Introduction
As a prominent feature, heart failure (HF) is
characterized by sympathetic hyperactivity (1). Following myocardial injury, increased
sympathetic activity has been widely identified, even prior to the
onset of HF (2). The association
between inflammation and HF was investigated by Levine et al
(3). However, there are few
reports describing inflammation and HF in the paraventricular
nucleus (PVN).
Sympathetic activity is widely regulated and
includes the renin angiotensin system (RAS) (4). The RAS is important in the
progression of HF. In the PVN, the inhibition of angiotensin
receptor-type 1 (AT-1R) can improve sympathetic activity in HF
(5). Further investigation has
shown that increased cytokines in the PVN enhance the production of
reactive oxygen species, which further exacerbates the
sympathoexcitatory effects (6). In
previous studies, the expression of several inflammatory cytokines,
in addition to interleukin (IL)-1β (7), tumor necrosis factor-α (TNF-α)
(8) and IL-10 (9), have been reported to be altered in
HF. IL-6 is the prototypal inflammatory cytokine and shows high
expression in patients with HF (10,11).
The present study investigated the hypothesis that increased IL-6
contributes to the progression of HF via the AT1-R pathway.
Decreased neuronal nitric oxide synthase (nNOS) has
also been identified in rats with HF (12,13).
In addition, the antagonistic mechanism of nitric oxide (NO) is
disrupted by angiotensin II-induced sympathetic hyperactivity,
which then increases the production of superoxide, indicating the
cross talk between the production of nNOS and the RAS mechanism
(14–16). However, whether enhanced cytokine
production and AT-1Rs in the PVN contribute to HF through
regulating nNOS in rats remains to be fully elucidated. The present
study also investigated alterations in nNOS in the PVN, including
RAS-related hormones.
Materials and methods
Experimental animals
Male Sprague-Dawley rats (weight, 200–250 g; age,
6–8 weeks; n=50), were purchased from Shanghai Experimental Animal
Breeding Co. (Shanghai, China). Following adaptation to the
environment for 1 week, the rats were randomly divided into five
groups: sham (n=10), vehicle (VEH; n=10), LOS (n=10), LOS+IL-6
(n=10) and IL-6 (n=10). In the sham group, the rats received no
treatment. The rats were housed in at 22±2°C and 40–60% humidity
and light-controlled room 12 h light/dark with free access to
water. The experiments conformed to the Guide for the Care and Use
of Laboratory Animals published by the US National Institutes of
Health (17).
General experimental protocol
Animals were anesthetized with intravenous infusion
of sodium pentobarbital [50 mg/kg−1; intraperitoneal
(i.p.)]. The rats underwent implantation of PVN cannulae, following
which coronary artery ligation was performed to induce HF. At 24 h
post-surgery, they were administered with artificial cerebrospinal
fluid lasting for 4 weeks in the VEH group. At the same time, the
rats were administered with AT1-R antagonist, LOS (200 µg/day) in
the LOS group (n=10; Merck Millipore; Darmstadt, Germany). The rats
were administered with LOS (200 µg/day) and IL-6 (1 µg/day) in the
LOS+IL-6 group (n=10; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany). The rats were administered with IL-6 (1 µg/day) in the
IL-6 group (n=10). Osmotic mini-pumps were implanted subcutaneously
and connected with the cannulae for the continuous infusion of LOS,
IL-6 and LOS+IL-6 or artificial cerebrospinal fluid directly into
the PVN (0.1 ml/h pumping, once a day for 4 weeks). A stainless
steel double cannula (Plastics One, Inc., Roanoke, VA, USA) was
implanted into the PVN. Left ventricular (LV) function was assessed
using echocardiography 24 h following recovery from surgery. At 4
weeks, the rats were anesthetized for echocardiograph examination
and were then sacrificed by inducing an air embolism via
intravenous infusion of 1 ml air, tissue and plasma for further
analyses was subsequently obtained.
Implantation of PVN cannulae and
coronary ligation
Alzet miniosmotic pumps (model 2004; Durect
Corporation, Cupertino, CA, USA) were used to enable the continuous
infusion of drugs. The rats were placed into a stereotaxic
apparatus following anesthesia (sodium pentobarbital; 50
mg/kg−1; i.p.) The method for PVN cannulation has been
described previously (7). In
brief, a stainless steel double cannula with a center-to-center
distance of 0.5 mm was implanted into the PVN (2.0 mm posterior to
the bregma and 8.5 mm ventral from the skull surface). HF was
induced by ligation of the left anterior descending coronary
artery, as previously described. In the sham group, surgery was
performed in the same manner without ligating the coronary artery.
Following surgery, the animals were administered with benzathine
penicillin (30,000 units; IM).
Echocardiographic assessment of LV
function
Echocardiography was performed using an Acuson
Sequoia clinical imager (Siemens AG, Munich, Germany) fitted with
an 8-MHz sector-array probe, which generates 2-dimensional images
at a rate of 100/sec. The method for assessment LV function has
been described previously (7). The
animals were sedated with sodium pentobarbital (25 mg
kg−1; i.p.) to facilitate positioning for
echocardiographic examination. The animal was positioned in the
left lateral recumbent position to optimize the windows for
echocardiography. The anterior chest was shaved and pre-warmed
acoustic coupling gel was applied. Short-axis images were acquired
parallel to the mitral valve plane to obtain the largest
cross-sectional image of the LV. Long-axis views were obtained
perpendicular to the mitral valve plane. Images were stored for
subsequent offline analysis. LV end-diastolic volume (LVEDV), LV
end-systolic volume, LV ejection fraction (LVEF), LV stroke volume
and LV mass were computed. The region of the LV, which exhibited
akinesis was planimetered electronically and expressed as a
percentage of the total LV silhouette to estimate the size of the
ischemic zone. Only animals with large infarctions (ischemic zone
≥40%) were used in the examination.
Tissue microdissection
The animals were sacrificed by decapitation, and
their brains were rapidly removed and frozen on dry ice. Serial
sections (300 mm) of the brain were obtained using a cryostat
maintained at −10°C. The sections were transferred to coverslips,
which were placed on a cold stage set at −10°C. The PVN was
microdissected out using Palkovits' microdissection technique
(18).
