔΔCq). The qPCR performed 40 cycles
at 95°C for 5 sec, and then at 60°C for 20 sec.
Western blot analysis
Western blot analysis was used to detect protein
expression levels of RUNX2 and ALP. The BMMSCs isolated from
direct, indirect irradiated bones as well as the control group at
3, 7 and 14 days post-irradiation were cultured in osteogenic
inductive medium for a week. Then total cytoplasmic protein was
isolated with RIPA lysis buffer and supplemented with
phenylmethylsulfonylfluoride (PMSF) (both from Beyotime Institute
of Biotechnology). Next, the samples were centrifuged at 20,000 ×
g for 10 min at 4°C. The protein contents were determined
using a BCA protein assay kit (Beyotime) according to
manufacturer's instructions. Equal quantities of total proteins
were separated by 10% (w/v) Tris glycine SDS/PAGE (Beyotime) and
transferred to PVDF membranes (Millipore). The membranes were then
blocked with 5% (w/v) non-fat milk in Tris-buffered saline with
Tween-20 for an hour at room temperature. We then incubated the
membranes with an optimal concentration of the primary antibodies:
anti-RUNX2 (1:1000, cat. no. 8486S; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-ALP (1:1000, cat. no. sc-98652; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-Tubulin
(Beyotime) overnight at 4°C. Immunoreactive bands were detected
with anti-rabbit (Cell Signaling Technology, Inc.) or goat
anti-mouse (Beyotime) fluorescein-conjugated secondary antibody and
visualized with chemiluminescent substrate (BeyoECL Plus; Beyotime)
by Gel Imaging system (Omega Lum C; Aplegen, San Francisco, CA,
USA). The protein expression levels were quantified by the optical
density ratio of the target and loading control proteins using
Image-Pro Plus 6.0 software.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) for separate experiments. Statistical analysis was done using
a one-way ANOVA by SPSS 20.0 software (SPSS, Inc., Chicago, IL,
USA). Values of P<0.05 were considered to be statistically
significant.
Results
Cell morphology
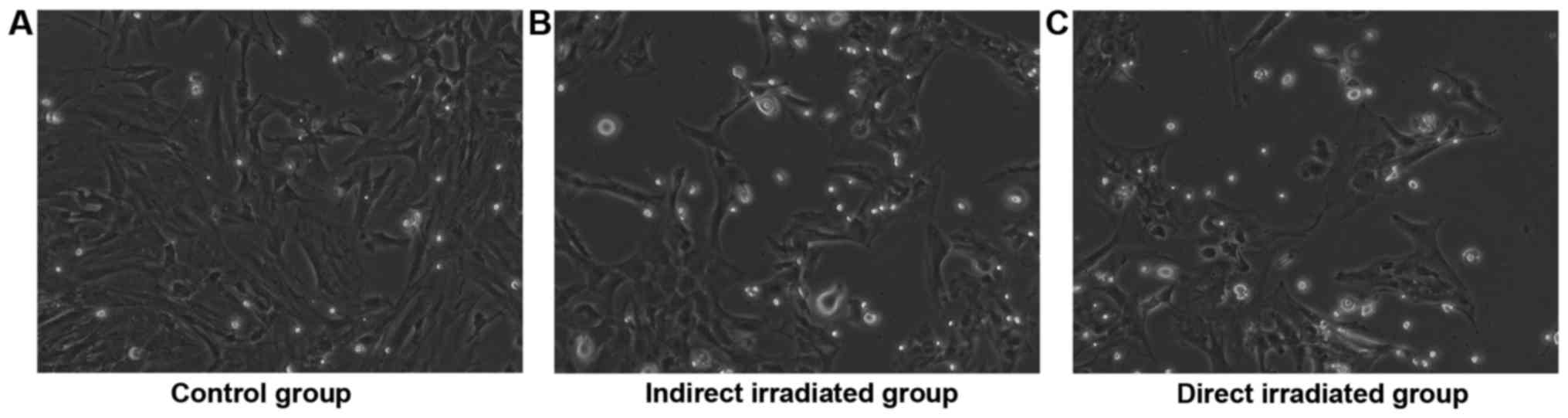
The BMMSCs isolated from the rats 3, 7 and 14 days
after local irradiation were named D3, D7 and D14, respectively.
Under a light microscope, no obvious morphological change was
observed 3 days post-irradiation in the direct and indirect
irradiated groups. However, there were significant morphological
changes in the cell shape in the direct and indirect irradiated
groups at 14 days post-irradiation compared to the control group.
In detail, the control group was presented with long spindle-shaped
adherent growth, with equal cytoplasm and oval nuclei (Fig. 1A), while the BMMSCs of direct and
indirect irradiated group were irregularly shaped, such as short
fusiform or polygons, with bigger cell bodies and increased
granules at 14 days post-irradiation (Fig. 1B and C).
