Introduction
Astemizole, used for many years as an H1-histamine
receptor antagonist, is a long-acting, non-sedating,
second-generation anti-histamine, which is currently used in
certain countries to treat allergy symptoms (1). However, astemizole has gained
attention as an antineoplastic drug (2) as it targets important ion channels
involved in cancer progression, such as potassium voltage-gated
channel subfamily H member 1 in breast cancer (3). Compared with calcitriol, the
antineoplastic effects of astemizole involve different mechanisms
of action, including antagonizing H1-histamine receptors (2), reducing P450-aromatase expression
(4) and inhibiting the release of
inflammatory mediators (5), and
this may further improve therapeutic efficacy when these drugs are
jointly administered. The reported therapeutic and toxic serum
levels of astemizole are 0.05 mg/ml (0.10 mM) and 14 mg/m (30.5
mM), respectively (6). Nishimoto
et al (6) previously
examined the effects of astemizole, a non-sedating antihistamine,
on ventricular activation and RT intervals in a canine myocardial
infarction model and Romero et al (7) investigated the impact of potassium
voltage-gated channel subfamily H member 2 channel kinetic
abnormalities on channel block and susceptibility to acquired long
QT syndrome. Therefore, the present study aimed to investigate the
effect of astemizole in cardiovascular diseases (CVDs).
CVDs are the leading causes of death and disability
among aged people worldwide (8),
and hypertension and arteriosclerosis have important roles in CVD.
Functional impairment of vascular endothelial cells is involved in
the establishment of hypertension and arteriosclerosis (9), therefore, the maintenance of vascular
endothelial function is important to prevent hypertension and
arteriosclerosis (10). A number
of diseases involve the generation of reactive oxygen species (ROS)
by vascular endothelial cells, including atherosclerosis,
hypercholesteremia and disseminated intravascular coagulation
(11,12). Increased levels of ROS in vascular
lesions have been demonstrated to have detrimental effects on a
number of processes, resulting in the peroxidation of membrane
lipids, endothelium-derived enzyme inactivation and the occurrence
of apoptosis (13). As one of the
most common forms ROS, hydrogen peroxide
(H2O2) crosses the plasma membrane easily and
produces a highly reactive radical, ŸOH, that damages cells and
tissues (9,14). The generation of
H2O2 is implicated in the progression of
atherosclerosis and H2O2 mediates various
cellular responses. Thus, H2O2 has been
extensively used as an oxidative stimulus to induce oxidative
stress in in vitro models. Falone et al (15) may have laid the foundation for the
development of non-invasive pulsed electromagnetic frequency-based
approaches aimed at elevating endogenous antioxidant properties in
cellular or tissue models. Furthermore, Venditti et al
(16) investigated substitution of
the oldest ROS-overproducing mitochondria with neoformed
mitochondria endowed with a smaller capacity to produce free
radicals. As the major type of endothelial cells, human umbilical
vein endothelial cells (HUVECs) are commonly accepted as a model
cell to investigate the mechanisms involved in the pathogenesis of
CVDs (17).
The present study, therefore, aimed to investigate
the protective effect of the histamine H1 receptor antagonist,
astemizole, on HUVECs. The present study investigated the action of
astemizole as an anti-ROS agent in HUVECs and demonstrated that
astemizole may exert its anti-ROS effect via
ROS/p53/p21Cip1/Waf1/p16INK4a signaling
pathways.
Materials and methods
Chemicals and reagents
Astemizole, H2O2,
dimethylsulfoxide (DMSO) and MTT were obtained from Sigma-Aldrich;
Merck Millipore (Darmstadt, Germany). The levels of malondialdehyde
(MDA) were measured using an MDA assay kit, which is based on the
thiobarbituric acid method to determine MDA in samples including
blood, urine and tissue. Superoxide dismutase (SOD) was measured
with a WST-1 Cell Proliferation and Cytotoxicity Assay kit,
glutathione peroxidase (GSH-Px) was measured with a GSH-PX assay
kit based on the colorimetric method, ROS was measured using a
Reactive Oxygen Species Assay kit. All kits were purchased from
Beyotime Institute of Biotechnology, Haimen, China. The following
primary antibodies were obtained: Anti-p16INK4a (sc-377412; 1:500),
anti-GAPDH (sc-32233; 1:2,000) (both from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-p21Cip1/Waf1 (610233; 1:1,000; BD
Pharmingen, San Diego, CA, USA), anti-p53 (9282, 1:1,000), horse
radish peroxidase (HRP)-linked anti-rabbit immunoglobulin (Ig)-G
(#7074; 1:5,000) and HRP-linked anti-mouse IgG (#7076; 1:5,000)
(both from Cell Signaling Technology, Inc., Danvers, MA, USA). All
other reagents used were of analytical grade.
