Introduction
Parasites can be divided within a diverse phylum
composed of protozoan and helminthes, and this phylum contains
numerous species that can infect humans and animals. Some parasitic
species can even infect plants, and have been affecting humans for
many years (1). Emerging parasitic
diseases in humans and animals, such as malaria, toxoplasmosis,
leishmaniasis, schistosomiasis, echinococcosis, trichinellosis and
cysticercosis, are global problems, which can lead to serious
nutritional deficiencies, reduce animal productivity, effect acute
tissue damage or even death (2–5).
Considering that parasites have diverse and complex
biological and ecological lifestyles, this raises questions as to
how can they maintain their fluid homeostasis under different
osmotic stress in vivo and in vitro, and also how
parasites regulate rapid water transport in moist and dry
environments. What is interesting about these parasites are the
channels and transporters (also known as permeases) in membranes
with essential biological functions involving in facilitating water
transport and osmoregulation, nutrient uptake, cytotoxic release
and host cellular adhesion (6,7).
A significant discovery concerning channels in cell
membranes awarded the Nobel Prize in Chemistry in 2003 to Peter
Agre, who was famed for identifying the functions of aquaporins
(AQPs). In the present paper, the authors review the amino acid
residue divergence and analyze the evolutionary relationship of
parasite aquaporins, and further discuss the possibility of
developmental drug design against helminths based on the
protein.
AQPs belong to the major intrinsic protein (MIP)
family and exist in almost all living organisms including animals,
plants, bacteria and viruses (8).
The aquaporin family is abundant in the plasma membrane (9). Proteomics analyses indicate that
aquaporins are the most plentiful proteins within the biological
tegument surface of Schistosoma mansoni (10). The common structure of AQPs
consists of 6 α-helical transmembrane domains (TM) and five
connecting loops (11). The N- and
C-terminal regions of AQPs, as well as B and D loops, are within
the cytoplasm.
The advanced structure of AQP proteins is often
compared to an hourglass, whereby the center of the pore has unique
Asn-Pro-Ala (NPA) sequence motifs in loops B and E; this is where
an isolated water molecule will transiently form hydrogen bonds
with two conserved Asn residues (12). The narrowest restricted site is
located at the entrance of the pore mouth with a diameter of 2.8 Å,
which may interact with passing solutes, bound by aromatic amino
acids and a widely conserved aromatic arginine (ar/R) filter
(12,13).
Re-construction of phylogenetic tree based
on aquaporin sequences
To investigate the evolutionary relationship of MIP
family proteins in parasites, the deduced polypeptides were
investigated by ClustalW multiple sequence alignment software and
the phylogenetic tree of parasites was re-constructed using the
neighbor-joining (NJ) and maximum likelihood (ML) methods, based on
the AQP sequences (Fig. 1). A
total of 44 AQP sequences were selected. The most outstanding
