Introduction
Age-related macular degeneration (AMD) is a leading
cause of blindness in developed countries (1,2).
Choroidal neovascularization (CNV) is a damaging complication of
AMD (3); however, the exact
mechanism underlying the development of CNV has not been
determined. The laser-induced CNV mouse model is a well-known model
of CNV, which has been widely used to determine the underlying
mechanisms involved in its development (4). In this model, the depletion of
macrophages by clodronate liposomes leads to a reduction in the
size of the induced CNVs, indicating that macrophages are essential
for their formation and progression (5,6). In
addition, it has been reported that blood-derived F4/80-positive
macrophages infiltrate the retina and activate Müller cells under
the CNVs in this model (7). This
indicates that the recruited blood-derived macrophages associate
with the laser-induced CNV rather than the resident microglia.
There are two subtypes of macrophages, M1 and M2
(8,9). M1, or pro-inflammatory macrophages,
are considered to be important for the destruction of tumor cells
and foreign organisms, whereas M2, or anti-inflammatory
macrophages, have been suggested to be primarily involved in
angiogenesis, wound healing, chronic infections, tumorigenesis and
tumor metastasis (10–14). It has been suggested that the
pathological shift of macrophage polarization may contribute to the
pathogenesis of AMD (15).
It has been reported that M1 and M2 macrophages
express different levels of angiogenic cytokines and growth factors
(14). M2 macrophages have been
revealed to promote angiogenesis in in vivo studies
(14,16). In addition, it has been
demonstrated that more M2-like macrophages accumulate at the site
of wet compared with dry AMD (15), suggesting that M2 macrophages may
be involved in the development of CNVs. M2 macrophages injected
into the eyes of mice promoted the progression of CNV lesions,
whereas M1 macrophages inhibited them. These findings indicated
that these macrophage subtypes have a causal role in AMD (17). The number of cluster of
differentiation (CD)80-positive M1 macrophages and CD206-positive
M2 macrophages have been demonstrated to be increased in the
posterior segment of eyes in a CNV model (17). However, the exact locations of M1
and M2 macrophages in eyes with CNVs remain undetermined.
The aim of the present study was therefore to
investigate the expression and distribution of the M1 and M2
macrophages in a mouse model of laser-induced CNV.
Materials and methods
Animals
A total of 50 male C57BL/6 J mice (age, 6–9 weeks)
were purchased from Kyudo (Tosu, Japan) were used in the present
study. All mice were housed in a pathogen-free facility with access
to food and water under controlled conditions (temperature, 23±1°C;
humidity, 55%; 12-h light/dark cycle). All experimental procedures
were approved by the Committee on the Ethics of Animal Experiments
of Kyushu University Graduate School of Medical Sciences (Fukuoka,
Japan), and animals were cared for according to the Association for
Research in Vision and Ophthalmology Statement for the Use of
Animals in Ophthalmic and Vision Research.
Laser-induced CNV mouse model
Prior to the laser-treatment, mice were anesthetized
by an intraperitoneal injection of a mixture of ketamine (Ketalar;
80 mg/kg; Daiichi-Sankyo, Co., Ltd., Tokyo, Japan) and xylazine
(Selactar; 10 mg/kg; Bayer AG, Leverkusen, Germany). CNV was
induced by laser photocoagulation as previously described (18,19).
In brief, the photocoagulations were placed around the optic disc
with a 532-nm diode laser (200 mW, 0.1 sec duration, 75 µm; Verdi;
Coherent, Inc., Santa Clara, CA, USA). A total of five spots were
burned in each eye; four for observation and one for
orientation.
Prior to sacrifice, mice were anesthetized as above.
The mice were sacrificed by cervical dislocation at 1–7 days after
laser-treatment. The retinas were isolated from the posterior
segment of the eyes, and flat-mounted for immunofluorescence
studies. For reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), 20 spots were burned on each eye, and the eyes
were enucleated at various time-points following laser
photocoagulation.
RT-qPCR analysis
RT-qPCR was performed as described previously
(16,20). Total RNA was extracted from the
retinal pigment epithelium (RPE)-choroid complexes or homogenized
retinas at the selected time-points. The MagDEA® RNA kit
(Precision System Science USA, Inc., Pleasanton, CA, USA) was used
to extract RNA according to the manufacturer's protocol. RNA
concentrations were measured and cDNA was subsequently synthesized
using a First Strand cDNA Synthesis kit (Roche Diagnostics GmbH,
Mannheim, Germany). qPCR was performed using the TaqMan®
gene expression assays listed below (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and a
LightCycler® 96 Real-Time PCR system (Roche Diagnostics
GmbH).
CD80 and CD86 were used as M1 macrophage markers
(21), and CD206 and CD163 were
used as M2 macrophage markers (22,23),
and F4/80 was used as a pan macrophage marker. The reference
numbers for the assays were as follows: Mm99999915_g1 (GAPDH),
Mm00802529_m1 (F4/80), Mm00485148_m1 (CD206), Mm00474091_m1
(CD163), Mm00711660_m1 (CD80), Mm00444543_m1 (CD86), Mm00433287_m1
[basic fibroblast growth factor (Fgf2)], Mm00435613_m1 [placental
growth factor (Pgf)], Mm00449032_g1 [thrombospondin 1 (Thbs1)] and
Mm00441242_m1 [monocyte chemoattractant protein-1 (Ccl2)]. GAPDH
served as an endogenous control. For the TaqMan assays, the
HotStart DNA polymerase was activated by an initial 10-min
incubation at 95°C, followed by 45 cycles of 95°C for 20 sec and
60°C for 40 sec. The detection of the probe, calculation of
quantitation cycles (24) and
further analysis were performed using the LightCycler®
96 Real-Time PCR system software (Roche Diagnostics GmbH). Four
samples were examined in each group.