Cell culture and treatment
HUVECs were purchased from Shanghai Zhong Qiao Xin
Zhou Biotechnology Co., Ltd (Shanghai, China). The cells were
maintained in endothelial cell medium (ECM; Corning Incorporated,
Corning, NY, USA) supplemented with 5% fetal bovine serum (Zhejiang
Tianhang Biotechnology Co., Ltd., Huzhou, China) and 1% endothelial
cell growth supplement in Poly-L-Lysine-pretreated flasks, at 37°C
in a 5% CO2 incubator. HUVECs were treated with
astemizole (0, 0.25, 0.50 or 1.00 µM) for 12 h at 4°C, or a PBS
vehicle control. The culture supernatant was subsequently removed
and the cells were exposed to H2O2 (200 µM)
diluted in ECM for 12 h at 37°C until further assays.
Cell viability measurement
Cell morphology was assessed using a light
microscope. The viability of HUVECs was measured using the MTT
assay. DMSO was used as the solvent. The absorbance at 490 nm in
each well was determined using a microplate autoreader (Bio-Rad
Laboratories, Inc. Hercules, CA, USA). The viability of HUVECs in
each well was presented as a percentage of the control group.
Measurement of MDA level and
activities of SOD and GSH-Px
The level of MDA and the activity of SOD and GSH-Px
in HUVECs were measured using MDA, SOD and GSH-Px detection kits
(Beyotime Institute of Biotechnology, Haimen, China), respectively,
according to the manufacturer's instructions.
Detection of ROS level
The level of intracellular ROS was determined using
an ROS assay kit (Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. The fluorescence intensity was
measured using a flow cytometer under an excitation wavelength of
488 nm. Analyses were performed using bivariate flow cytometry in a
BD FACSCanto II, equipped with BD FACSDiva (Becton-Dickinson, San
Jose, CA, USA).
Western blot analysis
Total protein samples were extracted from HUVECs
with radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology). Protein concentration was determined
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Protein samples (100 µg) were separated by SDS-PAGE
(10% polyacrylamide gels) and transferred to a nitrocellulose
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
subsequently blocked with milk powder at room temperature for 2 h
and incubated overnight at 4°C with the primary antibody. The
following day, membranes were washed and incubated with
HRP-conjugated secondary anti-rabbit or anti-mouse antibody for 1 h
at room temperature. Specific proteins were visualized using
enhanced chemiluminescence (ECL) detection with BeyoECL Plus
(Beyotime Institute of Biotechnology, Beijing, China). Western blot
bands were quantified using Odyssey v1.2 software (LI-COR
Biosciences, Lincoln, NE, USA) by measuring the band intensity
(area × optical density) for each group and normalizing to
GAPDH.
Statistical analysis
The data are presented as the mean ± standard error
of the mean or the mean ± standard deviation. Statistical
comparisons among multiple groups were performed by one-way
analysis of variance followed by Tukey's multiple comparison test.
P<0.05 was considered to indicate statistical significance.