Immunohistochemistry
The method for immunohistochemistry has been
previously described (19).
Paraffin sections of the artery were deparaffinized and endogenous
peroxidase activity was inactivated with 3%
H2O2 for 10 min. The Fra-like (Fra-LI)
primary antibody (1:200; cat. no. sc-271657; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or normal blocking serum was
added and incubated overnight. Biotin-conjugated goat anti-mouse
immunoglobulin G (IgG) (1:1,000; cat. no. 115-035-003; Jackson,
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was used as
the secondary antibody and incubated for 30 min at 4°C. An
avidin-biotin enzyme reagent was sequentially added and incubated
for 20 min. A peroxidase substrate was added and incubated until
desired stain intensity developed. Finally, the sections were
covered with a glass cover slip and observed under a light
microscope. The intensity of positive staining in tissue was
analyzed by integrated optical density (IOD) using Image-Pro Plus
software (Media Cybernetics, Rockville, MD, USA). The Fra-LI
expression was expressed as (IOD/area)x100 in accordance with a
previous study (20).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The method for mRNA analysis has been described
previously (19). Total RNA was
extracted from the artery specimens using TRIzol®
reagent according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The total RNA (1
µg) was used as a template to produce cDNA using an RT kit
(BioDev-Tech Co., Ltd., Beijing, China). The qPCR was performed by
monitoring the increase in fluorescence of the SYBR Green dye using
GreenMaster mix (Genaxxon BioScience, Ulm, Germany) according to
the manufacturer's protocol. The primer sets used to amplify nNOS
were 5′-gccatccagcataatgacccag-3′ (sense) and
5′-gagggtgatccaaagatgtcctc −3′ (antisense) (21). The primer sets used to amplify CRH
were 5′-cagaacaacagtgcgggctca-3′ (sense) and
5′-aaggcagacagggcgacagag-3′ (antisense) (22). The primer sets used to amplify
GAPDH were 5′-ccactttgtgaagctcatttcct-3′ (sense) and
5′-tcgtcctcctctggtgctct-3′ (antisense). PCR amplification was
performed with tag polymerase for 32 cycles at 95°C for 45 sec,
62°C for 30 sec and 72°C for 1 min (for CRH, nNOS and GAPDH). The
2−ΔΔCq method (23) was
used to determine relative changes in the gene expression of CRH,
nNOS and GAPDH. Values are expressed in relative quantities to the
mRNA expression in the control group.
Western blot analysis
The method for western blot analysis has been
described previously (19). The
specimens were washed with ice-cold PB and lysed for 20 min on ice
with lysis buffer (cat.no. SC-003; Invent, Lund, Sweden). Following
lysis, the lysates were centrifuged for 4 min at 394 × g and the
supernatants were collected in a fresh tube on ice. The protein
concentrations in each sample were determined using a BCA assay.
Total proteins (100 µg) were mixed with loading buffer with the
anionic denaturing detergent, sodium dodecyl sulfate (SDS), boiled
for 5 min and then resolved by 10% SDS polyacrylamide gel
electrophoresis. The proteins were transferred onto PVDF membranes.
Following blocking of the membranes in TBST containing non-fat milk
for 1 h at 4°C under agitation, the membranes were washed three
times in TBST and incubated for 2 h at 4°C with anti-rat nNOS
antibody (1:200 dilution; cat. no. NB120-3511; Novus Biologicals,
Ltd., Cambridge, UK), anti-rat CRH antibody (1:200 dilution; cat.
no. NBP1-42614; Novus Biologicals, Ltd.) or GAPDH monoclonal
antibody (1:200 dilution; cat. no. NB100-56875; Novus Biologicals,
Ltd.). Following washing three times in TBST, the membranes were
incubated with HRP-conjugated goat anti-rabbit IgG (1:1,000; cat.
no. AB501-01A; Novoprotein, Shanghai, China) at temperature for 1 h
at 4°C and then washed three times with TBST. The immunobands were
detected using a streptavidin amplification reagent (cat. no. WBKL
SOO 50; EMD Millipore, Billerica, MA, USA) according to the
manufacturer's protocol.
ELISA for IL-6 and NE
The method for the analysis of IL-6 and NE in plasma
has been previously described (19). From all animals, fresh blood
samples (3 ml) were obtained via the femoral vein and centrifuged
at 2,465 × g for 10 min at 4°C. The supernatant was placed in a
clean centrifuge tube and frozen at −20°C. Plasma concentrations of
IL-6 and NE were assayed using ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol. The
minimum detectable concentration of the kits was <1.0 pg/ml; the
variation in different boards was <15%. They were not
cross-reactive with other soluble structural analogues.
Statistical analysis
The data were analyzed using the SPSS 11.5 program
(SPSS, Inc., Chicago, IL, USA) for Windows. Quantitative data are
presented as the mean ± standard deviation. For comparison between
multiple groups, data was analyzed using one-way analysis of
variance, and with Student-Newman-Keuls post hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
LV function alterations
Echocardiography was used to evaluate alterations in
LV function (Table I). At 24 h,
the LVEDV, LVEDV/mass ratio and % infarction zone (IZ) were higher
in the VEH, LOS, IL-6, and LOS+IL-6 groups, compared with those in
the sham group. No differences were observed in these parameters
among the HF rats assigned to the LOS, IL-6, LOS+IL-6 or the VEH
treatment groups at 24 h (P>0.05). At 4 weeks, LVEDV and
LVEDV/mass ratio were higher, compared with the 24-h baseline
values in the LOS, LOS+IL-6 and vehicle-treated HF rats
(P<0.01). At 4 weeks, LVEF was higher in the HF rats in the LOS
and LOS+IL-6 treatment groups, compared with the HF rats in the VEH
or IL-6 treatment groups (P<0.01). The LVEDV and LVEDV/mass in
the HF rats, which received LOS or LOS+IL-6 were lower, compared
with those in the rats in the VEH and IL-6 treatment groups
(P<0.01), whereas no significant difference were observed
between the LOS and LOS+IL-6 groups (P>0.05).
 | Table I.Echocardiograph measures for
evaluation of LV function. |
Table I.