Immunofluorescence
Immunofluorescence staining was performed as
previously described (16,18,25),
with certain minor modifications. Briefly, eyes were enucleated and
fixed in 4% paraformaldehyde for 1 h, the corneas and muscles were
removed, and the posterior segments were placed in 4%
paraformaldehyde for a further 1 h. The lens, uvea and sclera were
excised, and the retina was isolated from certain posterior
segments. For other eyes, 20 µm sections were cut with a cryostat
(Leica CM1800; Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Following rinsing and blocking, the posterior segment of the eyes,
isolated retinas or cryostat sections were incubated with the
primary or conjugated antibodies overnight at 4°C. The following
secondary antibodies (1:200) were added for 1 h at room temperature
to detect anti-CD31 binding: Alexa Fluor® 488 chicken
anti-goat IgG (catalog no. A-21467), Alexa Fluor 546 donkey
anti-goat IgG (catalog no. A-11056) or Alexa Fluor 647 chicken
anti-goat IgG (catalog no. A-21469), all obtained from Thermo
Fisher Scientific, Inc. Hoechst 33342 (Molecular Probes; Thermo
Fisher Scientific, Inc.) was used to counterstain the nuclei in the
cryostat sections. Following rinsing with phosphate buffered saline
containing Tween-20 20, the cryostat sections and flat-mounts were
coverslipped using a PermaFluor aqueous mounting medium (Thermo
Fisher Scientific, Inc.).
The samples were incubated with the following
primary antibodies: Alexa Fluor 647 anti-mouse CD206 (1:100;
catalog no. 141712; BioLegend, Inc., San Diego, CA, USA),
fluorescein isothiocyanate anti-mouse CD80 (1:200; catalog no.
11-0801; eBioscience, Inc., San Diego, CA, USA),
fluorescein-labeled isolectin B4 (1:150; catalog no. FL-1201;
Vector Laboratories, Inc., Burlingame, CA, USA),
DyLight® 594-labeled isolectin B4 (1:150; catalog no.
DL-1207; Vector Laboratories, Inc.) or goat anti-mouse CD31 (1:20;
catalog no. AF3628; R&D Systems, Inc., Minneapolis, MN, USA).
The sections and flat-mounts were analyzed under a BZ-9000
fluorescence microscope (Keyence Corporation, Osaka, Japan)
(20,26,27).
Posterior segments of the eyes, containing connected retina and
choroid were examined and analyzed using a laser scanning confocal