Statistical values were calculated using SPSS 19.0 (IBM SPSS,
Armonk, NY, USA) and illustrated using Graph Pad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
Effects of astemizole on the viability
of H2O2-induced HUVECs
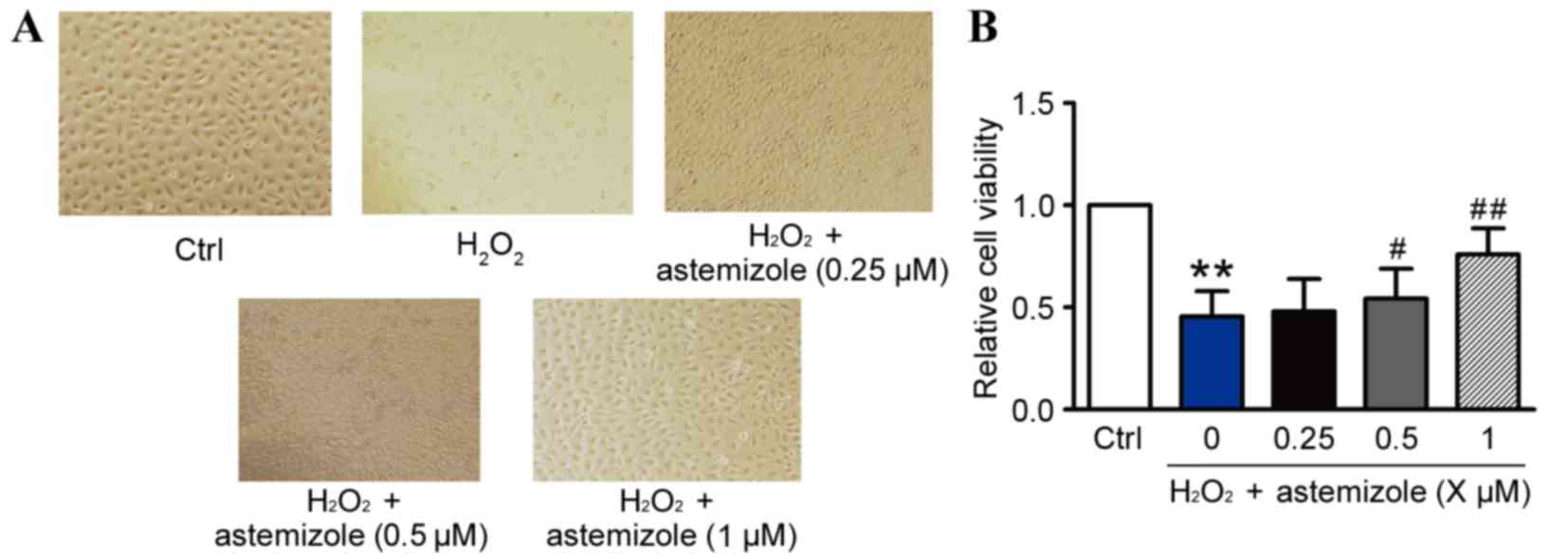
Visualization of the cells by light microscopy
revealed alterations to the shape and size of the HUVECs (Fig. 1A). H2O2
treatment caused injury to the HUVECs, however, astemizole
inhibited the detrimental effect of H2O2 and
promoted the normal morphology of HUVECs. The effects of astemizole
on the viability of HUVECs exposed to H2O2
were evaluated by MTT analysis (Fig.
1B). The survival rate of HUVECs was 48.92±3.48%, as a
percentage of control cell viability, following exposure to 200 µM
of H2O2 for 12 h (P<0.01; Fig. 1B). Pretreatment with astemizole
(0.5 or 1 µM) significantly increased the viability of HUVECs
exposed to H2O2, in a dose-dependent manner,
compared with H2O2 only (P<0.05 and
P<0.01, respectively; Fig. 1B).
However, pretreatment with 0.25 µM astemizole for 12 h did not
increase the cell viability compared with the
H2O2 only group (P>0.05; Fig. 1B). When incubated with 0.0625–2 µM
astemizole alone for 24 h, cell viability did not exhibit marked
changes compared with the control group, although significant
cytotoxicity was observed with 4 µM astemizole treatment for 24 h
(data not shown). Therefore, only concentrations of 0.5 and 1 mM
astemizole were used throughout subsequent experiments in this
study.
