|
1
|
Rodríguez LV, Alfonso Z, Zhang R, Leung J,
Wu B and Ignarro LJ: Clonogenic multipotent stem cells in human
adipose tissue differentiate into functional smooth muscle cells.
In: Proc Natl Acad Sci USA. 103. pp. 12167–12172. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai JY, Yoon CY, Yoo JJ, Wulf T and Atala
A: Phenotypic and functional characterization of in vivo tissue
engineered smooth muscle from normal and pathological bladders. J
Urol. 168:1853–1857. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silva GV, Litovsky S, Assad JA, Sousa AL,
Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, et al:
Mesenchymal stem cells differentiate into an endothelial phenotype,
enhance vascular density, and improve heart function in a canine
chronic ischemia model. Circulation. 111:150–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacou F, el Andalousi RB, Daussin PA,
Micallef JP, Levin JM, Chammas M, Casteilla L, Reyne Y and Nougues
J: Transplantation of adipose tissue-derived stromal cells
increases mass and functional capacity of damaged skeletal muscle.
Cell Transplant. 13:103–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou
GD, Liu W and Cao Y: Engineering of an elastic large muscular
vessel wall with pulsatile stimulation in bioreactor. Biomaterials.
29:1464–1472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y
and Cui L: A small diameter elastic blood vessel wall prepared
under pulsatile conditions from polyglycolic acid mesh and smooth
muscle cells differentiated from adipose-derived stem cells.
Biomaterials. 31:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cartwright MJ, Tchkonia T and Kirkland JL:
Aging in adipocytes: Potential impact of inherent, depot-specific
mechanisms. Exp Gerontol. 42:463–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weinzierl K, Hemprich A and Frerich B:
Bone engineering with adipose tissue derived stromal cells. J
Craniomaxillofac Surg. 34:466–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ashjian PA, Elbarbary AS, Edmonds B,
DeUgarte D, Zhu M, Zuk PA, Lorenz HP, Benhaim P and Hedrick HK: In
vitro differentiation of human processed lipoaspirate cells into
early neural progenitors. Plast Reconstr Surg. 111:1922–1931. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deslex S, Negrel R, Vannier C, Etienne J
and Ailhaud G: Differentiation of human adipocyte precursors in a
chemically defined serum-free medium. Int J Obes. 11:19–27.
1987.PubMed/NCBI
|
|
13
|
Wang C, Yin S, Cen L, Liu Q, Liu W, Cao Y
and Cui L: Differentiation of adipose-derived stem cells into
contractile smooth muscle cells induced by transforming growth
factor-beta1 and bone morphogenetic protein-4. Tissue Eng Part A.
16:1201–1213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lagna G, Ku MM, Nguyen PH, Neuman NA,
Davis BN and Hata A: Control of phenotypic plasticity of smooth
muscle cells by bone morphogenetic protein signaling through the
myocardin-related transcription factors. J Biol Chem.
282:37244–37255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida T, Sinha S, Dandré F, Wamhoff BR,
Hoofnagle MH, Kremer BE, Wang DZ, Olson EN and Owens GK: Myocardin
is a key regulator of CArG-dependent transcription of multiple
smooth muscle marker genes. Circ Res. 92:856–864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Wang DZ, Hockemeyer D, McAnally J,
Nordheim A and Olson EN: Myocardin and ternary complex factors
compete for SRF to control smooth muscle gene expression. Nature.