microscope (Nikon Corporation, Tokyo, Japan) to obtain
three-dimensional images.
Statistical analysis
All results are presented as the mean ± standard
error. Differences between groups were compared using Dunnett's
tests or Student's t-tests. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using JMP software version 10.0.2 (SAS Institute, Cary,
NC, USA).
Results
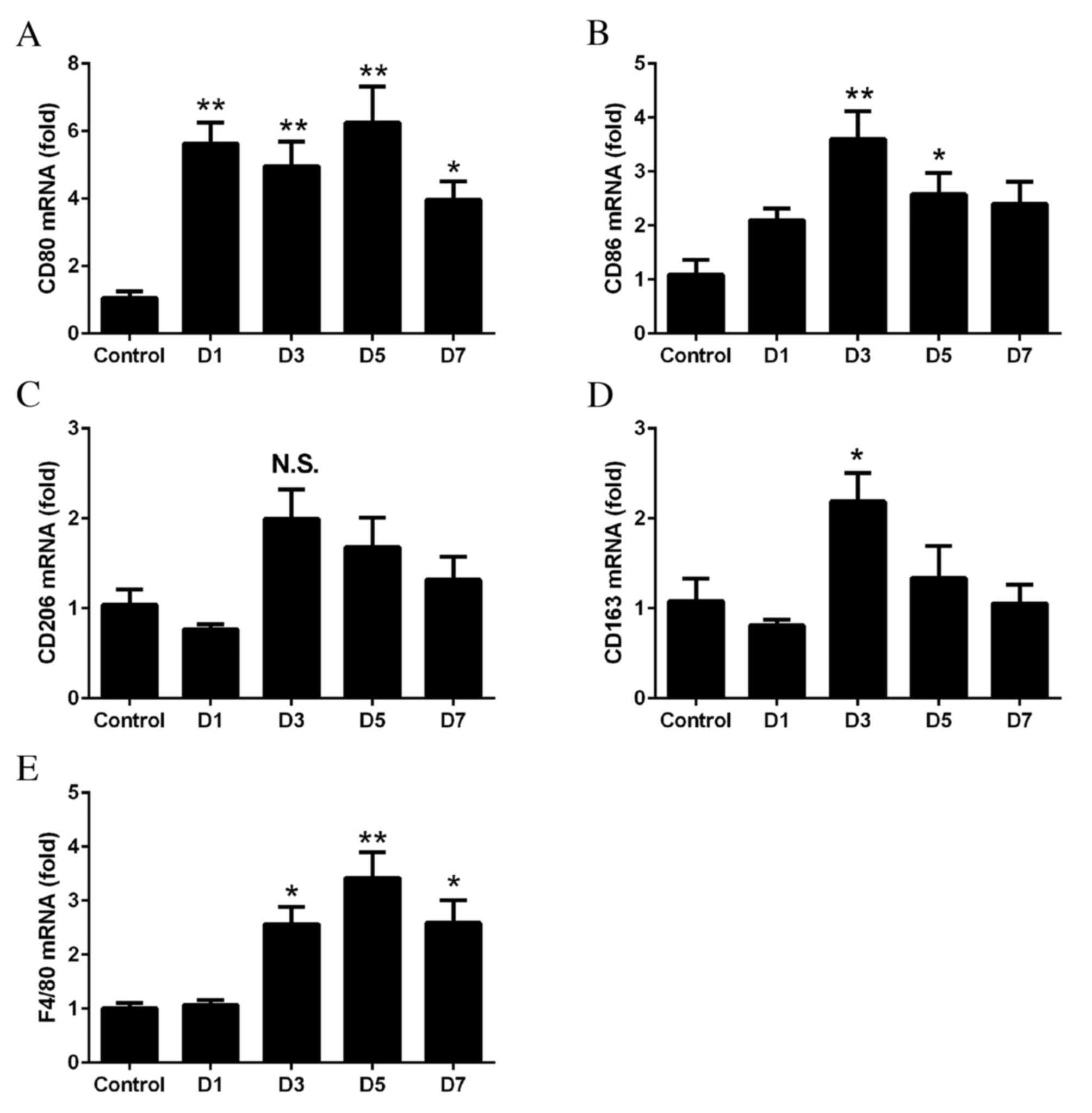
Recruitment of M1 macrophages to
RPE-choroid complexes of CNV
RT-qPCR was performed to determine whether M1 and M2
macrophages were associated with the development of laser-induced
CNV. The mRNA expressions levels of CD80, CD86, CD206, CD163 and
F4/80 in the RPE-choroid complex at days 1–7 following laser
treatment were compared with those in the untreated control
tissues. The mRNA expression levels of CD80 (day 1, P=0.0011; day
3, P=0.0041; day 5, P=0.0003; day 7, P=0.0311; Fig. 1A) and CD86 (day 3, P=0.0009; day 5,
P=0.0418; Fig. 1B) were
significantly upregulated following laser treatment. The mRNA
expression levels of the M2 markers CD206 (P>0.05; Fig. 1C) and CD163 (day 3, P=0.0291;
Fig. 1D) were increased to a
lesser extent. The mRNA expression levels of F4/80 increased from
days 3 to 7, with the degree of increase between that of the M1 and
M2 markers (day 3, P=0.013; day 5, P=0.0003; day 7, P=0.0119;
Fig. 1E).
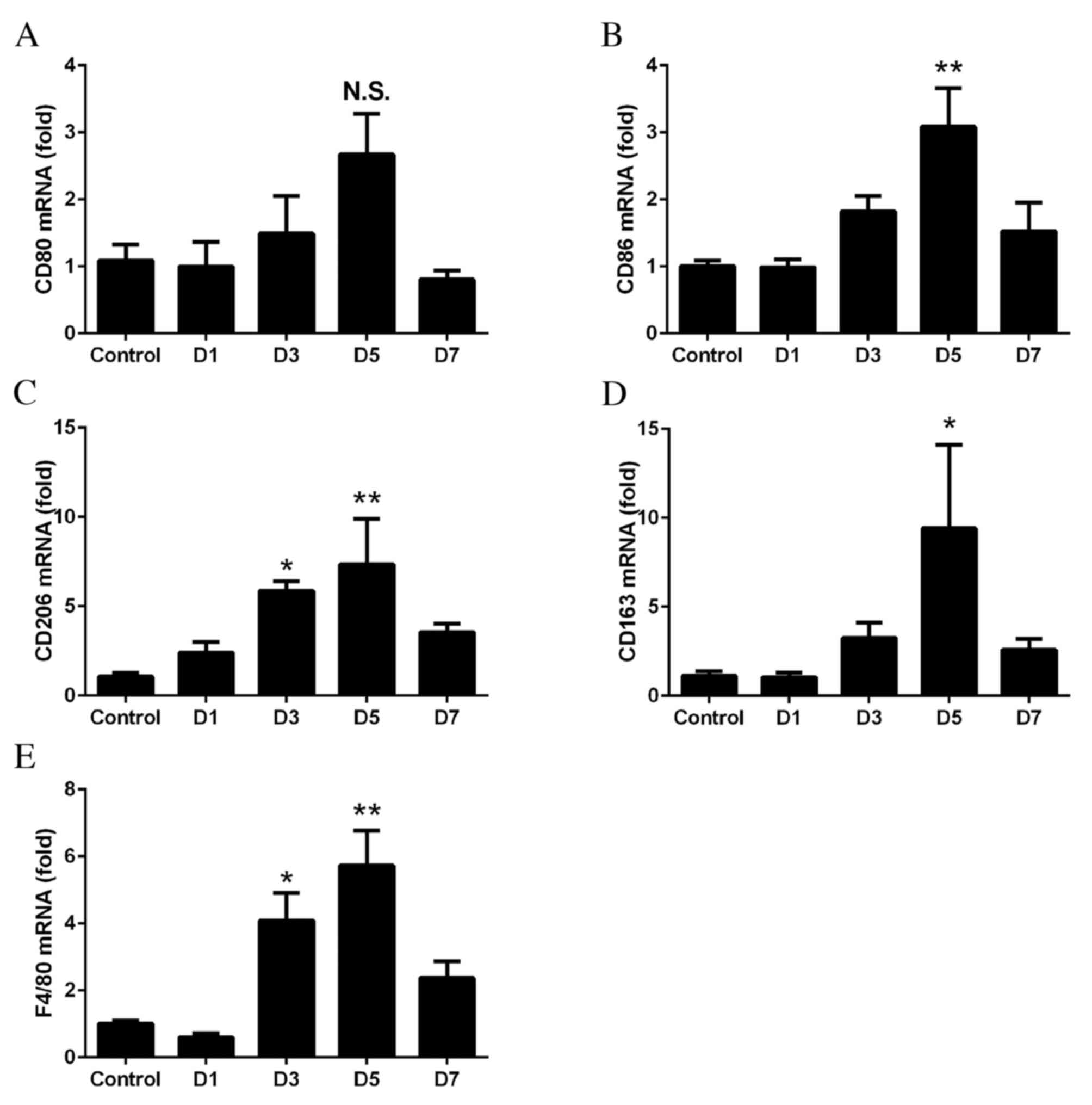
Recruitment of M2 macrophages in
retinas with CNV
To determine whether M1 and M2 macrophages were
recruited into the retinas, RT-qPCR was performed on the retinas at
days 1–7 following laser treatment. No significant differences were
observed in the mRNA expression levels of CD80 at anytime point
(P>0.05; Fig. 2A). However, the
expression levels of CD86 were significantly upregulated on day 5
(P=0.0024; Fig. 2B). The mRNA
expression levels of CD206 (day 3, P=0.0427; day 5, P=0.0077;
Fig. 2C) and CD163 (day 5,
P=0.0497; Fig. 2D) were
significantly increased, with the greatest fold-changes observed on
days 3 and 5. The mRNA expression levels of F4/80 significantly
increased from days 3 to 5, with the degree of increase between
that of M1 and M2 (day 3, P=0.0138; day 5, P=0.0004; Fig. 2E).