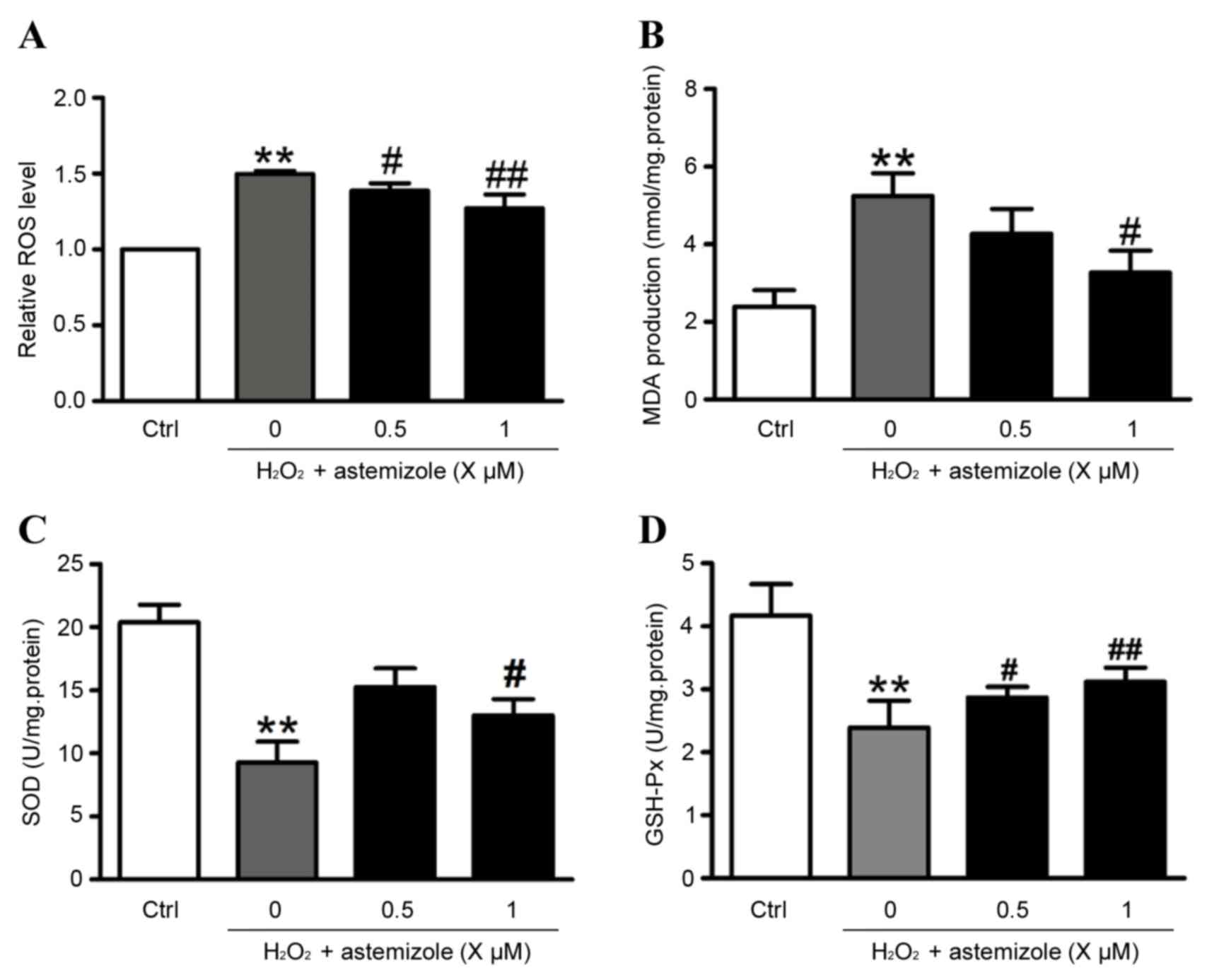
Astemizole inhibits oxidative stress
in HUVECs
Levels of ROS and MDA were increased when cells were
exposed to H2O2 (200 µM for 12 h) compared
with control cells (P<0.01; Fig. 2A
and B, respectively). However, the HUVECs co-cultured with 0.5
or 1 µM astemizole exhibited significant decreases in ROS and MDA
levels compared with H2O2 only-treated cells
(Fig. 2A and B). Additionally, the
activities of SOD and GSH-Px were reduced following exposure to
H2O2 (200 µM, 12 h) compared with the control
(P<0.01; Fig. 2C and D).
However, groups pretreated with 1 µM astermizole prior to
H2O2 exposure exhibited significantly
increased activities of SOD and GSH-Px compared with the
H2O2 only-treated cells (Fig. 2C and D).