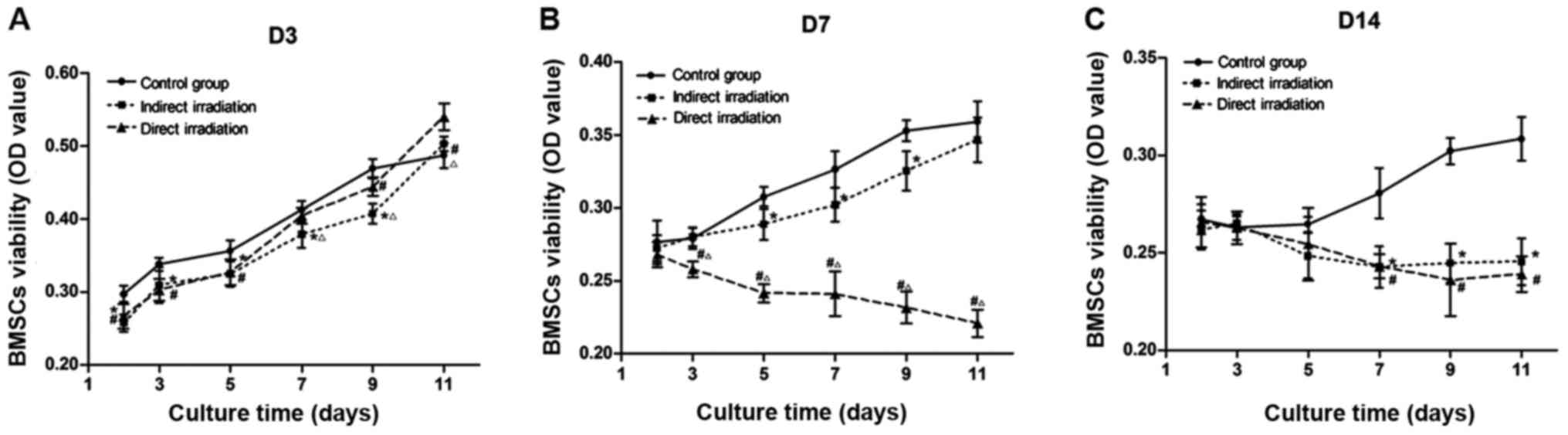
Cell proliferation
The proliferation ability of BMMSCs showed different
trends at 3, 7 and 14 days post-irradiation. At 3 days
post-irradiation, the proliferation ability of direct and indirect
irradiated group decreased slightly, with the decrease rate
reaching 20.9 and 32.0%, respectively (after 7 days culture).
However, at 7 days post-irradiation, the proliferation of BMMSCs in
the direct irradiated group was damaged significantly by 35.9%, and
the viability of BMMSCs in the indirect irradiated group was still
impaired (Fig. 2B). At 14 days
post-irradiation, the proliferation of BMMSCs obtained from direct
and indirect irradiated bones were both markedly inhibited by 40.4
and 39.9%, respectively (Fig.
2C).
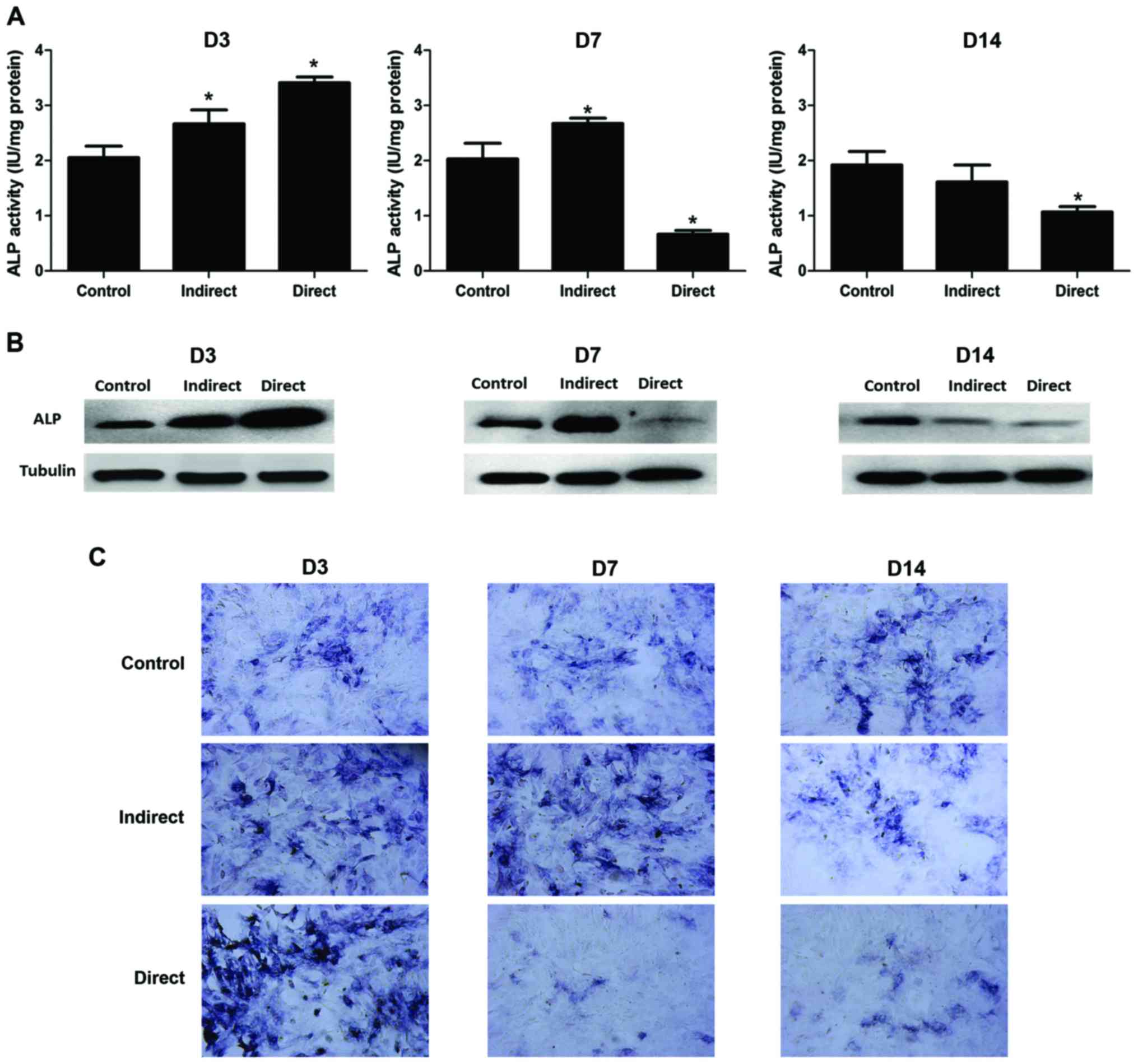
Osteogenic potential of BMMSCs
ALP is a marker for early osteoblast
differentiation, and its activity was analyzed to reflect the early
osteogenic potential of BMMSCs. The results of ALP activity
normalized by protein content (IU/mg protein) were shown as
Fig. 3A, and the ALP staining was