428:185–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miano JM: Channeling to myocardin. Circ
Res. 95:340–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
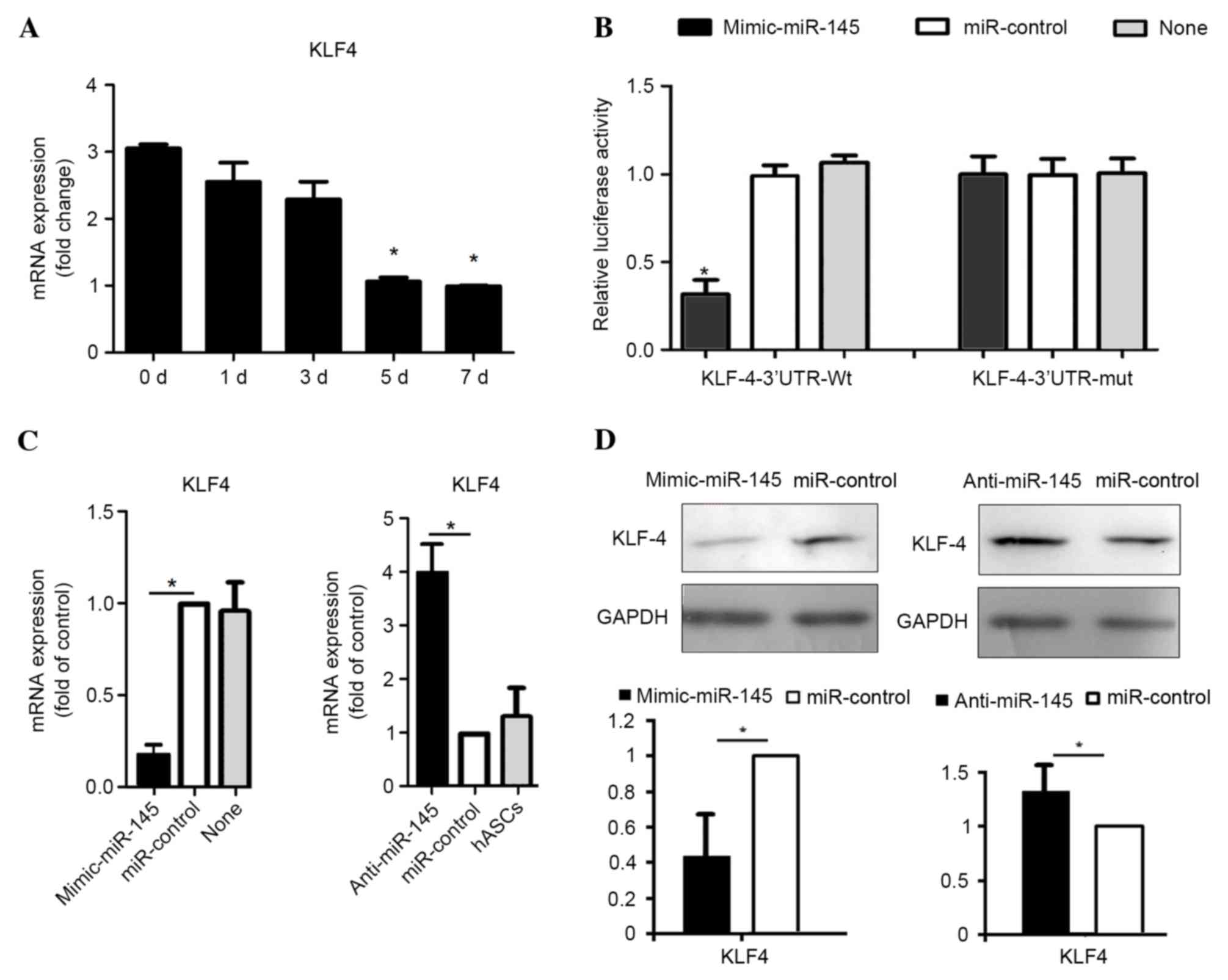
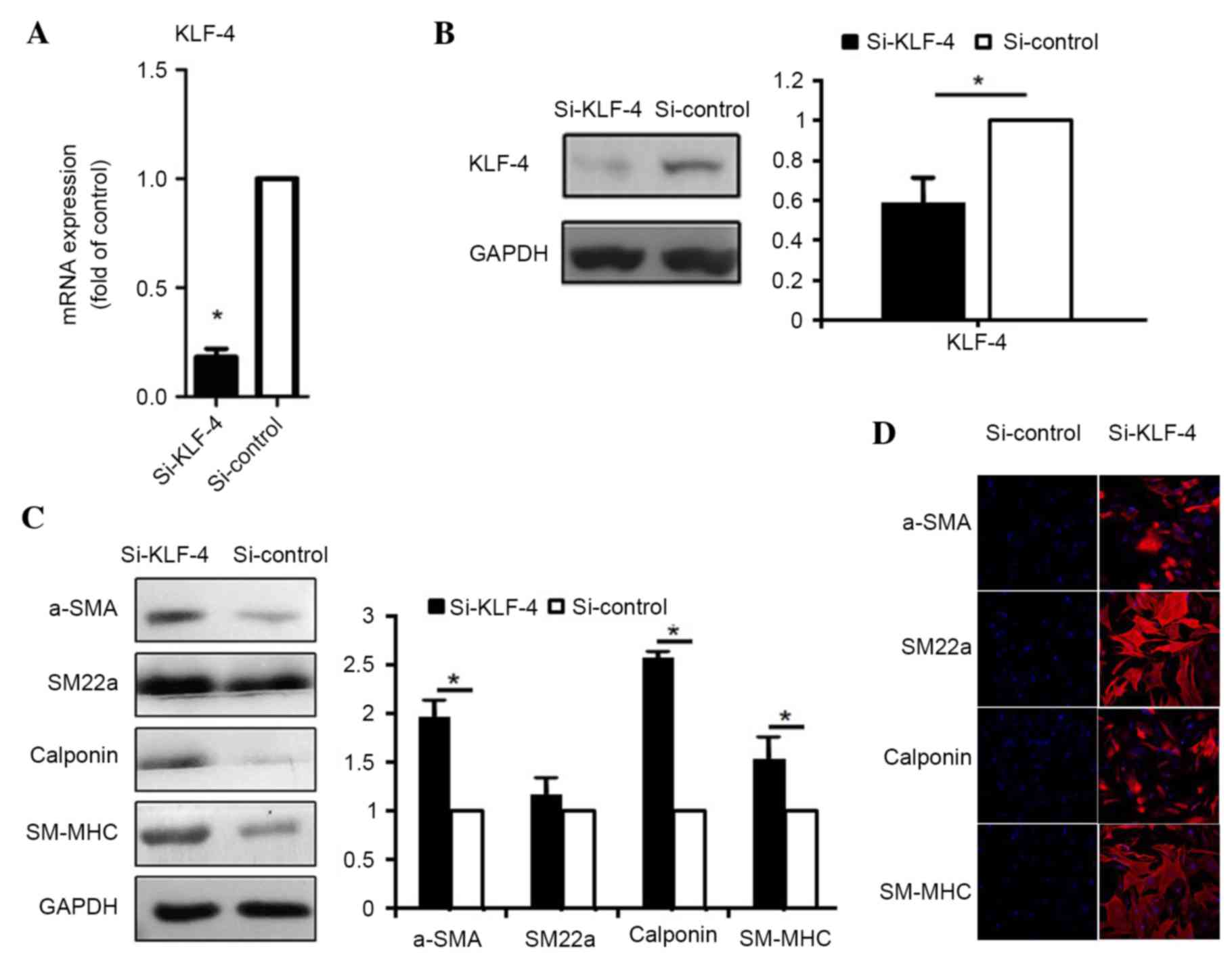
Liu Y, Sinha S, McDonald OG, Shang Y,
Hoofnagle MH and Owens GK: Kruppel-like factor 4 abrogates
myocardin-induced activation of smooth muscle gene expression. J
Biol Chem. 280:9719–9727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davis-Dusenbery BN, Chan MC, Reno KE,
Weisman AS, Layne MD, Lagna G and Hata A: Down-regulation of