Echocardiograph measures for
evaluation of LV function.
| Measurement | SHAM | VEH | LOS | LOS+IL-6 | IL-6 |
|---|
| 24 h |
|
|
|
|
|
| IZ (%) | – | 47±5 | 46±5 | 46±4 | 46±7 |
| LVEF | 0.78±0.04 | 0.38±0.04 | 0.37±0.05 | 0.37±0.06 | 0.36±0.05 |
| LVEDV | 0.35±0.03 | 0.75±0.05 | 0.77±0.07 | 0.78±0.06 | 0.76±0.08 |
| LVEDV/mass | 0.53±0.05 | 1.12±0.08 | 1.09±0.08 | 1.06±0.07 | 1.08±0.05 |
| 4 weeks |
|
|
|
|
|
| IZ (%) | – | 48±6 | 47±4 | 47±4 | 47±5 |
| LVEF | 0.79±0.07 | 0.32±0.05 | 0.42±0.06 | 0.41±0.05 | 0.26±0.05 |
| LVEDV | 0.36±0.05 | 1.41±0.04 | 1.05±0.05 | 1.06±0.04 | 1.66±0.05 |
| LVEDV/mass | 0.54±0.05 | 1.74±0.05 | 1.24±0.05 | 1.22±0.06 | 1.96±0.06 |
Location and expression of Fra-LI
activity
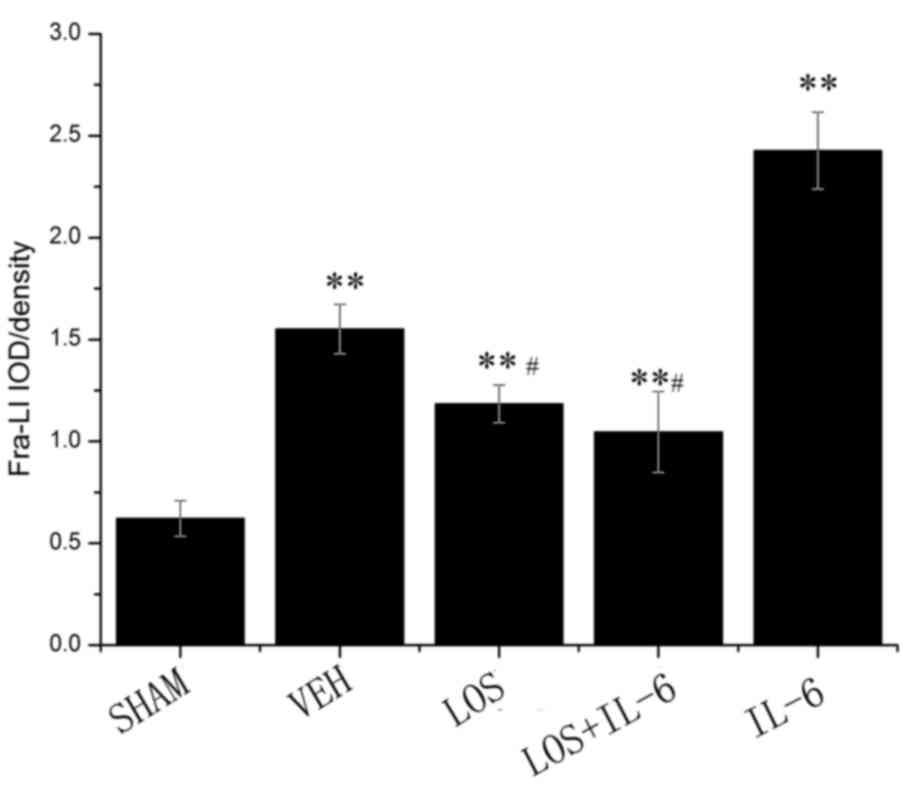
The immunohistochemistry showed that the staining
intensity of Fra-LI was minimal in the sham group (Fig. 1). Fra-LI activity is an indicator
of chronic neuronal excitation. Lower levels of Fra-LI
immunostaining were detected in the LOS and LOS+IL-6 groups,
compared with those in the VEH and IL-6 groups (P<0.01). The HF
rats treated with IL-6 had increased Fra-LI-positive PVN neurons,
compared with those in the VEH, LOS and LOS+IL-6 groups
(P<0.01). No significant differences were observed between the
LOS and LOS+IL-6 groups (P>0.05; Fig. 2).