shown as Fig. 3C. In direct
irradiated groups, a transient increased ALP activity was observed
at 3 days post-irradiation, then a sharp drop of ALP activity was
observed at 7 days post-irradiation, the decrease rate reaching
67.2% compared with the control group, and only a little recovery
was observed at 14 days post-irradiation compared to 7 days
post-irradiation. Interestingly, in indirect irradiated groups, ALP
activity was found to increase both at 3 and 7 days
post-irradiation, with the increase rate reaching 29.9 and 31.3%,
respectively (P<0.05), and a decline of ALP activity was
observed only at 14 days post-irradiation, (decrease rate reaching
16.0%, P<0.05). The results of western blot analysis were shown
as Fig. 3B, and the results were
well consistent with ALP activity and staining.
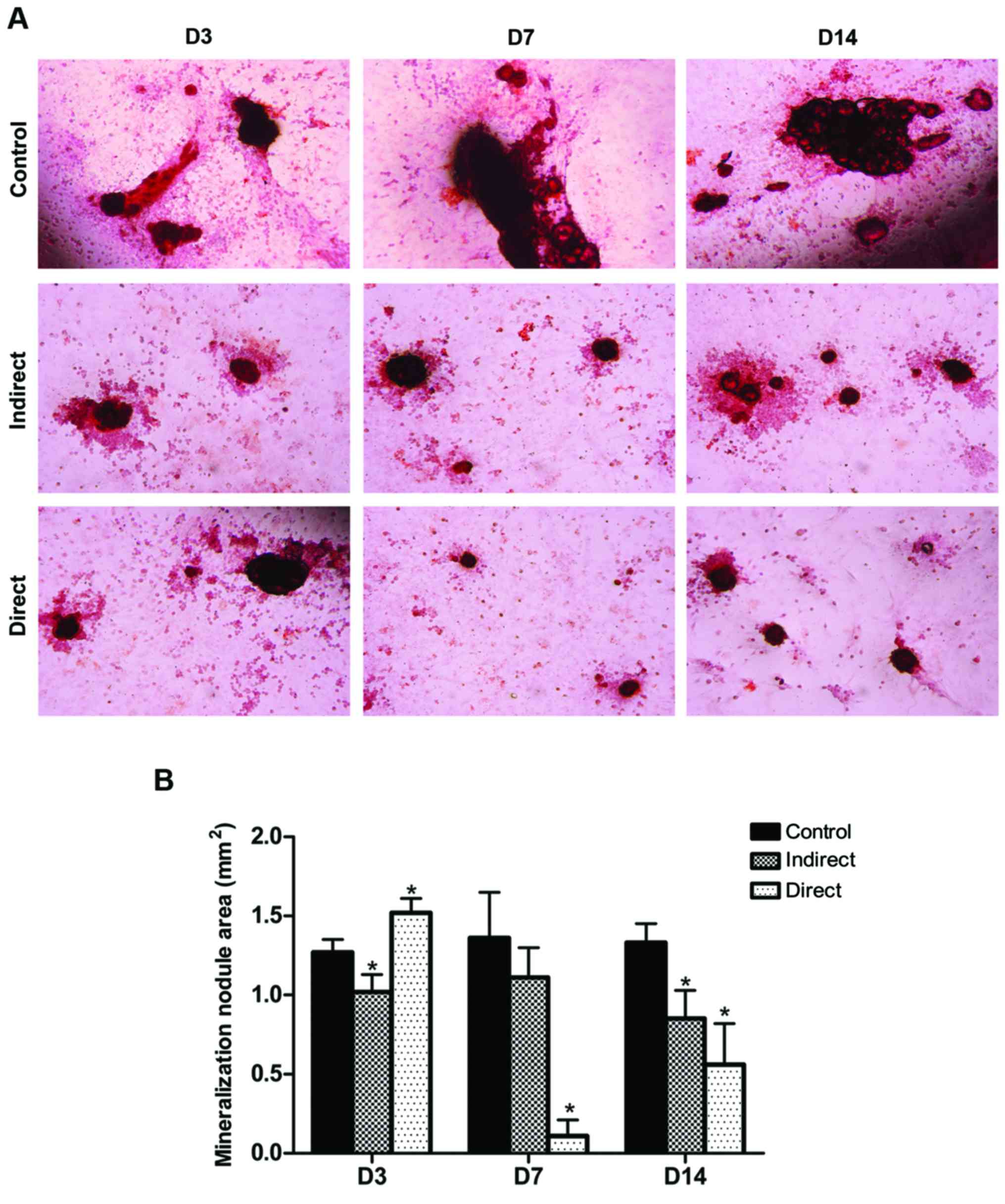
Mineralization ability
Alizarin Red staining was applied to reflect the
capacity of mineralization of osteoblast in vitro (Fig. 4A), and the area of bone
mineralization nodules quantified by Simple PCI 5.2.1. imaging
software were showed as Fig. 4B.