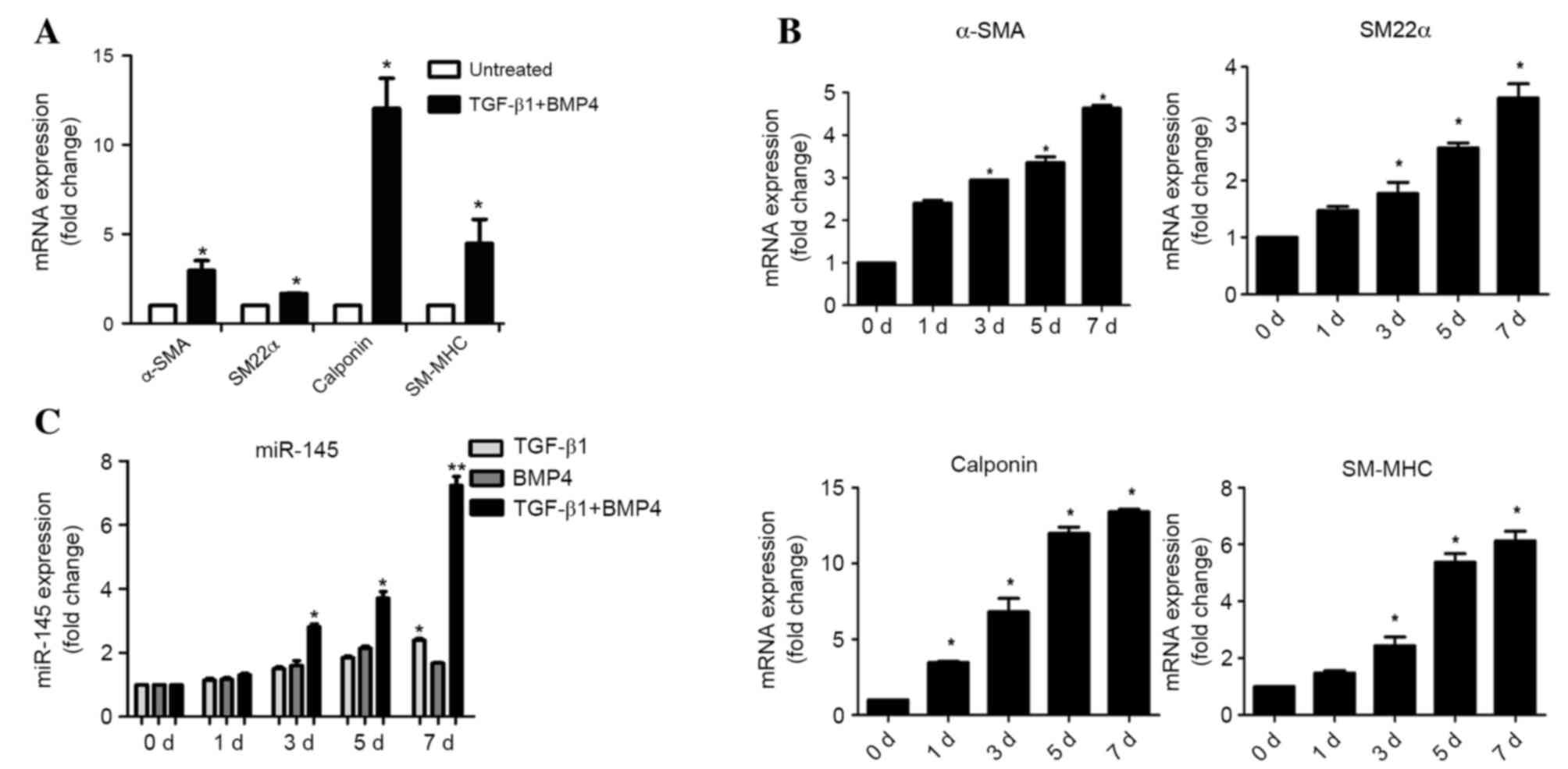
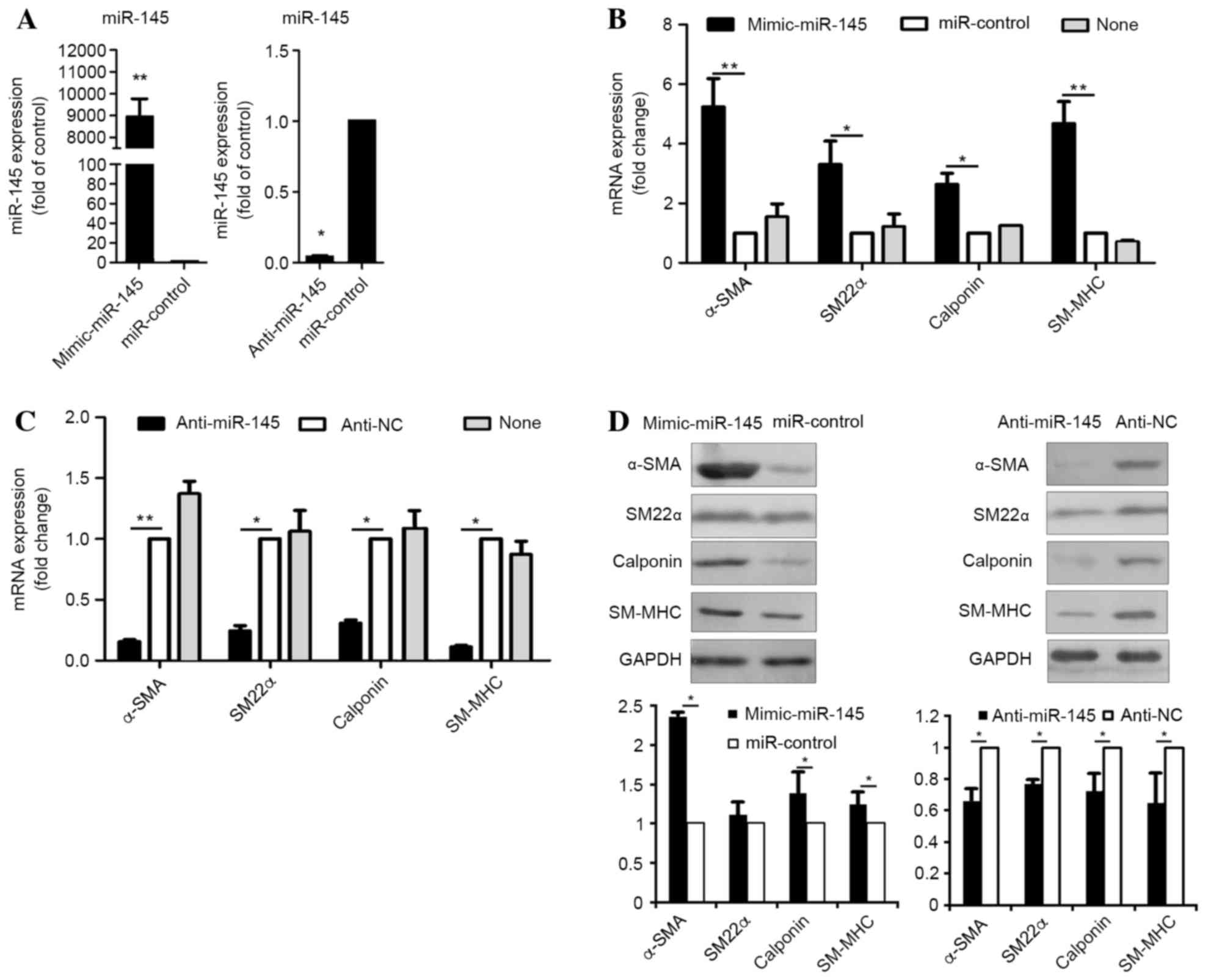
Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for
modulation of vascular smooth muscle cell phenotype by transforming
growth factor-beta and bone morphogenetic protein 4. J Biol Chem.
286:28097–29110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatfield SD, Shcherbata HR, Fischer KA,
Nakahara K, Carthew RW and Ruohola-Baker H: Stem cell division is
regulated by the microRNA pathway. Nature. 435:974–978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanellopoulou C, Muljo SA, Kung AL,
Ganesan S, Drapkin R, Jenuwein T, Livingston DM and Rajewsky K:
Dicer deficient mouse embryonic stem cells are defective in
differentiation and centromeric silencing. Genes Dev. 19:489–501.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cordes KR, Sheehy NT, White MP, Berry EC,
Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN and Srivastava D:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009.PubMed/NCBI
|
|
25
|
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu
Q, Deitch EA, Huo Y, Delphin ES and Zhang C: MicroRNA-145, a novel
smooth muscle cell phenotypic marker and modulator, controls
vascular neointimal lesion formation. Circ Res. 105:158–166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boettger T, Beetz N, Kostin S, Schneider
J, Krüger M, Hein L and Braun T: Acquisition of the contractile
phenotype by murine arterial smooth muscle cells depends on the
Mir143/145 gene cluster. J Clin Invest. 119:2634–2647. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xin M, Small EM, Sutherland LB, Qi X,
McAnally J, Plato CF, Richardson JA, Bassel-Duby R and Olson EN:
MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and
responsiveness of smooth muscle cells to injury. Genes Dev.