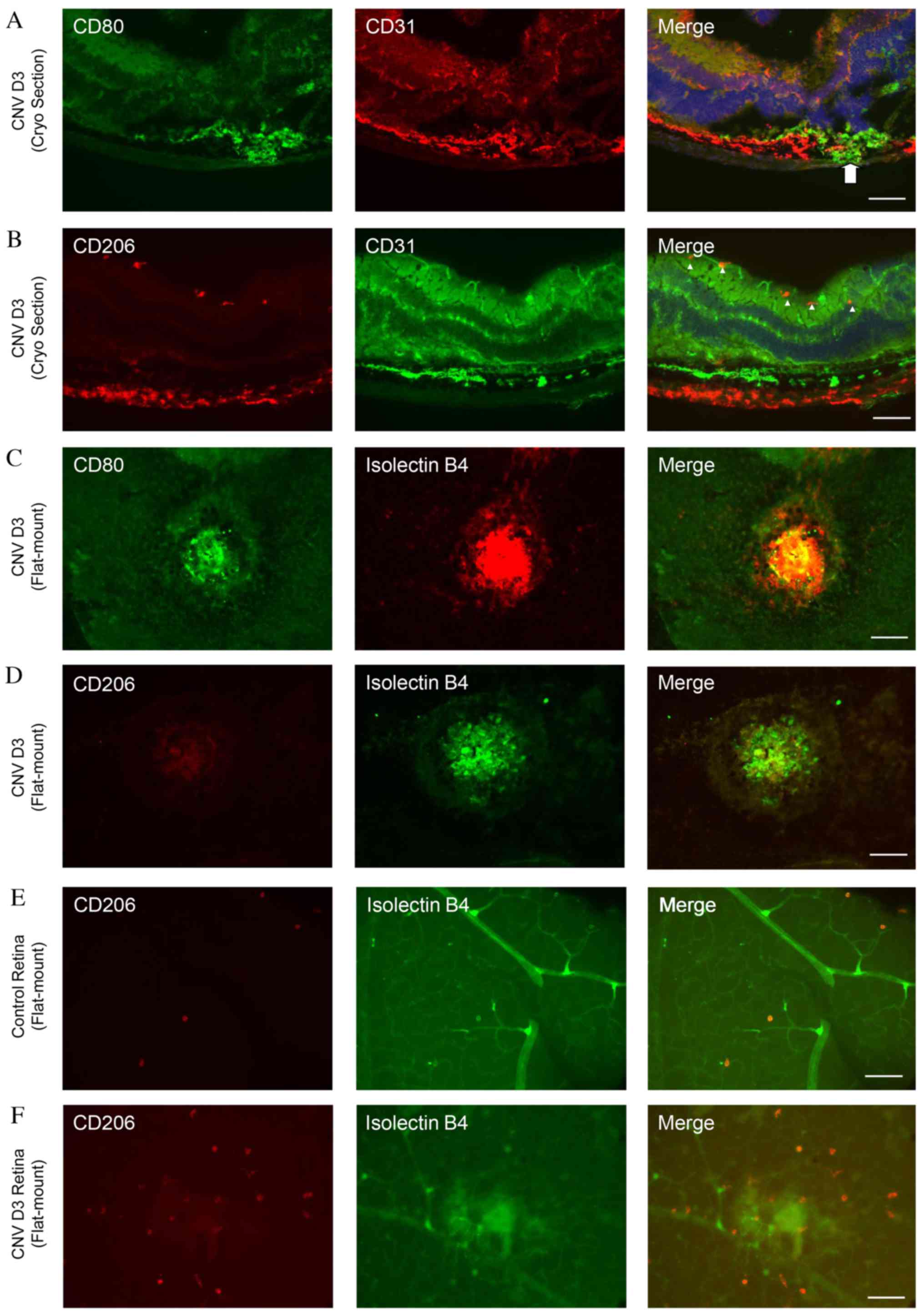
Predominance of M1 macrophages in
choroid and M2 macrophages in retina following laser-induced
CNV
To determine the distributions of M1 and M2
macrophages in the retina, double immunofluorescence staining of
cryosections was performed on day 3 following laser
photocoagulation using antibodies against CD31 and CD80 or CD206.
The CD80-positive cells were located around the site of
laser-photocoagulations (Fig. 3A).
By contrast, numerous CD206-positive cells were detected in the
inner layer of the retina around the CD31-positive vessels
(Fig. 3B).
Double immunofluorescence staining with isolectin B4
and CD80 or CD206 was additionally performed in flat-mounted
retinas and choroid following laser photocoagulation. Numerous
CD80-positive cells were observed in the laser-injured areas
(Fig. 3C); however, very few
CD206-positive cells were present (Fig. 3D). By contrast, the number of
CD206-positive cells increased markedly in laser-treated retinas
compared with controls (Fig. 3E and
F).