Typical nodular structure of mineralized tissue with clear cell
accumulation around the nodules can be observed in the control
group. In the direct irradiated group, a slight increase of
staining for mineralized nodules were observed 3 days
post-irradiation (the increase rate reaching 19.6%), while almost
no mineralization structure was observed 7 days post-irradiation
(the area of bone mineralization nodules decreased by 91.9%), and
only a little recovery was observed at 14 days post-irradiation
(the decrease rate reaching 57.9%). Additionally, in the indirect
irradiated group, there has been a modest decrease of the
calcium-richer mineralized matrix at 3, 7 and 14 days
post-irradiation, with the decrease rate reaching 19.7, 27.9 and
36.1%, respectively.
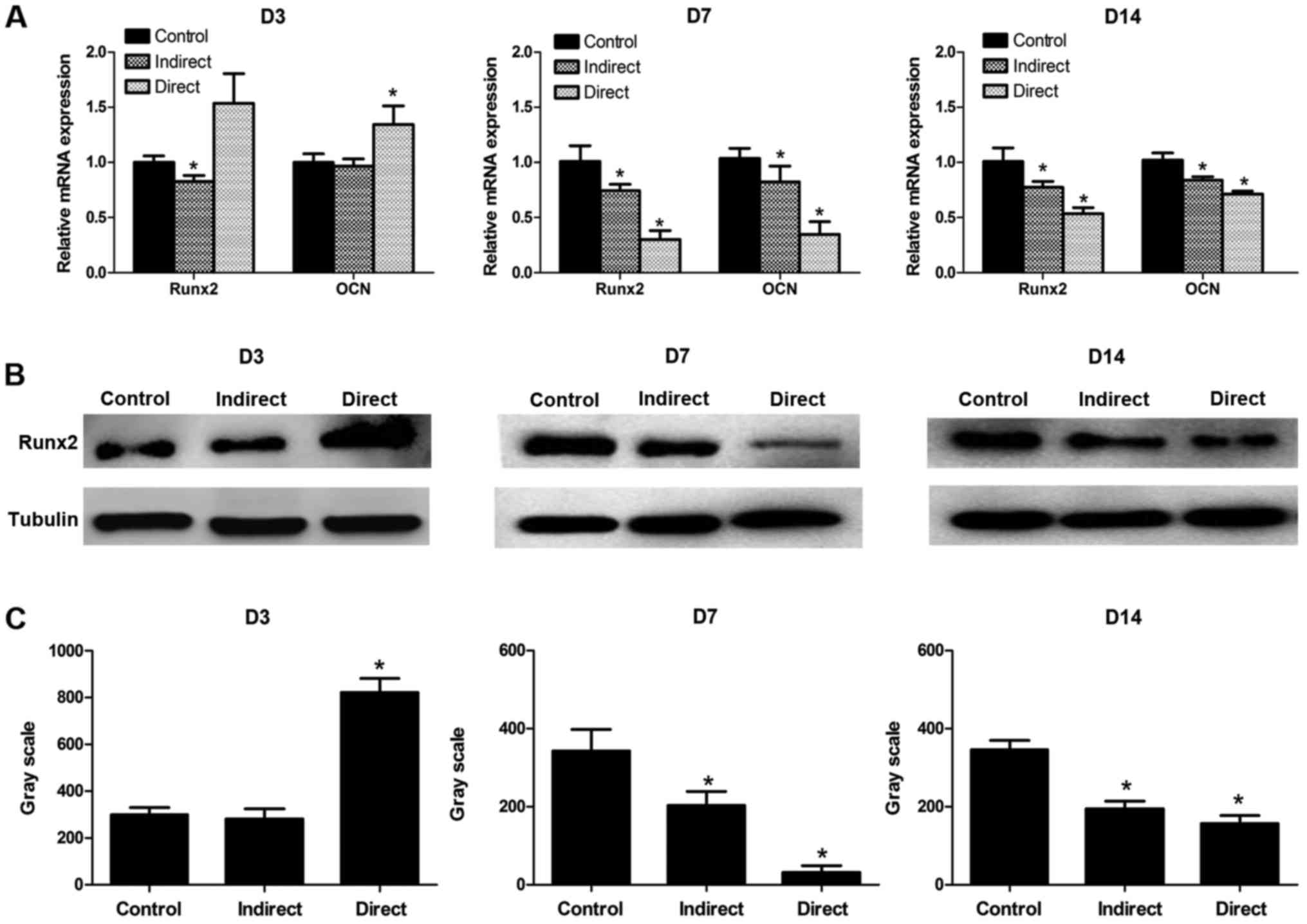
Differential expression of lineage-specific genes
and proteins. By using the real-time PCR, we found the expressions
of osteogenesis-related genes RUNX2 (Runt-related transcription
factor 2) and OCN (osteocalcin) altered in BMMSCs after irradiation
(Fig. 5A). In the direct
irradiated group, the mRNA expression levels of RUNX2 and OCN were
clearly higher than that of the control group at 3 days
post-irradiation, while a sharp decrease was observed at 7 days
post-irradiation, and no obvious recovery was found at 14 days
post-irradiation. In other words, in the direct irradiated group
there has been a more powerful osteogenic potential at 3 days
post-irradiation, but the enhanced potential decreased at 7 and 14
days post-irradiation. In the indirect irradiated group, the
expressions of RUNX2 and OCN dropped in varying degrees at 3, 7 and
14 days post-irradiation. Thus, the osteogenic potential of BMMSCs
obtained from indirect irradiated bones was impaired at early
stages of irradiation. The results of western blot analysis were
well consistent with the gene expression levels (Fig. 5B). The protein expression of RUNX2
at D3, D7, D14 groups were shown as Fig. 5B with the control groups showed the
basic level. The optical density of the protein bands of RUNX2
(related to tubulin) was quantified, and the results were shown as
Fig. 5C.