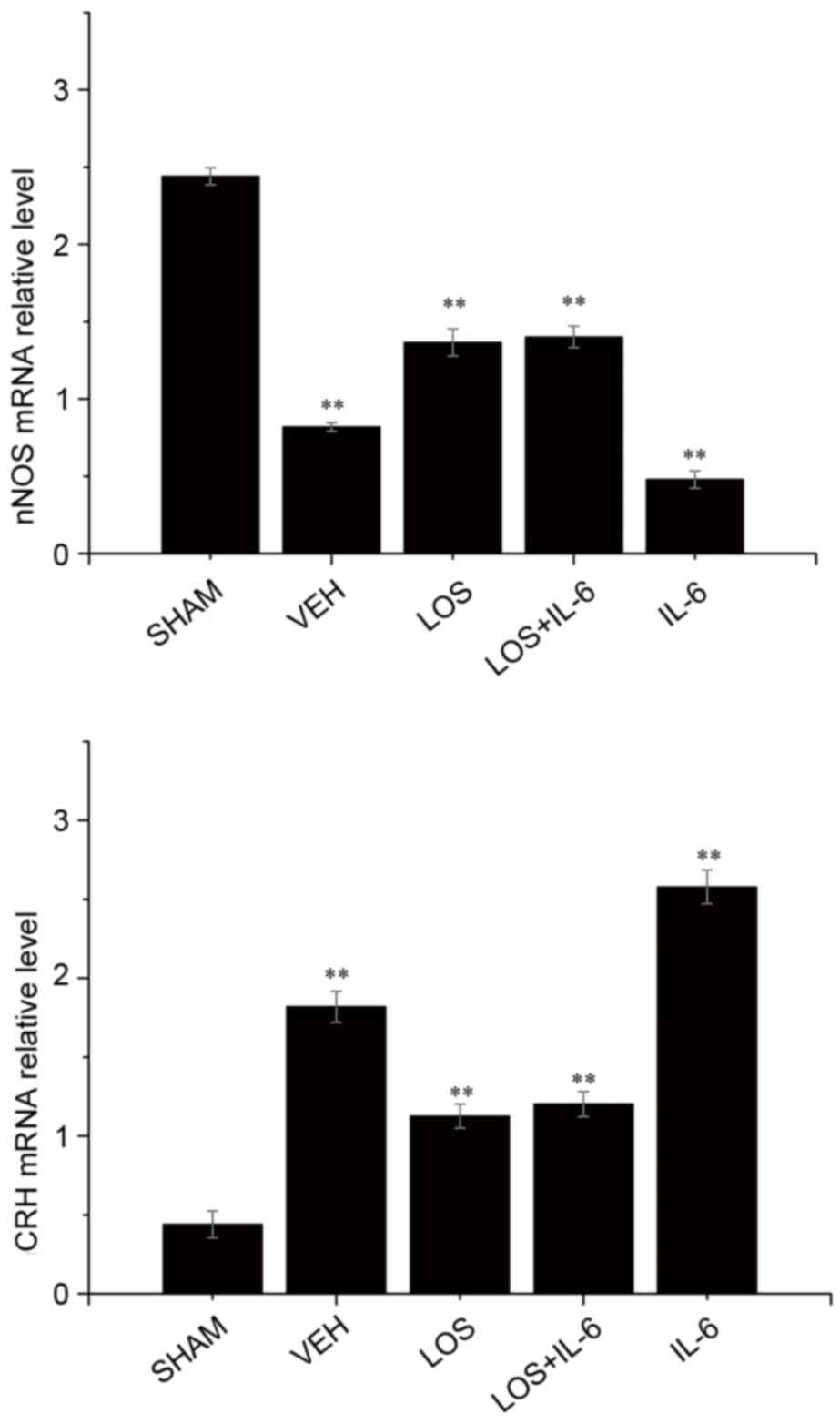
nNOS gene expression
In order to investigate the mechanism responsible
for alterations in the HF mice, mRNA levels of nNOS were analyzed
using RT-qPCR analysis (Fig. 3).
Compared with the VEH group, the mRNA expression levels of nNOS
were significantly increased in the sham group (P<0.01), LOS
group (P<0.05) and LOS+IL-6 group (P<0.05). The HF rats
treated with IL-6 had fewer nNOS-positive neurons, compared with
the rats treated with LOS+IL-6 (P<0.01). No significant
difference the expression of nNOS in PVN neurons was observed
between the LOS group and LOS+IL-6 group (P>0.05).
Gene expression of CRH
Compared with the sham group, the mRNA expression of
CRH was significantly increased in the LOS group, LOS+IL-6 group
and VEH group (P<0.01). By contrast, the mRNA expression levels
of CRH were decreased in the LOS and LOS+IL-6 groups, compared with
that in the IL-6 group (P<0.01). The rats treated with IL-6 had
a higher number of CRH-positive neurons, compared with the rats
treated with LOS+IL-6 or VEH (P<0.01). No significant difference
in the gene expression of CRH in PVN neurons was observed between
the LOS group and LOS+IL-6 group (P>0.05; Fig. 3).
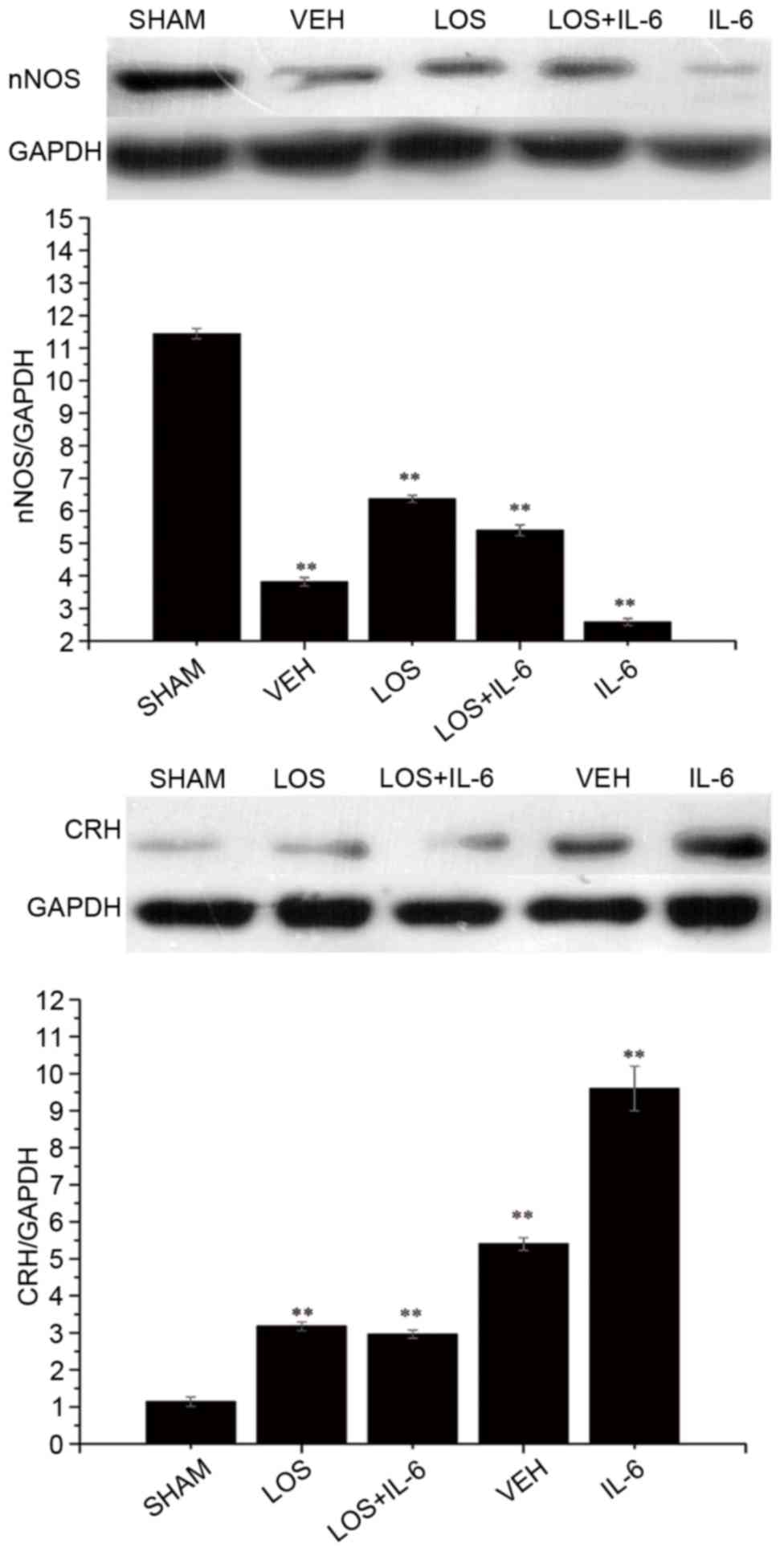
Protein expression of nNOS
Western blot analysis was used to examine whether
the protein levels of nNOS correlated with alterations in its mRNA
levels (Fig. 4). Compared with the
VEH group and IL-6 group, significant increases in the protein
expression of nNOS were observed in the sham group, LOS group and
LOS+IL-6 group in the blots (P<0.01). The rats treated with IL-6
exhibited lower expression of nNOS, compared with the rats treated
with LOS+IL-6 (P<0.05). No differences in the protein expression
of nNOS were found between the LOS group and LOS+IL-6 group
(P>0.05).