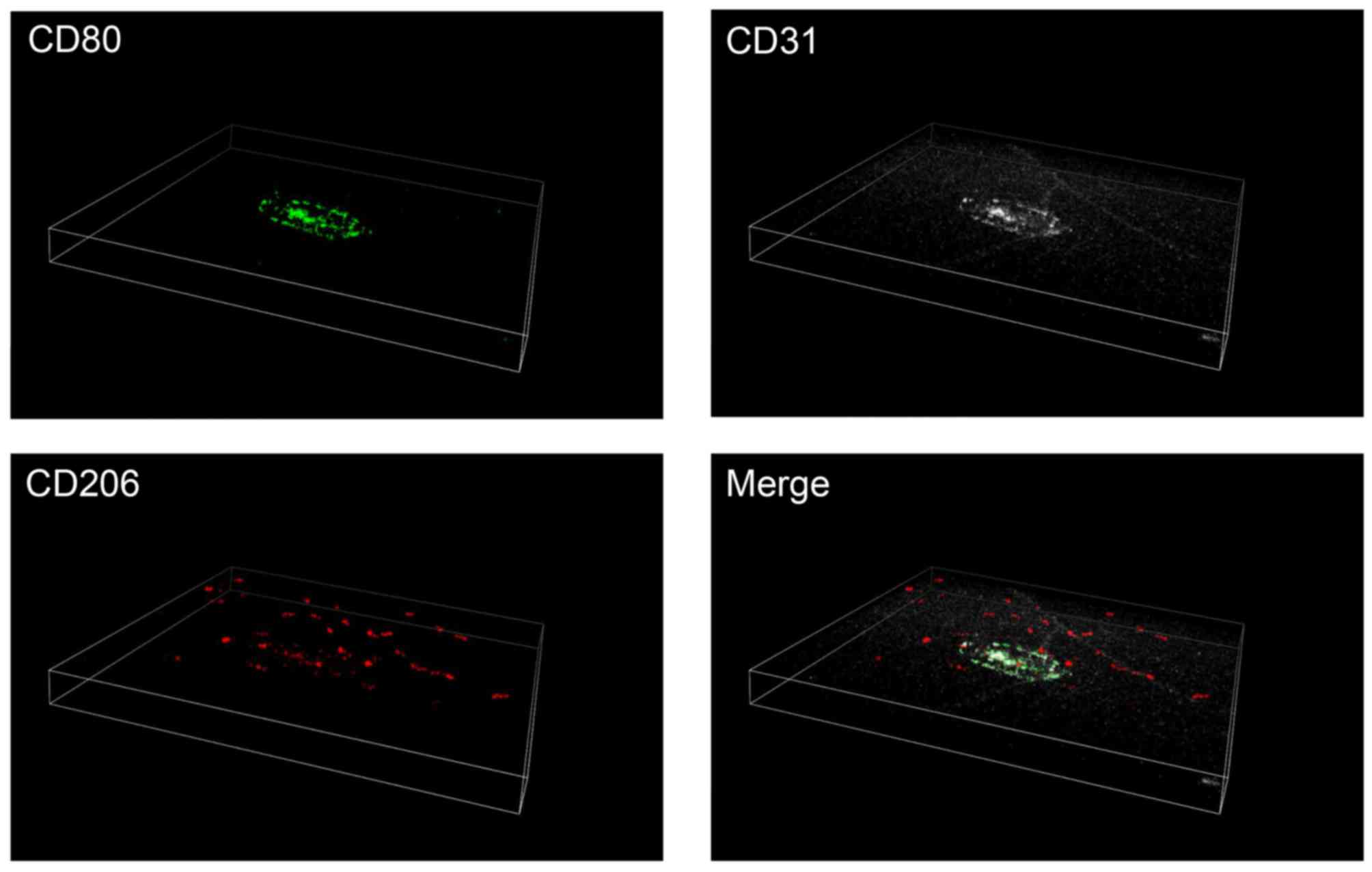
Distribution of M1 and M2 macrophages
in three dimensional images
To further confirm the differential distribution of
M1 and M2 macrophages in the retina and choroid, triple
immunofluorescence staining was performed in the posterior segments
of the eyes, with intact retina and choroid. Consistent with the
findings presented in Fig. 3,
CD80-positive M1 macrophages were observed in and around the CNV
lasered areas. By contrast, the majority of the CD206-positive M2
macrophages were observed in the upper layer of the retina
(Fig. 4).
mRNA expression levels of M1 and M2
macrophage-associated cytokines in RPE-choroid complex and retina
following CNV
To determine the synthesis of cytokines by M1 and M2
macrophages in the laser-induced CNV model, RT-qPCR was performed
to examine the mRNA expression levels of various angiogenic factors
using cDNA from the retina and RPE-choroid complex obtained at day
3 following laser treatment. The mRNA expression levels of Thbs1
increased in the RPE-choroid complex following CNV (P=0.0026;
Fig. 5A) compared with control
untreated tissues, whereas Pgf decreased significantly (P=0.0006;
Fig. 5B). mRNA expression levels
of Fgf2 (P=0.0132; Fig. 5C) and
Ccl2 (P=0.0002; Fig. 5D)
significantly increased in the RPE-choroid complex following laser
treatment. In the retina, no significant differences were observed
in Thbs1 mRNA expression levels (Fig.
5E); however, the mRNA expression levels of Pgf (P=0.0006;
Fig. 5F), Fgf2 (P=0.0009; Fig. 5G) and Ccl2 (P=0.0060; Fig. 5H) all increased significantly in
the laser-treated compared with control untreated tissues.