Discussion
Clinical studies (26,27)
and murine model experiments (28)
have proved that irradiation can cause bone loss and other bone
complications, which considered to be a type of late radiation
damage (3,4,29).
In preclinical models, sublethal irradiation may lead to impaired
bone formation and increased osteoclast activity, thereby
contributing to a rapid collapse in bone quantity and quality
(30–33). Currently, clinical treatment
strategy usually adopted irradiation with fractionation dose of 2
Gy every time (34,35) rather than disposable high dose
treatment. According to our pre-experiment, too high dose of
irradiation would result in BMMSCs' death. Therefore, we built a
local irradiated rat model with 2 Gy irradiation dose to
investigate the effect of irradiation on BMMSCs. It is worth
mentioning that we specially selected male SD rats to avoid
estrogen affecting the results of the experiment (36,37).
And in the future, combine irradiation with the ovariectomized rat
model to investigate the effects of irradiation on BMMSCs will be a
promising research. In our local irradiated rat model, we found
that ionizing radiation can lead to radioactive bone injury and
indirect effects by affecting BMMSCs' proliferation and osteogenic
differentiation ability.
In present study, as the results of MTT assay shown,
the proliferation of BMMSCs in the direct irradiated group had no
significant change at 3 days post-irradiation, whereas there was a
sharp decline at 7 days post-irradiation, and no obvious recovery
was observed at 14 days post-irradiation. Although the
proliferation of BMMSCs in the indirect irradiated group has no
much difference with that of the control group at 3 days
post-irradiation, it declined at 7 days post-irradiation, and the
downward trend was more obvious at 14 days post-irradiation.
Therefore, we infer that the machinery of self-renewal of BMMSCs
may have been injured after irradiation, which deserves further
investigation. At the same time, the decreased proliferation of
BMMSCs may associate with the damage of the microenvironment of
BMMSCs. As an important component of the microenvironment of
BMMSCs, vasculatures were also seriously damaged after irradiation,
which may be a potential mechanism lead to subsequent impairment of
bone formation (19).
Simultaneously, the impairment of angiogenesis is also an important
factor lead to the poor nutrition and decreased number of BMMSCs
(38,39). On the other hand, we thought
irradiation may cause the shrink of the BM mesenchymal
stem/progenitor cells pool (40).
In addition, it has been proved that the levels of free radicals
were dramatically increased after irradiation, which may have great
relationships with the survival and self-renewal of BMMSCs.
Consistent with our study, some research found that irradiation can
reduce the number of osteoblasts from the irradiated area at 1 and
4 weeks after irradiation (19).
It is now well established that the
osteodifferentiation of BMMSCs is marked by sequential stages of
cellular proliferation and bone extracellular matrix maturation,
ALP activity peaks at the end of the proliferation stage and before
matrix maturation (41). Since
active osteoblast has high expression of ALP (42), ALP is regarded as a transient early
marker of osteodifferentiation. In present study, ALP activity was
analyzed to indicate the early osteogenic differentiation potential
of BMMSCs. As shown by our results, an enhanced ALP activity in the
direct irradiated group was observed at 3 days post-irradiation,
while declines were found at 7 and 14 days post-irradiation. This
indicates that, as a kind of stress response after irradiation, an
increase in early differentiation ability was seen in BMMSCs from
the direct irradiated group, but the early differentiation
potential decreased as time elapsed. Surprisingly, an increase in
ALP activity was found in the indirect irradiated group at both 3
and 7 days post-irradiation, and it finally dropped at 14 days
post-irradiation. We assumed that BMMSCs in the indirect irradiated
group made corresponding compensation with the early osteogenic
differentiation ability of BMMSCs in the direct irradiated group
decreasing over time. However, the early osteogenic differentiation
ability of indirect irradiated group declined in the long-term.