Protein expression of CRH
Western blot analysis was used to examine whether
the protein levels of CRH were correlated with alterations in its
mRNA levels (Fig. 4). Compared
with the sham group, significant increases in the expression levels
of CRH were observed in the VEH group, LOS group, LOS+IL-6 group
and IL-6 group in the blots (P<0.01). The rats treated with IL-6
exhibited higher expression of CRH, compared with the rats treated
with LOS+IL-6 (P<0.01). No difference in the protein expression
of CRH was observed between the LOS group and LOS+IL-6 group
(P>0.05).
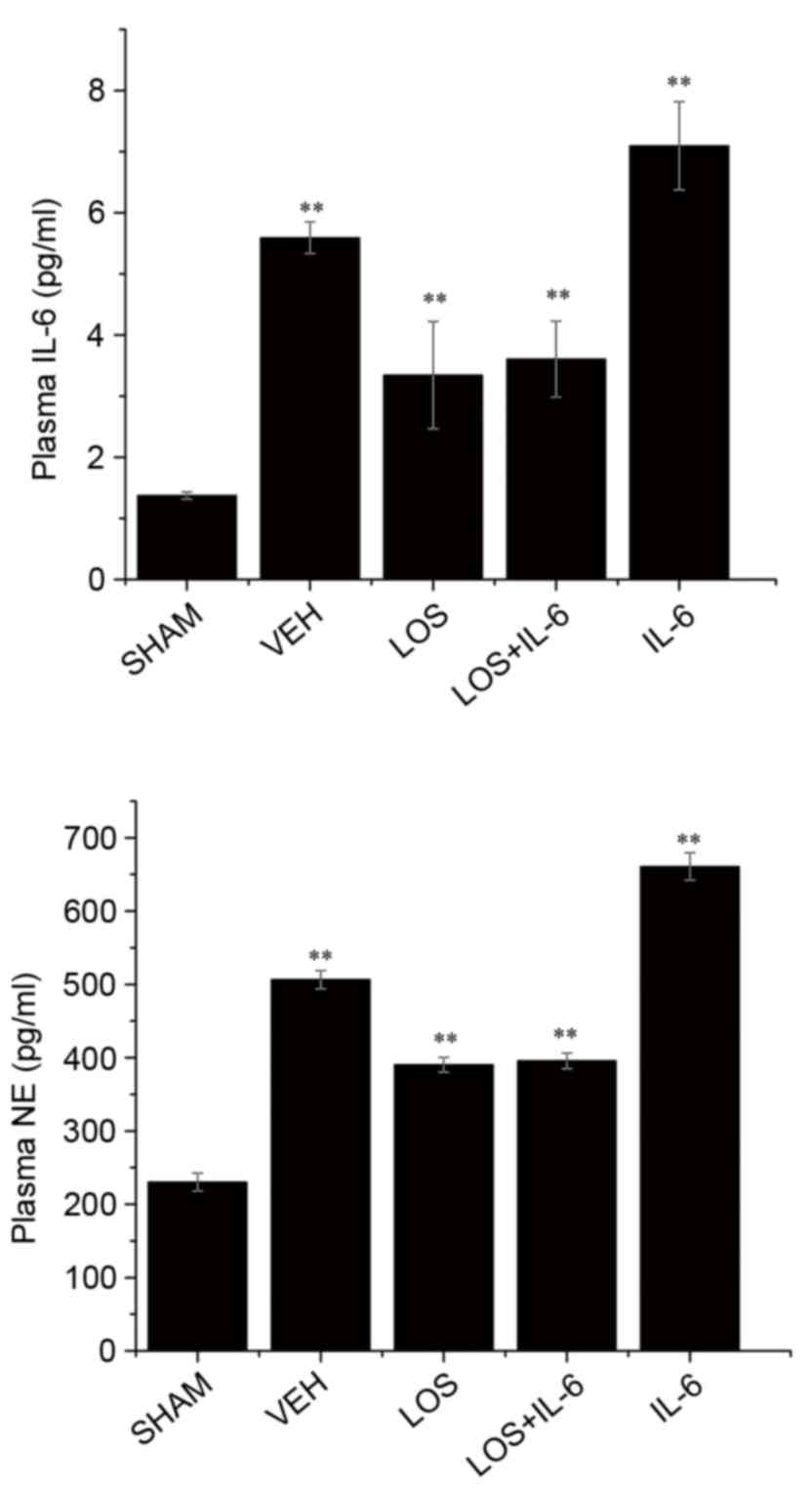
Serum levels of IL-6 and NE
Compared with the sham group, the levels of IL-6 and
NE were significantly increased following treatment (P<0.01;
Fig. 5). Compared with the VEH
group, treatment with LOS and LOS+IL-6 significantly decreased the
expression of IL-6 and NE (P<0.01), and treatment with IL-6
increased the levels of IL-6 and NE (P<0.05). No significant
differences were observed in the levels of IL-6 or NE between the
LOS group and LOS+IL-6 group (P<0.05; Fig. 5).