23:2166–2178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kozomara A and Griffiths-Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42(Database Issue): D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reczko M, Maragkakis M, Alexiou P,
Papadopoulos GL and Hatzigeorgiou AG: Accurate microRNA target
prediction using detailed binding site accessibility and machine
learning on proteomics data. Front Genet. 2:1032012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sartore S, Chiavegato A, Faggin E, Franch
R, Puato M, Ausoni S and Pauletto P: Contribution of adventitial
fibroblasts to neointima formation and vascular remodeling: From
innocent bystander to active participant. Circ Res. 89:1111–1121.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walsh K and Takahashi A: Transcriptional
regulation of vascular smooth muscle cell phenotype. Z Kardiol. 90
Suppl 3:S12–S16. 2001. View Article : Google Scholar
|
|
33
|
Hirschi KK, Lai L, Belaguli NS, Dean DA,
Schwartz RJ and Zimmer WE: Transforming growth factor-beta
induction of smooth muscle cell phenotype requires transcriptional
and post-transcriptional control of serum response factor. J Biol
Chem. 277:6287–6295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohnaka M, Marui A, Yamahara K, Minakata K,
Yamazaki K, Kumagai M, Masumoto H, Tanaka S, Ikeda T and Sakata R:
Effect of microRNA-145 to prevent vein graft disease in rabbits by
regulation of smooth muscle cell phenotype. J Thorac Cardiovasc
Surg. 148:676–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salmon M, Gomez D, Greene E, Shankman L
and Owens GK: Cooperative binding of KLF4, pELK-1 and HDAC2 to a
G/C repressor element in the SM22α promoter mediates
transcriptional silencing during SMC phenotypic switching in vivo.
Circ Res. 111:685–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshida T, Kaestner KH and Owens GK:
Conditional deletion of Krüppel-like factor 4 delays downregulation
of smooth muscle cell differentiation markers but accelerates
neointimal formation following vascular injury. Circ Res.
102:1548–1557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cherepanova OA, Pidkovka NA, Sarmento OF,
Yoshida T, Gan Q, Adiguzel E, Bendeck MP, Berliner J, Leitinger N
and Owens GK: Oxidized phospholipids induce type VIII collagen
expression and vascular smooth muscle cell migration. Circ Res.
104:609–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pidkovka NA, Cherepanova OA, Yoshida T,
Alexander MR, Deaton RA, Thomas JA, Leitinger N and Owens GK:
Oxidized phospholipids induce phenotypic switching of vascular
smooth muscle cells in vitro and in vivo. Circ Res. 101:792–801.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Sinha S and Owens G: A transforming
growth factor-beta control element required for SM alpha-actin
expression in vivo also partially mediates GKLF-dependent
transcriptional repression. J Biol Chem. 278:48004–48011. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li HX, Han M, Bernier M, Zheng B, Sun SG,
Su M, Zhang R, Fu JR and Wen JK: Krüppel-like factor 4 promotes
differentiation by transforming growth factor-beta
receptor-mediated Smad and p38 MAPK signaling in vascular smooth
muscle cells. J Biol Chem. 285:17846–17856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davis BN, Hilyard AC, Lagna G and Hata A:
SMAD proteins control DROSHA-mediated microRNA maturation. Nature.
454:56–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Davis BN, Hilyard AC, Nguyen PH, Lagna G
and Hata A: Induction of microRNA-221 by platelet-derived growth
factor signaling is critical for modulation of vascular smooth
muscle phenotype. J Biol Chem. 284:3728–3738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu X, Cheng Y, Zhang S, Lin Y, Yang J and
Zhang C: A necessary role of miR-221 and miR-222 in vascular smooth
muscle cell proliferation and neointimal hyperplasia. Circ Res.
104:476–487. 2009. View Article : Google Scholar : PubMed/NCBI
|