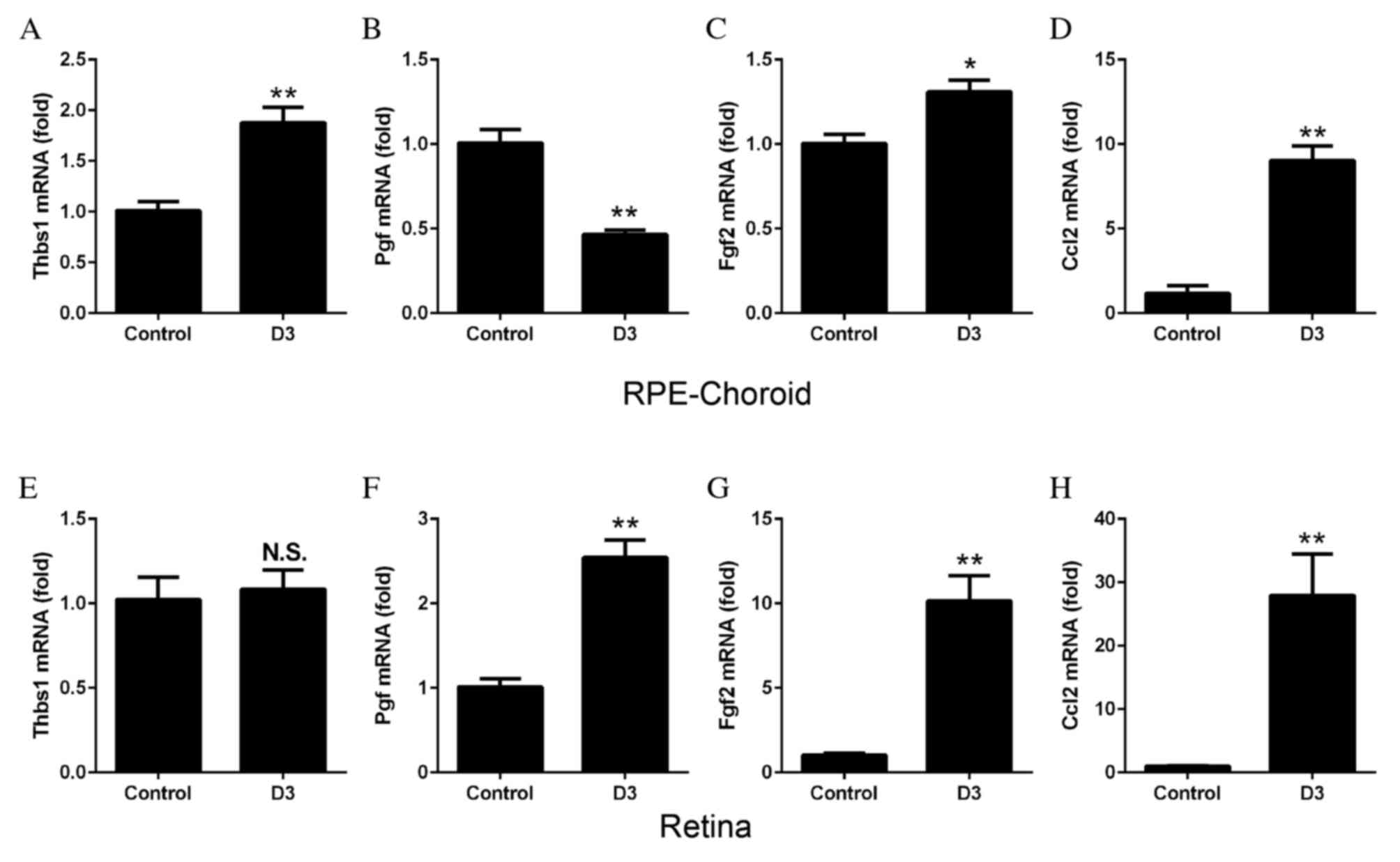 | Figure 5.mRNA expression levels of angiogenic
factors, as determined by reverse transcription-quantitative
polymerase chain reaction. RPE-choroid complexes or retinas were
harvested from mice 3 days following laser-induced choroidal
neovascularization. In the RPE-choroid complexes, the mRNA
expression levels of (A) Thbs1 were significantly increased
following laser treatment, compared with untreated control tissues.
Expression levels of (B) Pgf were decreased, (C) Fgf2 were
increased and (D) Ccl2 were increased. In the retinas, no
significant differences were observed in mRNA expression levels of
(E) Thbs1 between control and laser-treated tissues; however, the
mRNA expression levels of (F) Pgf, (G) Fgf2 and (H) Ccl2 were all
significantly increased following laser treatment. Data are
expressed as the mean ± standard error (n=4). **P<0.01 and
*P<0.05 vs. control. RPE, retinal pigment epithelium; Thbs1,
thrombospondin 1; Pgf, placental growth factor; Fgf2, basic
fibroblast growth; Ccl2, monocyte chemoattractant protein-1; D,
day; NS, non-significant. |
Discussion
It has recently been reported that the number of
macrophages increases in choroid following laser photocoagulation
in mice (4). The results of the
present study demonstrated that the mRNA expression levels of M1
and M2 macrophage markers increased significantly in the
RPE-choroid and retina following laser photocoagulation in a mouse
model of CNV. However, the distributions of the M1 and M2
macrophages differed; M1 macrophages were detected to a greater
extent in the RPE-choroid, whereas M2 macrophages were primarily
located in the retina.
The expression of THBS1 has been demonstrated to be
increased in M1 macrophages compared with monocytes, whereas M2
macrophages produce greater quantities of FGF2, PGF and CCL2
(14). It has been reported that
THBS1 is an inhibitor of neovascularization in tumors (28). In the present study, Thbs1 mRNA
expression levels increased significantly in the RPE-choroid
complexes, consistent with the predominance of M1 macrophages
around the laser-induced areas. By contrast, Fgf2 and Pgf mRNA
expression levels increased primarily in the retinas, which is
consistent with the presence of greater numbers of M2 macrophages
in the retinas of the CNV model. Ccl2 mRNA expression levels
increased markedly in the retina and RPE-choroid complex, which may
be due to the fact it is associated with the recruitment of the two
macrophage subsets. These data further support the results of the
immunofluorescence staining demonstrating the differential
distribution of M1 and M2 macrophages.