Discussion
Despite modern advances in technology, HF remains a
leading contributor to morbidity and mortality rates worldwide
(24). Sympathoexcitation is a
pathophysiological hallmark of HF (25). Studies have indicated that
excitation is negatively correlated with the prognosis of HF
(26,27). However, the precise mechanism
underlying HF remains to be fully elucidated. In the present study,
it was found that IL-6 was associated with AT1-R in rats with HF.
Treatment with LOS led to increased nNOS, decreased CRH and NE, and
improved heart function. The effect of IL-6 was opposite to that of
LOS in HF, and treatment with LOS reduced the damaging effects.
It has been reported that enhanced sympathetic nerve
activity (SNA) and inflammatory cytokines lead to severe
ventricular arrhythmias, which is the major contributor to rates of
mortality among patients with HF (28,29).
The increase in SNA in HF is due to an imbalance between inhibitory
and excitatory mechanisms within specific areas in the central
nervous system (CNS), including the PVN of the hypothalamus
(30). SNA to the kidneys results
in renal vasoconstriction, increased renal sodium retention and
increased renin release, and consequently to elevated levels of
angiotensin II and aldosterone (31). An increasing number of experimental
studies have suggested that PVN is key in the progression of HF
(26). Studies have found that CRH
and NE contribute to sympathoexcitation in rats with
ischemia-induced HF (32,33). The results of the present study are
consistent with these previous studies. LOS, as an angiotensin
receptor inhibitor, has been used in clinical management for
several years (34); it decreases
plasma renin activity and inhibits the conversion of
angiotensinogen to angiotensin I. In the present study, it was
found that IL-6 stimulated the expression of CRH and NE, and led to
worsening heart function. The microinjection of IL-6 and LOS in
combination attenuated the effects of IL-6. Taken together, the
results indicated that IL-6 increased sympathetic activity to
simulate the progression of HF via the AT1-R pathway.
Previous studies have suggested that repressing the
production of cytokines improves sympathetic activity in rats with
HF (35). For example, it was
found that the production of TNF-α and IL-1β leads to abnormal LV
dysfunction and remodeling in the progression of HF (7,36,37).
IL-6 is known to regulate inflammatory reactions, immunity and
neural development (38,39). Higher expression levels of IL-6 in
plasma and cardiac tissue directly correlate with HF (40,41).
There are few reports describing the association between IL-6 and
PVN in HF, although IL-6 receptor is increased in the PVN of rats
with HF (42). In the present
study, it was also found that plasma levels of IL-6 were elevated
in HF rats, which correlated with the severity of heart function.
In accordance with these findings, the present study identified
that treatment with LOS improved cytokine-induced diastolic and
systolic dysfunction in HF rats. Su et al (43) revealed that AT1-R inhibitor
significantly reduces the level of IL-6 in macrophages. These data
indicate the cross talk between cytokines and AT1-R in HF. In the
present study, treatment with IL-6 deteriorated heart function,
whereas the AT1-R inhibitor alleviated the dysfunction induced by
IL-6. This suggested that AT1-R may be a target site for IL-6 in
PVN.
In addition to interaction with cytokines, AT1-R
inhibitor has also been reported to interact with NO in the PVN of
animals with HF. NO is a gaseous neuromodulator substance, which is
key in the regulation of sympathetic tone (44,45)
and protection of endothelial function (46). However, the role of nNOS in the
pathophysiology of HF remains to be fully elucidated. The reduced
production of NO may lead to amplification of the angiotensin II
signal and enhance sympathetic activity (47). In the neurons of the PVN, nNOS
exerts an important function in controlling central sympathetic
outflow and enhancing sympathetic activity in HF (48). In the present study, it was found
that LOS significantly increased the expression of nNOS and
improved LV function, whereas IL-6 had adverse effects. Therefore,
in addition to inhibiting SNA, LOS increased the expression of
nNOS, which delayed the process of HF.
Taken together, the results of the present study
demonstrated that the interaction between AT1-R and IL-6 is
important in HF, and treatment with LOS significantly attenuated
the effects caused by IL-6, which was accompanied by imbalance in
sympathoexcitation and nNOS in the PVN.
Acknowledgements
This study was supported by the Jinan Science and
Technology Project (grant no. 201201051) and Shandong Provincial
Natural Science Foundation, China (grant no. ZR2014HP021).
References
|
1
|
Francis GS, Benedict C, Johnstone DE,
Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E and Yusuf
S: Comparison of neuroendocrine activation in patients with left
ventricular dysfunction with and without congestive heart failure.
A substudy of the studies of left ventricular dysfunction (SOLVD).
circulation. 82:1724–1729. 1990. View Article : Google Scholar
|
|
2
|
Zhang ZH, Francis J, Weiss RM and Felder
RB: The renin-angiotensin-aldosterone system excites hypothalamic
paraventricular nucleus neurons in heart failure. Am J Physiol
Heart Circ Physiol. 283:H423–H433. 2002. View Article : Google Scholar
|
|
3
|
Levine B, Kalman J, Mayer L, Fillit HM and
Packer M: Elevated circulating levels of tumor necrosis factor in
severe chronic heart failure. N Engl J Med. 323:236–241. 1990.
View Article : Google Scholar
|
|
4
|
Francis J, Chu Y, Johnson AK, Weiss RM and
Felder RB: Acute myocardial infarction induces hypothalamic
cytokine synthesis. Am J Physiol Heart Circ Physiol.