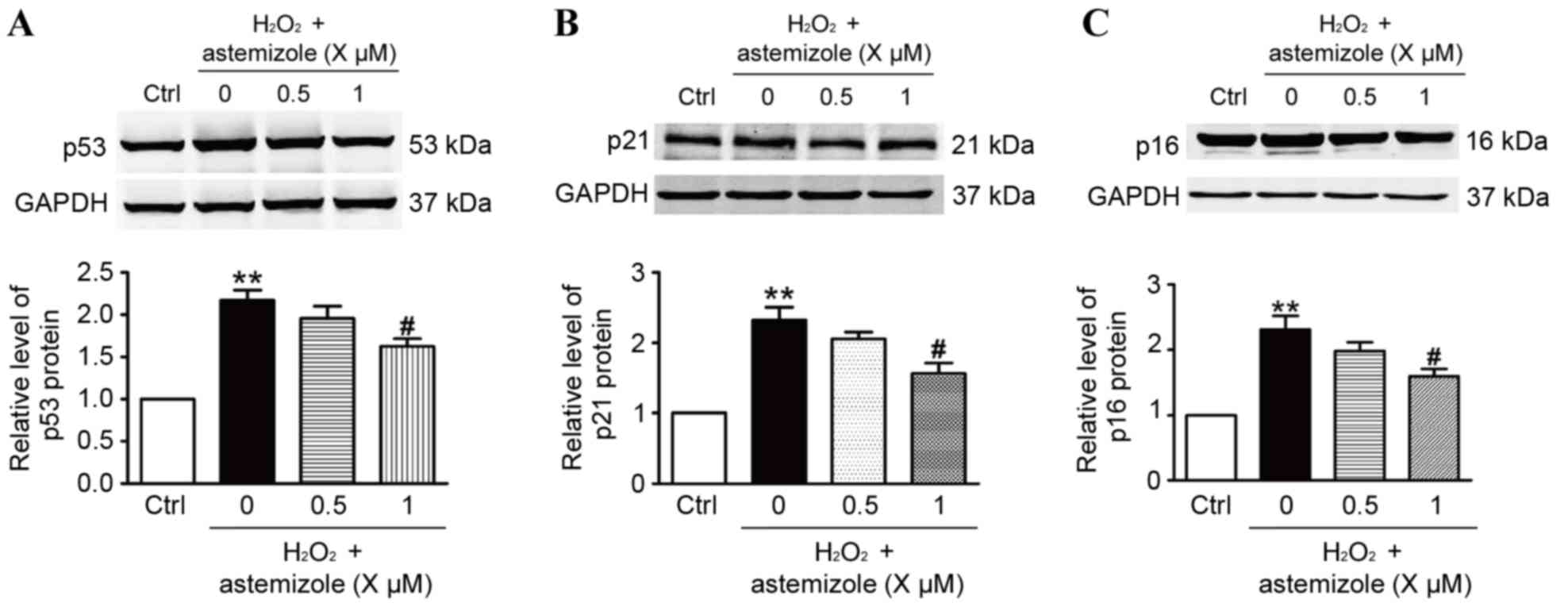
Astemizole decreases expression of
p53, p21Cip1/Waf1 and p16INK4a in HUVECs
To investigate the mechanisms that mediate the
antioxidative effect of astemizole, the present study performed
western blot analysis. The results demonstrated that p53,
p21Cip1/Waf1 and p16INK4a protein expression was significantly
increased in HUVECs treated with H2O2
compared with the control group (Fig.
3). The p53 pathway is implicated in numerous diseases,
including cancer (18), senescence
(19) and heart failure (20). In addition, Pietrzkowicz et
al (21) previously
demonstrated the effects of the H1-receptor antagonists,
terfenadine and astemizole, on the CV system of humans. In the
present study, pretreatment with 1 µM astemizole significantly
decreased the expression of p53, p21Cip1/Waf1 and p16INK4a compared
with the H2O2 only-treated cells (P<0.05;
Fig. 3A-C, respectively). The
results indicate that changes in p53, p21Cip1/Waf1 and p16INK4a may
have an important role in the ability of astemizole to protect
HUVECs against H2O2-induced injury.
Discussion
Astemizole possesses a variety of unique properties
and has also gained attention as a potential antineoplastic drug as
it targets important ion channels involved in cancer progression.