Caicedo et al (7) reported that bone marrow-derived
F4/80-positive macrophages infiltrated retinas and activated Müller
cells leading to photoreceptor degeneration in a mouse model of
CNV. Evidence suggests that retinal microglia and macrophages may
migrate from the inner layer to the subretinal space to infiltrate
the CNVs (29–31). These pathological alterations are
essential for the development of CNVs, indicating that retinal
microglia and macrophages may have a marked effect on the
pathogenesis of CNVs. As M2 macrophages were the predominant
subtype in the retinas in the present study, the M2 phenotype may
comprise a large number of the macrophages and microglia observed
in the retina in previous studies (7,29–31).
Retinal degeneration is associated with the
development of CNVs in a rat model (32), indicating that retinal pathologies
are relevant to the development of CNVs. M1 and M2 macrophages
secrete different levels of molecules, including THBS1, FGF2, PGF
and CCL2, as demonstrated in the present study, and it is possible
that the function of each phenotype is partially based on these
secreted molecules. M2 macrophages in the retina may therefore be
relevant to the pathogenesis of CNVs. By contrast, M1 macrophages
were located primarily in the RPE-choroid and may have a more
direct effect in inhibiting the development of CNVs. There may be a
conversion from M2 to M1 following the migration of macrophages
from the retina to the choroid around the CNVs.
Macrophages are recognized to exist as two distinct
subtypes, M1 pro-inflammatory and M2 anti-inflammatory (8,9,14).
In addition, it has been reported that monocytes/macrophages may be
differentiated by their expression of phenotypic markers, including
C-C chemokine receptor 2+Ly6Chigh
(pro-inflammatory) and CX3C chemokine receptor
1+Ly6Clow (anti-inflammatory) (33–35).
Therefore, further studies are required to determine the
contribution of these monocyte subsets to laser-induced CNVs.
In conclusion, the results of the present study
demonstrated that M1 and M2 macrophages are recruited in response
to laser-induced CNV. However, the distribution of these two cell
subtypes differed; M1 macrophages were present primarily in
RPE-choroid and M2 macrophages in the retina. Therefore, M1
macrophages may be more directly involved in laser-induced CNVs.
These findings support and expand upon the results of previous
studies on the functional roles of M1 and M2 macrophages in
CNVs.
Acknowledgements
The present study was supported in part by the Japan
Society for the Promotion of Science Grants-in-Aid for Scientific
Research (B; grant nos. 15H04995 and 26293374), Grants-in-Aid for
Challenging Exploratory Research (grant no. 16K15734), the Takeda
Science Foundation and the China Scholarship Council (to Y.Z.). The
authors thank Ms. Masayo Eto, Ms. Kinuko Sasada, and Ms. Hiroko
Miura (Kyushu University) for their excellent technical
assistance.
References
|
1
|
Klein R, Peto T, Bird A and Vannewkirk MR:
The epidemiology of agerelated macular degeneration. Am J
Ophthalmol. 137:4864952004. View Article : Google Scholar
|
|
2
|
Wong IY, Koo SC and Chan CW: Prevention of
agerelated macular degeneration. Int Ophthalmol. 31:73822011.
View Article : Google Scholar
|
|
3
|
Ambati J, Ambati BK, Yoo SH, Ianchulev S
and Adamis AP: Agerelated macular degeneration: Etiology,
pathogenesis, and therapeutic strategies. Surv Ophthalmol.
48:2572932003. View Article : Google Scholar
|
|
4
|
Lambert V, Lecomte J, Hansen S, Blacher S,
Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart
JM, et al: Laser-induced choroidal neovascularization model to
study age-related macular degeneration in mice. Nat Protoc.
8:2197–2211. 2013. View Article : Google Scholar
|
|
5
|
Heidmann DG Espinosa, Suner IJ, Hernandez
EP, Monroy D, Csaky KG and Cousins SW: Macrophage depletion
diminishes lesion size and severity in experimental choroidal
neovascularization. Invest Ophthalmol Vis Sci. 44:358635922003.
|
|
6
|
Sakurai E, Anand A, Ambati BK, van Rooijen
N and Ambati J: Macrophage depletion inhibits experimental
choroidal neovascularization. Invest Ophthalmol Vis Sci.
44:357835852003. View Article : Google Scholar
|
|
7
|
Caicedo A, Heidmann DG Espinosa, Pina Y,
Hernandez EP and Cousins SW: Bloodderived macrophages infiltrate
the retina and activate Muller glial cells under experimental
choroidal neovascularization. Exp Eye Res. 81:38472005. View Article : Google Scholar
|
|
8
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol.
25:6776862004. View Article : Google Scholar
|
|
9
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Front Biosci.
13:4534612008. View
Article : Google Scholar
|
|
10
|
Delavary B Mahdavian, van der Veer WM, Van
Egmond M, Niessen FB and Beelen RHJ: Macrophages in skin injury and
repair. Immunobiology. 216:753–762. 2011. View Article : Google Scholar
|
|
11
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:5936042010. View Article : Google Scholar
|
|
12
|
Mantovani A, Schioppa T, Porta C, Allavena
P and Sica A: Role of tumorassociated macrophages in tumor
progression and invasion. Cancer Metastasis Rev. 25:3153222006.
View Article : Google Scholar
|
|
13
|
Ho VW and Sly LM: Derivation and
characterization of murine alternatively activated (M2)
macrophages. Methods Mol Biol. 531:1731852009.
|
|
14
|
Jetten N, Verbruggen S, Gijbels MJ, Post
MJ, De Winther MP and Donners MM: Antiinflammatory M2, but not
proinflammatory M1 macrophages promote angiogenesis in vivo.
Angiogenesis. 17:1091182014. View Article : Google Scholar
|
|
15
|
Cao X, Shen D, Patel MM, Tuo J, Johnson
TM, Olsen TW and Chan CC: Macrophage polarization in the maculae of
age-related macular degeneration: A pilot study. Pathol Int.