286:H2264–H2271. 2004. View Article : Google Scholar
|
|
5
|
Guggilam A, Haque M, Kerut EK, McIlwain E,
Lucchesi P, Seghal I and Francis J: TNF-alpha blockade decreases
oxidative stress in the paraventricular nucleus and attenuates
sympathoexcitation in heart failure rats. Am J Physiol Heart Circ
Physiol. 293:H599–H609. 2007. View Article : Google Scholar
|
|
6
|
Dawson VL and Dawson TM: Nitric oxide
neurotoxicity. J Chem Neuroanat. 10:179–190. 1996. View Article : Google Scholar
|
|
7
|
Liu Q, Wang T, Yu H, Liu B and Jia R:
Interaction between interleukin-1 beta and angiotensin II receptor
1 in hypothalamic paraventricular nucleus contributes to
progression of heart failure. J Interferon Cytokine Res.
34:870–875. 2014. View Article : Google Scholar :
|
|
8
|
Wei P, Yang XJ, Fu Q, Han B, Ling L, Bai
J, Zong B and Jiang CY: Intermedin attenuates myocardial infarction
through activation of autophagy in a rat model of ischemic heart
failure via both cAMP and MAPK/ERK1/2 pathways. Int J Clin Exp
Pathol. 8:9836–9844. 2015.
|
|
9
|
Duan HY, Liu DM, Qian P, Wang SL, Yan LJ,
Wu JT, Yang HT, Fan XW and Chu YJ: Effect of atorvastatin on plasma
NT-proBNP and inflammatory cytokine expression in patients with
heart failure. Genet Mol Res. 14:15739–15748. 2015. View Article : Google Scholar
|
|
10
|
Demissei BG, Valente MA, Cleland JG,
O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison
B, Givertz MM, et al: Optimizing clinical use of biomarkers in
high-risk acute heart failure patients. Eur J Heart Fail.
18:269–280. 2016. View
Article : Google Scholar
|
|
11
|
Bielecka-Dabrowa A, von Haehling S, Aronow
WS, Ahmed MI, Rysz J and Banach M: Heart failure biomarkers in
patients with dilated cardiomyopathy. Int J Cardiol. 168:2404–2410.
2013. View Article : Google Scholar
|
|
12
|
Patel KP, Zhang K, Zucker IH and Krukoff
TL: Decreased gene expression of neuronal nitric oxide synthase in
hypothalamus and brainstem of rats in heart failure. Brain Res.
734:109–115. 1996. View Article : Google Scholar
|
|
13
|
Li BF, Liu YF, Cheng Y, Zhang KZ, Li TM
and Zhao N: Protective effect of inducible nitric oxide synthase
inhibitor on pancreas transplantation in rats. World J
Gastroenterol. 13:6066–6071. 2007. View Article : Google Scholar :
|
|
14
|
Rajagopalan S, Kurz S, Münzel T, Tarpey M,
Freeman BA, Griendling KK and Harrison DG: Angiotensin II-mediated
hypertension in the rat increases vascular superoxide production
via membrane NADH/NADPH oxidase activation. Contribution to
alterations of vasomotor tone. J Clin Invest. 97:1916–1923. 1996.
View Article : Google Scholar :
|
|
15
|
Jang JH, Chun JN, Godo S, Wu G, Shimokawa
H, Jin CZ, Jeon JH, Kim SJ, Jin ZH and Zhang YH: ROS and
endothelial nitric oxide synthase (eNOS)-dependent trafficking of
angiotensin II type 2 receptor begets neuronal NOS in cardiac
myocytes. Basic Res Cardiol. 110:212015. View Article : Google Scholar :
|
|
16
|
Ratliff BB, Sekulic M, Rodebaugh J and
Solhaug MJ: Angiotensin II regulates NOS expression in afferent
arterioles of the developing porcine kidney. Pediatr Res. 68:29–34.
2010. View Article : Google Scholar :
|
|
17
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. Washington (DC): National Academies Press (US);
1996
|
|
18
|
Kumar SM Mohan, Kumar PS Mohan and Quadri
SK: Specificity of interleukin-1beta-induced changes in monoamine
concentrations in hypothalamic nuclei: Blockade by interleukin-1
receptor antagonist. Brain Res Bull. 47:29–34. 1998. View Article : Google Scholar
|
|
19
|
Cao H, Wen G and Li H: Role of peroxisome
proliferator-activated receptor α in atherosclerosis. Mol Med Rep.
9:1755–1760. 2014.
|
|
20
|
Campioli E, Batarseh A, Li J and
Papadopoulos V: The endocrine disruptor mono-(2-ethylhexyl)
phthalate affects the differentiation of human liposarcoma cells
(SW 872). PLoS One. 6:e287502011. View Article : Google Scholar :
|
|
21
|
Sousa LE, Magalhães WG, Bezerra FS, Santos
RA, Campagnole-Santos MJ, Isoldi MC and Alzamora AC: Exercise
training restores oxidative stress and nitric oxide synthases in
the rostral ventrolateral medulla of renovascular hypertensive
rats. Free Radic Res. 49:1335–1343. 2015. View Article : Google Scholar
|
|
22
|
Ge JF, Xu YY, Qin G, Peng YN, Zhang CF,
Liu XR, Liang LC, Wang ZZ and Chen FH: Depression-like behavior
induced by Nesfatin-1 in rats: Involvement of increased immune
activation and imbalance of synaptic vesicle proteins. Front
Neurosci. 9:4292015. View Article : Google Scholar :
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ,
Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et
al: Heart disease and stroke statistics-2011 update: A report from
the American Heart Association. Circulation. 123:e18–e29. 2011.