However, the effect of astemizole on oxidative stress in HUVECs
has, to the best of our knowledge, not been investigated
previously, and limited information is known about the effect of
its actions. The present study demonstrated that astemizole affects
HUVECs. Astemizole protected HUVECs exposed to
H2O2 in a dose-dependent manner. Hydrogen
peroxide caused significant increases in ROS, MDA, p53,
p21Cip1/Waf1 and p16INK4a, and decreases in
the antioxidant enzymes SOD and GSH-Px, as observed in HUVECs. In
addition, astemizole (1 µM) significantly reversed the aberrant
alterations induced by H2O2 in ROS levels,
endogenous antioxidant activities and the protein expression of
p53, p21Cip1/Waf1 and p16INK4a. The results
of the present study suggest that the
ROS/p53/p21Cip1/Waf1/p16INK4a signaling
cascade may be involved in CVDs. The present study demonstrates the
efficacy of astemizole against oxidative stress and provides a
potential novel strategy for slowing the progression of CVDs.
Oxidative stress has a major role in numerous
pathological conditions (20–24)
and the molecular mechanisms that control the cell response to ROS
have been extensively studied. Endothelial cells are involved in
several aspects of the generation and development of a number of
CVDs, including hypertension and arteriosclerosis. The present
study, via the use of a H2O2 model, has
provided information about the pathogenesis of CVDs;
H2O2-induced damage, and decreases in
cellular antioxidant ability, which may result in increased
apoptosis, were observed at H2O2
concentrations ≥200 mM. Agents that inhibit the production of ROS
or enhance cellular antioxidant defenses protect cells from the
damaging effects of oxygen radicals (25). The present study demonstrated that
astemizole scavenges intracellular ROS induced by
H2O2 (Fig.
2A) and also effectively increases the viability of endothelial
cells exposed to H2O2 (Fig. 1). These results indicate that the
protective effect is associated the inhibition of the production of
ROS or enhanced cellular antioxidant ability. In addition, the
present study demonstrated that increases in ROS and MDA levels,
and reduced activities of SOD and GSH-Px, have an important role in
CVDs (19). This imbalance between
enhanced oxidative stress and reduced antioxidant defense is
involved in the aging process (26). The results of the current study
demonstrated that pretreatment with astemizole (1 µM) caused
significant decreases in ROS, MDA and increases in antioxidant
enzymes SOD and GSH-Px, compared with H2O2
only-treated cells.
The p53 signaling pathway has important functions in
apoptosis, senescence and autophagy (19,27–29)
Fibroblast growth factor-23 induces cellular senescence in human
mesenchymal stem cells from skeletal muscle (30). A marine steroid derived from
Acropora formosa has been demonstrated to enhance
mitochondrial-mediated apoptosis in non-small cell lung cancer
cells (31). The results of the
present study demonstrate that astemizole may protect HUVECs that
are damaged by H2O2, and this anti-oxidative
stress effect appears to be conferred by effects on antioxidative
enzymes and p53/p21Cip1/Waf1/p16INK4a
signaling. The results are consistent with a study by Lee et
al (32), which demonstrated
that astemizole may be a novel biomarker for cardiotoxicity. The
present study demonstrated that p53, p21Cip1/Waf1 and
p16INK4a protein expression was higher following HUVEC
injury, indicating that the
p53/p21Cip1/Waf1/p16INK4a signaling pathway
may be involved in H2O2-induced HUVEC
injury.
The current study demonstrated that astemizole, at
low and safe concentrations, protected against
H2O2-induced damaged that is associated with
CVDs. Treatment with astemizole reduced expression of
ROS/p53/p21Cip1/Waf1/p16INK4a and had a
protective effect on endothelial cells. Further investigation is
required to determine whether astemizole may be used as an anti-CVD
agent in clinical patients.
References
|
1
|
Garcia-Quiroz J, Garcia-Becerra R, Barrera
D, Santos N, Avila E, Ordaz-Rosado D, Rivas-Suárez M, Halhali A,
Rodríguez P, Gamboa-Domínguez A, et al: Astemizole synergizes
calcitriol antiproliferative activity by inhibiting CYP24A1 and
upregulating VDR: A novel approach for breast cancer therapy. PLoS
One. 7:e450632012. View Article : Google Scholar :
|
|
2
|
Garcia-Quiroz J and Camacho J: Astemizole:
An old anti-histamine as a new promising anti-cancer drug.