61:528–535. 2011. View Article : Google Scholar :
|
|
16
|
Zhou Y, Yoshida S, Nakao S, Yoshimura T,
Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K,
et al: M2 macrophages enhance pathological neovascularization in
the mouse model of oxygen-induced retinopathy. Invest Ophthalmol
Vis Sci. 56:4767–4777. 2015. View Article : Google Scholar
|
|
17
|
Zandi S, Nakao S, Chun KH, Fiorina P, Sun
D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, et al:
ROCK-isoform-specific polarization of macrophages associated with
age-related macular degeneration. Cell Rep. 10:1173–1186. 2015.
View Article : Google Scholar :
|
|
18
|
Zhang H, Yang Y, Takeda A, Yoshimura T,
Oshima Y, Sonoda KH and Ishibashi T: A novel platelet-activating
factor receptor antagonist inhibits choroidal neovascularization
and subretinal fibrosis. PLoS One. 8:e681732013. View Article : Google Scholar :
|
|
19
|
Nakama T, Yoshida S, Ishikawa K, Kobayashi
Y, Zhou Y, Nakao S, Sassa Y, Oshima Y, Takao K, Shimahara A, et al:
Inhibition of choroidal fibrovascular membrane formation by new
class of RNA interference therapeutic agent targeting periostin.
Gene Ther. 22:127–137. 2015. View Article : Google Scholar
|
|
20
|
Ishikawa K, Yoshida S, Kadota K, Nakamura
T, Niiro H, Arakawa S, Yoshida A, Akashi K and Ishibashi T: Gene
expression profile of hyperoxic and hypoxic retinas in a mouse
model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci.
51:4307–4319. 2010. View Article : Google Scholar
|
|
21
|
Li W, Katz BP and Spinola SM: Haemophilus
ducreyiinduced interleukin10 promotes a mixed M1 and M2 activation
program in human macrophages. Infect Immun. 80:442644342012.
View Article : Google Scholar
|
|
22
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: A marker of alternative immunologic macrophage
activation. J Exp Med. 176:2872921992. View Article : Google Scholar
|
|
23
|
Bouhlel MA, Derudas B, Rigamonti E,
Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx
N, et al: PPARgamma activation primes human monocytes into
alternative M2 macrophages with anti-inflammatory properties. Cell
Metab. 6:137–143. 2007. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(Delta Delta C(T)) Method. Methods. 25:4024082001. View Article : Google Scholar
|
|
25
|
Ishikawa K, Yoshida S, Nakao S, Sassa Y,
Asato R, Kohno R, Arima M, Kita T, Yoshida A, Ohuchida K and
Ishibashi T: Bone marrow-derived monocyte lineage cells recruited
by MIP-1β promote physiological revascularization in mouse model of
oxygen-induced retinopathy. Lab Invest. 92:91–101. 2012. View Article : Google Scholar
|
|
26
|
Arima M, Yoshida S, Nakama T, Ishikawa K,
Nakao S, Yoshimura T, Asato R, Sassa Y, Kita T, Enaida H, et al:
Involvement of periostin in regression of hyaloidvascular system
during ocular development. Invest Ophthalmol Vis Sci. 53:6495–6503.
2012. View Article : Google Scholar
|
|
27
|
Ishikawa K, Yoshida S, Nakao S, Nakama T,
Kita T, Asato R, Sassa Y, Arita R, Miyazaki M, Enaida H, et al:
Periostin promotes the generation of fibrous membranes in
proliferative vitreoretinopathy. FASEB J. 28:131–142. 2014.
View Article : Google Scholar
|
|
28
|
Kazerounian S, Yee KO and Lawler J:
Thrombospondins in cancer. Cell Mol Life Sci. 65:7007122008.
View Article : Google Scholar
|
|
29
|
Combadiére C, Feumi C, Raoul W, Keller N,
Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E, et
al: CX3CR1-dependent subretinal microglia cell accumulation is
associated with cardinal features of age-related macular
degeneration. J Clin Invest. 117:2920–2928. 2007. View Article : Google Scholar :
|
|
30
|
Ma W, Zhao L, Fontainhas AM, Fariss RN and
Wong WT: Microglia in the mouse retina alter the structure and
function of retinal pigmented epithelial cells: A potential
cellular interaction relevant to AMD. PLoS One. 4:e79452009.
View Article : Google Scholar :
|
|
31
|
Huang H, Parlier R, Shen JK, Lutty GA and
Vinores SA: VEGF receptor blockade markedly reduces retinal
microglia/macrophage infiltration into laserinduced CNV. PLoS One.
8:e718082013. View Article : Google Scholar :
|
|
32
|
Albert DM, Neekhra A, Wang S, Darjatmoko
SR, Sorenson CM, Dubielzig RR and Sheibani N: Development of
choroidal neovascularization in rats with advanced intense cyclic
light-induced retinal degeneration. Arch Ophthalmol. 128:212–222.
2010. View Article : Google Scholar :
|
|
33
|
Auffray C, Fogg DK, Narni-Mancinelli E,
Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L,
Abitbol M, et al: CX3CR1+ CD115+ CD135+ common macrophage/DC
precursors and the role of CX3CR1 in their response to
inflammation. J Exp Med. 206:595–606. 2009. View Article : Google Scholar :
|
|
34
|
Nahrendorf M, Swirski FK, Aikawa E,
Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R
and Pittet MJ: The healing myocardium sequentially mobilizes two
monocyte subsets with divergent and complementary functions. J Exp
Med. 204:3037–3047. 2007. View Article : Google Scholar :
|
|
35
|
Benakis C, Bonilla L Garcia, Iadecola C
and Anrather J: The role of microglia and myeloid immune cells in
acute cerebral ischemia. Front Cell Neurosci. 8:4612015. View Article : Google Scholar :
|