View Article : Google Scholar
|
|
25
|
Watson AM, Hood SG and May CN: Mechanisms
of sympathetic activation in heart failure. Clin Exp Pharmacol
Physiol. 33:1269–1274. 2006. View Article : Google Scholar
|
|
26
|
Floras JS: Sympathetic activation in human
heart failure: dIverse mechanisms, therapeutic opportunities. Acta
Physiol Scand. 177:391–398. 2003. View Article : Google Scholar
|
|
27
|
Gao L, Wang W, Liu D and Zucker IH:
Exercise training normalizes sympathetic outflow by central
antioxidant mechanisms in rabbits with pacing-induced chronic heart
failure. Circulation. 115:3095–3102. 2007. View Article : Google Scholar
|
|
28
|
Aronson D and Burger AJ: Concomitant
beta-blocker therapy is associated with a lower occurrence of
ventricular arrhythmias in patients with decompensated heart
failure. J Card Fail. 8:79–85. 2002. View Article : Google Scholar
|
|
29
|
Kowalewski M, Urban M, Mroczko B and
Szmitkowski M: Proinflammatory cytokines (IL-6, TNF-alpha) and
cardiac troponin I (cTnI) in serum of young people with ventricular
arrhythmias. Pol Arch Med Wewn. 108:647–651. 2002.(In Polish).
|
|
30
|
Kang YM, Yang Q, Yu XJ, Qi J, Zhang Y, Li
HB, Su Q and Zhu GQ: Hypothalamic paraventricular nucleus
activation contributes to neurohumoral excitation in rats with
heart failure. Regen Med Res. 2:22014. View Article : Google Scholar :
|
|
31
|
Ramchandra R and Barrett CJ: Regulation of
the renal sympathetic nerves in heart failure. Front Physiol.
6:2382015. View Article : Google Scholar :
|
|
32
|
Kang YM, Zhang AQ, Zhao XF, Cardinale JP,
Elks C, Cao XM, Zhang ZW and Francis J: Paraventricular nucleus
corticotrophin releasing hormone contributes to sympathoexcitation
via interaction with neurotransmitters in heart failure. Basic Res
Cardiol. 106:473–483. 2011. View Article : Google Scholar :
|
|
33
|
Yildiz M, Hasdemir H, Turkkan C,
Astarcioglu MA, Alper AT, Sahin A and Ozkan M: Acute effects of
cardiac resynchronization therapy on arterial distensibility and
serum norepinephrine levels in advanced heart failure. Cardiol J.
20:304–309. 2013. View Article : Google Scholar
|
|
34
|
Timmermans PB, Duncia JV, Carini DJ, Chiu
AT, Wong PC, Wexler RR and Smith RD: Discovery of losartan, the
first angiotensin II receptor antagonist. J Hum Hypertens. 9 Suppl
5:S3–S18. 1995.
|
|
35
|
Wang Y, Patel KP, Cornish KG, Channon KM
and Zucker IH: nNOS gene transfer to RVLM improves baroreflex
function in rats with chronic heart failure. Am J Physiol Heart
Circ Physiol. 285:H1660–H1667. 2003. View Article : Google Scholar
|
|
36
|
Mann DL: Inflammatory mediators and the
failing heart: Past, present, and the foreseeable future. Circ Res.
91:988–998. 2002. View Article : Google Scholar
|
|
37
|
Anker SD and von Haehling S: Inflammatory
mediators in chronic heart failure: An overview. Heart. 90:464–470.
2004. View Article : Google Scholar :
|
|
38
|
Romano M, Sironi M, Toniatti C,
Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, Van
Hinsbergh V, Sozzani S, et al: Role of IL-6 and its soluble
receptor in induction of chemokines and leukocyte recruitment.
Immunity. 6:315–325. 1997. View Article : Google Scholar
|
|
39
|
Marshall LF: Head injury: Recent past,
present, and future. Neurosurgery. 47:546–561. 2000. View Article : Google Scholar
|
|
40
|
Deten A, Volz HC, Briest W and Zimmer HG:
Cardiac cytokine expression is upregulated in the acute phase after
myocardial infarction. Experimental studies in rats. Cardiovasc
Res. 55:329–340. 2002. View Article : Google Scholar
|
|
41
|
Torre-Amione G, Kapadia S, Benedict C,
Oral H, Young JB and Mann DL: Proinflammatory cytokine levels in
patients with depressed left ventricular ejection fraction: A
report from the studies of left ventricular dysfunction (SOLVD). J
Am Coll Cardiol. 27:1201–1206. 1996. View Article : Google Scholar
|
|
42
|
Helwig BG, Musch T, Craig RA and Kenney
MJ: Increased interleukin-6 receptor expression in the
paraventricular nucleus of rats with heart failure. Am J Physiol
Regul Integr Comp Physiol. 292:R1165–1173. 2007. View Article : Google Scholar
|
|
43
|
Su Q, Liu JJ, Cui W, Shi XL, Guo J, Li HB,
Huo CJ, Miao YW, Zhang M, Yang Q and Kang YM: Alpha lipoic acid
supplementation attenuates reactive oxygen species in hypothalamic
paraventricular nucleus and sympathoexcitation in high salt-induced
hypertension. Toxicol Lett. 241:152–158. 2016. View Article : Google Scholar
|
|
44
|
Stojanović M, Šćepanović L, Hrnčić D,
Rašić-Marković A, Djuric D and Stanojlović O: Multidisciplinary
approach to nitric oxide signaling: Focus on the gastrointestinal
and the central nervous system. Vojnosanitetski Pregled.
72:619–624. 2015. View Article : Google Scholar
|
|
45
|
Cheng WH, Lu PJ, Ho WY, Tung CS, Cheng PW,
Hsiao M and Tseng CJ: Angiotensin II inhibits neuronal nitric oxide
synthase activation through the ERK1/2-RSK signaling pathway to
modulate central control of blood pressure. Circ Res. 106:788–795.
2010. View Article : Google Scholar
|
|
46
|
Capettini LS, Cortes SF, Silva JF,
Alvarez-Leite JI and Lemos VS: Decreased production of neuronal
NOS-derived hydrogen peroxide contributes to endothelial
dysfunction in atherosclerosis. Br J Pharmacol. 164:1738–1748.
2011. View Article : Google Scholar :
|
|
47
|
Liu JL, Murakami H and Zucker IH:
Angiotensin II-nitric oxide interaction on sympathetic outflow in
conscious rabbits. Circ Res. 82:496–502. 1998. View Article : Google Scholar
|
|
48
|
Zhang K, Zucker IH and Patel KP: Altered
number of diaphorase (NOS) positive neurons in the hypothalamus of
rats with heart failure. Brain Res. 786:219–225. 1998. View Article : Google Scholar
|