Anticancer Agents Med Chem. 11:307–314. 2011. View Article : Google Scholar
|
|
3
|
Garcia-Quiroz J, Garcia-Becerra R,
Santos-Martinez N, Barrera D, Ordaz-Rosado D, Avila E, Halhali A,
Villanueva O, Ibarra-Sánchez MJ, Esparza-López J, et al: In vivo
dual targeting of the oncogenic Ether-à-go-go-1 potassium channel
by calcitriol and astemizole results in enhanced antineoplastic
effects in breast tumors. BMC Cancer. 14:7452014. View Article : Google Scholar :
|
|
4
|
Sanderson JT: The steroid hormone
biosynthesis pathway as a target for endocrine-disrupting
chemicals. Toxicol Sci. 94:3–21. 2006. View Article : Google Scholar
|
|
5
|
Fischer MJ, Paulussen JJ, Kok-Van Esterik
JA, Van der Heijden VS, De Mol NJ and Janssen LH: Effects of the
anti-allergics astemizole and norastemizole on Fc epsilon RI
receptor-mediated signal transduction processes. Eur J Pharmacol.
322:97–105. 1997. View Article : Google Scholar
|
|
6
|
Nishimoto M, Hashimoto H, Ohmura T, Ikeda
Y, Watanabe S, Ohashi K, Umemura K and Nakashima M: Effects of
astemizole on ventricular activation delay and RT intervals in a
canine myocardial infarction model. Biol Pharm Bull. 20:1020–1023.
1997. View Article : Google Scholar
|
|
7
|
Romero L, Trenor B, Yang PC, Saiz J and
Clancy CE: In silico screening of the impact of hERG channel
kinetic abnormalities on channel block and susceptibility to
acquired long QT syndrome. J Mol Cell Cardiol. 87:271–282. 2015.
View Article : Google Scholar :
|
|
8
|
Cai H: Hydrogen peroxide regulation of
endothelial function: Origins, mechanisms, and consequences.
Cardiovasc Res. 68:26–36. 2005. View Article : Google Scholar
|
|
9
|
Liu L, Gu L, Ma Q, Zhu D and Huang X:
Resveratrol attenuates hydrogen peroxide-induced apoptosis in human
umbilical vein endothelial cells. Eur Rev Med Pharmacol Sci.
17:88–94. 2013.
|
|
10
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar
|
|
11
|
Pandian RP, Kutala VK, Liaugminas A,
Parinandi NL and Kuppusamy P: Lipopolysaccharide-induced
alterations in oxygen consumption and radical generation in
endothelial cells. Mol Cell Biochem. 278:119–127. 2005. View Article : Google Scholar
|
|
12
|
Fasanaro P, Magenta A, Zaccagnini G,
Cicchillitti L, Fucile S, Eusebi F, Biglioli P, Capogrossi MC and
Martelli F: Cyclin D1 degradation enhances endothelial cell
survival upon oxidative stress. FASEB J. 20:1242–1244. 2006.
View Article : Google Scholar
|
|
13
|
Xu HB and Huang ZQ: Icariin enhances
endothelial nitric-oxide synthase expression on human endothelial
cells in vitro. Vascul Pharmacol. 47:18–24. 2007. View Article : Google Scholar
|
|
14
|
Kamata H and Hirata H: Redox regulation of
cellular signalling. Cell Signal. 11:1–14. 1999. View Article : Google Scholar
|
|
15
|
Falone S, Marchesi N, Osera C, Fassina L,
Comincini S, Amadio M and Pascale A: Pulsed electromagnetic field
(PEMF) prevents pro-oxidant effects of H2O2
in SK-N-BE(2) human neuroblastoma cells. Int J Radiat Biol.
92:281–286. 2016. View Article : Google Scholar
|
|
16
|
Venditti P, Costagliola IR and Di Meo S:
H2O2 production and response to stress
conditions by mitochondrial fractions from rat liver. J Bioenerg
Biomembr. 34:115–125. 2002. View Article : Google Scholar
|
|
17
|
Xia Z, Liu M, Wu Y, Sharma V, Luo T,
Ouyang J and McNeill JH: N-acetylcysteine attenuates
TNF-alpha-induced human vascular endothelial cell apoptosis and
restores eNOS expression. Eur J Pharmacol. 550:134–142. 2006.