RUNX2, a differentiation regulator in the osteoblast
lineage, is necessary for osteoblast differentiation. Its
expression is maintained postnatally in fully differentiated
osteoblasts and is crucial for regulating the rate of bone matrix
deposition (43,44). OCN is a marker only expressed by
fully differentiated osteoblasts before the mature phase (42). As the results of RT-PCR and
Western-blot in direct irradiated group showed at 3 days
post-irradiation, the expression levels of RUNX2 and OCN were
consistently defected following a transient enhanced expression.
The mineral deposition showed an identical trend. In accord with
our findings, it has been found that irradiation could alter
BMMSCs' osteogenic potential in a murine TBI (total body
irradiation) model (40). The
downregulated RUNX2 potentially affects the EZH2 expression in
mature osteoblasts, which may be one of the most prominent
epigenetic enzymes during the osteogenic differentiation of BMMSCs.
A recent study proved that the expression of EZH2 increased after
irradiation (20). We assumed that
the decreased expression of RUNX2 may influence the osteogenic
potential and bone formation through LncRNA-ANCR/EZH2/RUNX2
feedback loop (45). In
particular, our study also emphasized the osteogenic
differentiation process of BMMSCs was accelerated in the early
phase (3 days post-irradiation) and delayed thereafter.
But in the indirect irradiated group, our results
showed that the mRNA expression of RUNX2 and OCN declined at 3 days
post-irradiation, and no significant recovery was found at 7 and 14
days post-irradiation. The results of western blot analysis and
mineralization assay in indirect irradiated group were well consist
with the results mentioned above. It may relate to some cytokines
secreted from the irradiated area or that irradiation may cause
some systemic response. We inferred that irradiation altered the
osteoblast differentiation program of the BMMSCs in the distal
limbs without direct irradiation, further causing indirect effects
of radioactive bone injuries. Overall, it was proved that the
differentiation potential of BMMSCs obtained from direct and
indirect irradiated bone tissues were both impaired after
irradiation. Therefore, it could be inferred that radioactive bone
injury may be associated with the dysfunction of BMMSCs. This
highlights the importance of preventing BMMSCs' injuries from
radiotherapy to accelerate cancer patients' recovery from
radioactive bone injuries.
By comparing results of the direct and indirect
irradiated groups, we found that although the function of BMMSCs in
the indirect irradiated group was impaired (11), it was less severe than the direct
irradiated group. Meanwhile, the results of changes in ALP
activity, mineral deposition, along with the mRNA and protein
expressions suggested that irradiation may alter the osteoblast
differentiation program of the BMMSCs in the distal limbs without
direct irradiation, further causing indirect effects of radioactive
bone injuries. Other studies also revealed that irradiation can
affect BMMSCs indirectly in the proximal femur without direct
irradiation (11,19). However, further research on how
irradiation intervene the differentiation signal pathway of BMMSCs
in indirect irradiated area are still need in order to determine
the underlying mechanisms of govern BMMSCs' proliferation and
differentiation, providing us more effective approaches to making
progress in stem cell-related therapies. In this study, the results
give us a clue that the complications involving radioactive bone
injuries after radiotherapy may be associated with the dysfunction
of BMMSCs. Our results also highlight the importance of enhancing
applications of BMMSCs in cell therapy and regenerative medicine
(15,16,46,47).
The life qualities of patients after radiotherapy
were deeply affected by radioactive bone injuries. It has been
reported that osteoporosis may be relevant to stem-cell dysfunction
(48), and it has also been proved
that irradiation may influence bone formation by interfering with
BMMSCs' proliferation and osteogenic potential (17,19).
On the other hand, some studies suggested that the mobilization of
stem cells in the circulation can occur in response to irradiation
(49–51). Interestingly, hematopoietic stem
cells in the circulation can differentiate into osteoclast
precursors, gathered onto bone remodeling surfaces and
differentiated into osteoclasts. It is still unclear if the changes
in the hematopoietic stem cells after irradiation related to the
numbers and activities of osteoclasts. And the relationship between
hematopoietic stem cells and bone resorption need further
investigation.
In conclusion, the indirect effects of radioactive
bone injury at the BMMSCs' level were explored in a local
irradiated rat model. Our results suggest that radioactive bone
injury may relate to the BMMSCs' dysfunction, and irradiation has
indirect effects on the proliferation and osteogenic ability of
BMMSCs, which may be an important mechanism leading to subsequent
impairment of bone formation after radiotherapy.
Acknowledgements
The present study was sponsored by the Shanghai
Natural Science Fund (grant no. 14ZR1401600) and Shanghai Municipal
Commission of Health (grant nos. 2013ZYJB0801 and 20154Y0202).
References
|
1
|
Johansson S, Svensson H and Denekamp J:
Timescale of evolution of late radiation injury after postoperative
radiotherapy of breast cancer patients. Int J Radiat Oncol Biol
Phys. 48:745–750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Williams HJ and Davies AM: The effect of
X-rays on bone: A pictorial review. Eur Radiol. 16:619–633. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baxter NN, Habermann EB, Tepper JE, Durham
SB and Virnig BA: Risk of pelvic fractures in older women following
pelvic irradiation. JAMA. 294:2587–2593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmeler KM, Jhingran A, Iyer RB, Sun CC,
Eifel PJ, Soliman PT, Ramirez PT, Frumovitz M, Bodurka DC and Sood
AK: Pelvic fractures after radiotherapy for cervical cancer:
Implications for survivors. Cancer. 116:625–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Overgaard M: Spontaneous radiation-induced
rib fractures in breast cancer patients treated with postmastectomy
irradiation. A clinical radiobiological analysis of the influence
of fraction size and dose-response relationships on late bone
damage. Acta Oncol. 27:117–122. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nambu A, Onishi H, Aoki S, Tominaga L,
Kuriyama K, Araya M, Saito R, Maehata Y, Komiyama T, Marino K, et
al: Rib fracture after stereotactic radiotherapy for primary lung
cancer: Prevalence, degree of clinical symptoms, and risk factors.
BMC Cancer. 13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choo R, Lukka H, Cheung P, Corbett T,
Briones-Urbina R, Vieth R, Ehrlich L, Kiss A and Danjoux C:
Randomized, double-blinded, placebo-controlled, trial of
risedronate for the prevention of bone mineral density loss in
nonmetastatic prostate cancer patients receiving radiation therapy
plus androgen deprivation therapy. Int J Radiat Oncol Biol Phys.
85:1239–1245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondo H, Yumoto K, Alwood JS, Mojarrab R,
Wang A, Almeida EA, Searby ND, Limoli CL and Globus RK: Oxidative
stress and gamma radiation-induced cancellous bone loss with
musculoskeletal disuse. J Appl Physiol (1985). 108:152–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia D, Gaddy D, Suva LJ and Corry PM:
Rapid loss of bone mass and strength in mice after abdominal
irradiation. Radiat Res. 176:624–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morgan WF and Sowa MB: Non-targeted
effects induced by ionizing radiation: Mechanisms and potential
impact on radiation induced health effects. Cancer Lett. 356:17–21.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernandez-Palomo C, Bräuer-Krisch E,
Laissue J, Vukmirovic D, Blattmann H, Seymour C, Schültke E and
Mothersill C: Use of synchrotron medical microbeam irradiation to
investigate radiation-induced bystander and abscopal effects in
vivo. Phys Med. 31:584–595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okonogi N, Saitoh J, Suzuki Y, Noda SE,
Ohno T, Oike T, Ohkubo Y, Ando K, Sato H and Nakano T: Changes in
bone mineral density in uterine cervical cancer patients after
radiation therapy. Int J Radiat Oncol Biol Phys. 87:968–974. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang JH, Song SH, Lee JK, Lee NW and Lee
KW: Bone mineral density after concurrent chemoradiation in
patients with uterine cervical cancer. Menopause. 17:416–420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mori S: Bone quality and strength relating
with bone remodeling. Clin Calcium. 26:17–27. 2016.(In Japanese).
PubMed/NCBI
|
|
15
|
Caplan AI and Bruder SP: Mesenchymal stem
cells: Building blocks for molecular medicine in the 21st century.
Trends Mol Med. 7:259–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lda S Meirelles, Fontes AM, Covas DT and
Caplan AI: Mechanisms involved in the therapeutic properties of
mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zhu G, Wang J and Chen J:
Irradiation alters the differentiation potential of bone marrow
mesenchymal stem cells. Mol Med Rep. 13:213–223. 2016.PubMed/NCBI
|
|
18
|
Islam MS, Stemig ME, Takahashi Y and Hui
SK: Radiation response of mesenchymal stem cells derived from bone
marrow and human pluripotent stem cells. J Radiat Res. 56:269–277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao X, Wu X, Frassica D, Yu B, Pang L,
Xian L, Wan M, Lei W, Armour M, Tryggestad E, et al: Irradiation
induces bone injury by damaging bone marrow microenvironment for
stem cells. Proc Natl Acad Sci USA. 108:pp. 1609–1614. 2011;
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo C, Li C, Yang K, Kang H, Xu X, Xu X
and Deng L: Increased EZH2 and decreased osteoblastogenesis during
local irradiation-induced bone loss in rats. Sci Rep. 6:313182016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou JF, Zhang H, Yuan X, Li J, Wei YJ and
Hu SS: In vitro effects of low-level laser irradiation for bone
marrow mesenchymal stem cells: Proliferation, growth factors
secretion and myogenic differentiation. Lasers Surg Med.
40:726–733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soleimani M, Abbasnia E, Fathi M, Sahraei
H, Fathi Y and Kaka G: The effects of low-level laser irradiation
on differentiation and proliferation of human bone marrow
mesenchymal stem cells into neurons and osteoblasts-an in vitro
study. Lasers Med Sci. 27:423–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choong PF, Mok PL, Cheong SK, Leong CF and
Then KY: Generating neuron-like cells from BM-derived mesenchymal
stromal cells in vitro. Cytotherapy. 9:170–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khadra M, Lyngstadaas SP, Haanaes HR and
Mustafa K: Effect of laser therapy on attachment, proliferation and
differentiation of human osteoblast-like cells cultured on titanium
implant material. Biomaterials. 26:3503–3509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ozawa Y, Shimizu N, Kariya G and Abiko Y:
Low-energy laser irradiation stimulates bone nodule formation at
early stages of cell culture in rat calvarialcells. Bone.