View Article : Google Scholar
|
|
18
|
Ormenisan C, Kubik M, Legrand S, Kraemer
D, Smotherman C and Masood S: The potential of ki67 and p53
assessment in development of individualized targeted therapy in
breast cancer patients. Pathologica. 107:177–180. 2015.
|
|
19
|
Zhang M, Liu D, Li S, Chang L, Zhang Y,
Liu R, Sun F, Duan W, Du W, Wu Y, et al: Bone marrow mesenchymal
stem cell transplantation retards the natural senescence of rat
hearts. Stem Cells Transl Med. 4:494–502. 2015. View Article : Google Scholar :
|
|
20
|
Oka T, Morita H and Komuro I: Novel
molecular mechanisms and regeneration therapy for heart failure. J
Mol Cell Cardiol. 92:46–51. 2016. View Article : Google Scholar
|
|
21
|
Pietrzkowicz M and Grzelewska-Rzymowska I:
The effect of new H1-receptor antagonists on cardiovascular system.
Pol Merkur Lekarski. 5:360–362. 1998.(In Polish).
|
|
22
|
Griendling KK and FitzGerald GA: Oxidative
stress and cardiovascular injury: Part II: Animal and human
studies. Circulation. 108:2034–2040. 2003. View Article : Google Scholar
|
|
23
|
Griendling KK and FitzGerald GA: Oxidative
stress and cardiovascular injury: Part I: Basic mechanisms and in
vivo monitoring of ROS. Circulation. 108:1912–1916. 2003.
View Article : Google Scholar
|
|
24
|
Giustarini D, Dalle-Donne I, Tsikas D and
Rossi R: Oxidative stress and human diseases: Origin, link,
measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci.
46:241–281. 2009. View Article : Google Scholar
|
|
25
|
Wang YK and Huang ZQ: Protective effects
of icariin on human umbilical vein endothelial cell injury induced
by H2O2 in vitro. Pharmacol Res. 52:174–182.
2005. View Article : Google Scholar
|
|
26
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar :
|
|
27
|
Tseng CY, Wang JS, Chang YJ, Chang JF and
Chao MW: Exposure to high-dose diesel exhaust particles induces
intracellular oxidative stress and causes endothelial apoptosis in
cultured in vitro capillary tube cells. Cardiovasc Toxicol.
15:345–354. 2015. View Article : Google Scholar
|
|
28
|
Xu LL, Liu ML, Wang JL, Yu M and Chen JX:
Saligenin cyclic-o-tolyl phosphate (SCOTP) induces autophagy of rat
spermatogonial stem cells. Reprod Toxicol. 60:62–68. 2016.
View Article : Google Scholar
|
|
29
|
Song YM, Lee WK, Lee YH, Kang ES, Cha BS
and Lee BW: Metformin restores parkin-mediated mitophagy,
suppressed by cytosolic p53. Int J Mol Sci. 17:pii: E1222016.
View Article : Google Scholar
|
|
30
|
Sato C, Iso Y, Mizukami T, Otabe K, Sasai
M, Kurata M, Sanbe T, Sekiya I, Miyazaki A and Suzuki H: Fibroblast
growth factor-23 induces cellular senescence in human mesenchymal
stem cells from skeletal muscle. Biochem Biophys Res Commun.
470:657–662. 2016. View Article : Google Scholar
|
|
31
|
Vaikundamoorthy R, Sundaramoorthy R,
Krishnamoorthy V, Vilwanathan R and Rajendran R: Marine steroid
derived from Acropora formosa enhances mitochondrial-mediated
apoptosis in non-small cell lung cancer cells. Tumour Biol.
37:10517–10531. 2016. View Article : Google Scholar
|
|
32
|
Lee EH, Oh JH, Park HJ, Kim DG, Lee JH,
Kim CY, Kwon MS and Yoon S: Simultaneous gene expression signature
of heart and peripheral blood mononuclear cells in
astemizole-treated rats. Arch Toxicol. 84:609–618. 2010. View Article : Google Scholar
|