22:347–54. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galotto M, Berisso G, Delfino L, Podesta
M, Ottaggio L, Dallorso S, Dufour C, Ferrara GB, Abbondandolo A,
Dini G, et al: Stromal damage as consequence of high-dose
chemo/radiotherapy in bone marrow transplant recipients. Exp
Hematol. 27:1460–1466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hopewell JW: Radiation-therapy effects on
bone density. Med Pediatr Oncol. 41:208–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamilton SA, Pecaut MJ, Gridley DS, Travis
ND, Bandstra ER, Willey JS, Nelson GA and Bateman TA: A murine
model for bone loss from therapeutic and space-relevant sources of
radiation. J Appl Physiol (1985). 101:789–793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grigsby PW, Roberts HL and Perez CA:
Femoral neck fracture following groin irradiation. Int J
RadiatOncol Biol Phys. 32:63–67. 1995. View Article : Google Scholar
|
|
30
|
Bonyadi M, Waldman SD, Liu D, Aubin JE,
Grynpas MD and Stanford WL: Mesenchymal progenitor self-renewal
deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null
mice. Proc Natl Acad Sci USA. 100:pp. 5840–5845. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Willey JS, Livingston EW, Robbins ME,
Bourland JD, Tirado-Lee L, Smith-Sielicki H and Bateman TA:
Risedronate prevents early radiation-induced osteoporosis in mice
at multiple skeletal locations. Bone. 46:101–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kondo H, Searby ND, Mojarrab R, Phillips
J, Alwood J, Yumoto K, Almeida EA, Limoli CL and Globus RK:
Total-body irradiation of postpubertal mice with (137)Cs acutely
compromises the microarchitecture of cancellous bone and increases
osteoclasts. Radiat Res. 171:283–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Willey JS, Lloyd SA, Robbins ME, Bourland
JD, Smith-Sielicki H, Bowman LC, Norrdin RW and Bateman TA: Early
increase in osteoclast number in mice after whole-body irradiation
with 2 Gy X rays. Radiat Res. 170:388–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zwahlen DR, Bischoff LI, Gruber G, Sumila
M and Schneider U: Estimation of second cancer risk after
radiotherapy for rectal cancer: Comparison of 3D conformal
radiotherapy and volumetric modulated arc therapy using different
high dose fractionation schemes. Radiat Oncol. 11:1492016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rades D, Sehmisch L, Bajrovic A, Janssen S
and Schild SE: Comparison of 20×2 Gy and 12×3 Gy for Whole-brain
Irradiation of multiple brain metastases from malignant melanoma.
In Vivo. 30:917–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piao H, Chu X, Lv W and Zhao Y:
Involvement of receptor-interacting protein 140 in
estrogen-mediated osteoclasts differentiation, apoptosis, and bone
resorption. J Physiol Sci. 67:141–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chuang SC, Chen CH, Fu YC, Tai IC, Li CJ,
Chang LF, Ho ML and Chang JK: Estrogen receptor mediates
simvastatin-stimulated osteogenic effects in bone marrow
mesenchymal stem cells. Biochem Pharmacol. 98:453–464. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deng Z, Huang H, Wu X, Wu M, He G and Guo
J: Distinct expression of various angiogenesis factors in mice
brain after whole-brain irradiation by X-ray. Neurochem Res.
42:625–633. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kleibeuker EA, Fokas E, Allen PD,
Kersemans V, Griffioen AW, Beech J, Im JH, Smart SC, Castricum KC,
van den Berg J, et al: Low dose angiostatic treatment counteracts
radiotherapy-induced tumor perfusion and enhances the anti-tumor
effect. Oncotarget. 7:76613–76627. 2016.PubMed/NCBI
|
|
40
|
Ma J, Shi M, Li J, Chen B, Wang H, Li B,
Hu J, Cao Y, Fang B and Zhao RC: Senescence-unrelated impediment of
osteogenesis from Flk1+ bone marrow mesenchymal stem cells induced
by total body irradiation and its contribution to long-term bone
and hematopoietic injury. Haematologica. 92:889–896. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: an in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo X, Chen J, Song WX, Tang N, Luo J,
Deng ZL, Sharff KA, He G, Bi Y, He BC, et al: Osteogenic BMPs
promote tumor growth of human osteosarcomas that harbor
differentiation defects. Lab Invest. 88:1264–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physio. 143:420–430. 1990.
View Article : Google Scholar
|
|
44
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu L and Xu PC: Downregulated LncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wagner J, Kean T, Young R, Dennis JE and
Caplan AI: Optimizing mesenchymal stem cell-based therapeutics.
Curr Opin Biotechnol. 20:531–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Filip S, Mokrý J and Hruska I: Adult stem
cells and their importance in cell therapy. Folia Biol (Praha).
49:9–14. 2003.PubMed/NCBI
|
|
48
|
Green DE, Adler BJ, Chan ME and Rubin CT:
Devastation of adult stem cell pools by irradiation precedes
collapse of trabecular bone quality and quantity. J Bone Miner Res.
27:749–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Harrison DE, Astle CM and Delaittre JA:
Loss of proliferative capacity in immunohemopoietic stem cells
caused by serial transplantation rather than aging. J Exp Med.
147:1526–1531. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Harrison DE and Astle CM: Loss of stem
cell repopulating ability upon transplantation. Effects of donor
age, cell number, and transplantation procedure. J Exp Med.
156:1767–1779. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Werts ED, Gibson DP, Knapp SA and DeGowin
RL: Stromal cell migration precedes hemopoietic repopulation of the
bone marrow after irradiation. Radiat Res. 81:20–30. 1980.
View Article : Google Scholar : PubMed/NCBI
|