phylogenetic pattern is divided into two distinct clusters that are
orthodox aquaporins and aquaglyceroporins (GLPs). The orthodox
aquaporin, also named classical aquaporin, only allow the passage
of water, while the aquaglyceroporins can also transport uncharged
molecules like polyols, urea and metalloid besides water (14). In the phylogenic tree, a
subcellular-aquaporin group is also formed as an independent
cluster, due to the unusual conserved NPA motifs (15,16).
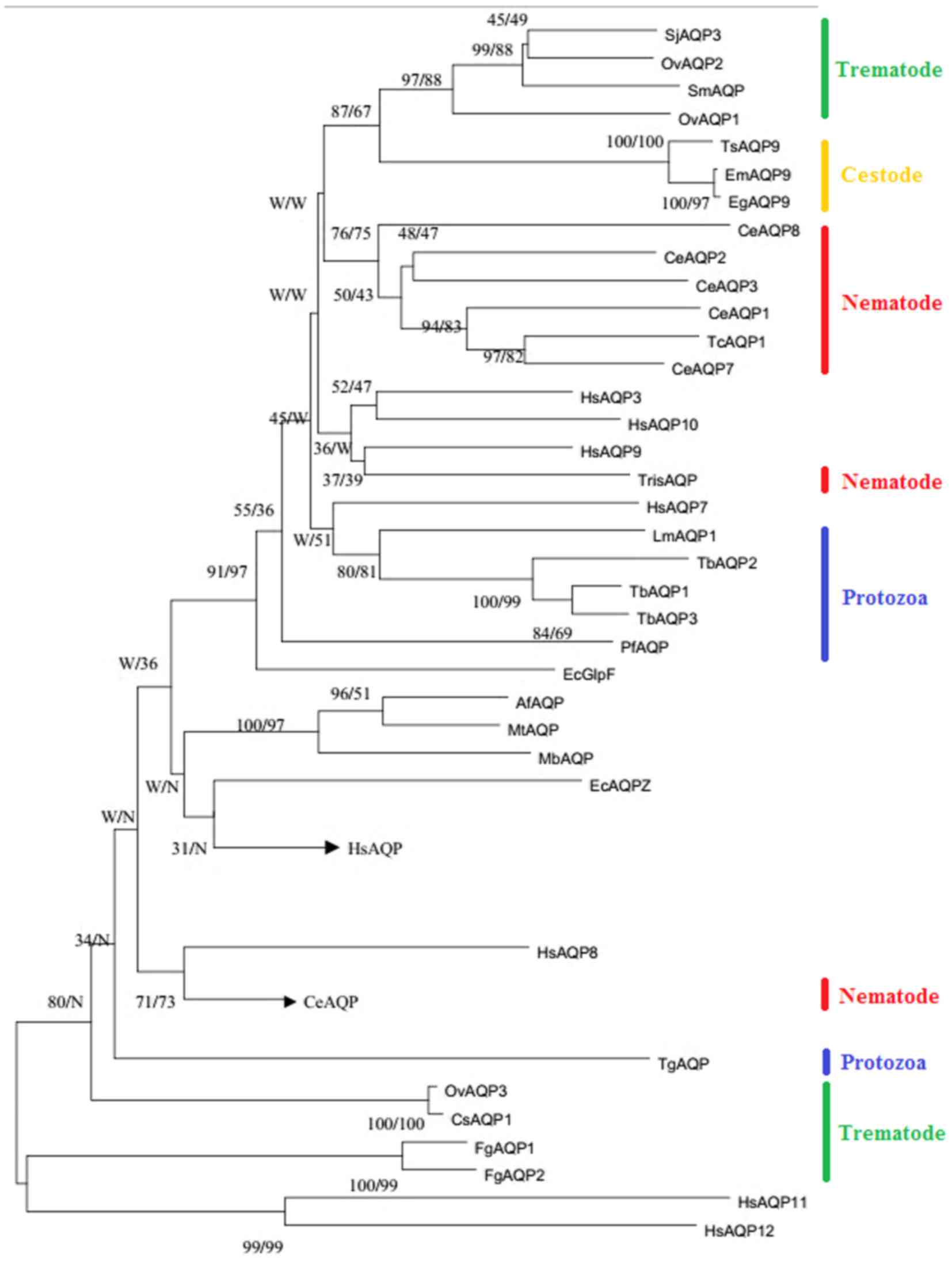 | Figure 1.The phylogenetic tree re-constructed
using NJ and ML methods to elucidate the evolutionary relationship
between parasitic aquaporin sequences. The accession numbers for
each sequence are as follows: PfAQP (AJ413249); LmAQP1 (AY567835);
TbAQP1-3 (AJ697889, AJ697890, AJ697891); TgAQP (Toxoplasma gondii);
SjAQP3 (CPRT0000005211); SmAQP (EU780065); FgAQP1-2 (HM748645,
HM748644); TcAQP1 (AF067963); CeAQP1-8 (CCD66276, CAA84633,
CAA22259, CAA94770, CAA94903, CCF23336, CCD66489, CCD68566);
HsAQP1-12 (NP_932,766, AAB31999, CAG46822, CAG46819, NP_0,01643,
CAI13303, AAH40630, CAG46824, CAH70483, Q8NBQ7, Q8IXF9); TrisAQP
(unclear); EcGlpF (CDZ22687); EcAQPZ (AAC43518); EmAQP9 (CDS35949);
EgAQP9 (CDS22736); TsAQP9 (000,547100); AfAQP (NP_07,0255); MbAQP
(ZP_00,077803); MtAQP (AB055880); OvAQP1-3 (KF697690, KF697691,
KM359766); CsAQP1 (GAA33659). The numbers indicate bootstrap values
resulting from different analyses in the order NJ/ML. NJ,
neighbor-joining; ML, maximum likelihood; AQP, aquaporin; Pf,
Plasmodium falciparum; Lm, Leishmania major; Tb, Trypanosoma
brucei; Tg, Toxoplasma gondii; Sj, Schistosoma japonicum; Sm,
Schistosoma mansoni; Fg, Fasciola gigantica; Tc, Toxocara canis;
Ce, Caenorhabditis elegans; Hs, Homo sapiens; Tris, Trichinella
spiralis; Ec, Escherichia coli; Em, Echinococcus multilocularis;
Eg, Echinococcus granulosus; Ts, Taenia solium; Af, archaeoglobus
fulgidus; Mb, Methanosarcina barkeri; Mt, Methanothermobacter
thermautotrophicus; Ov, Opisthorchis viverrini; Cs, Clonorchis
sinensis. |
Unlike the aquaporins in plants and mammalian
animals, the protein in parasites seems to have fewer isoforms
(17), and thus, the few channels
tend to be multifunctional. The assembled functions in a single
protein are not only due to the simple physiological structures of
parasites, but also due to the probability of a self-protection
strategy that parasites use to reduce excessive antigen exposure to
the host-parasite interface; this avoids activating the host immune
response system.
As revealed from the phylogenetic tree in the
present study, GLP group can be further divided into four major
GLPs sub-groups: i) Protozoon GLPs+Homo sapiens (Hs)
AQP7 and Escherichia coli (Ec) GlpF, ii) nematode
GLPs + HsAQP3,9,10, iii) trematode GLPs, and iv) cestode
GLPs. These parasites possess at least one multi-functional
aquaporin. It is noteworthy that both FgAQP1 and
FgAQP2 were integrated into in a single branch belonging
to subcellular-aquaporin group, both of which contain a mutated TAA
motif in the B loop (18). FgAQPs
are primarily expressed in tegumental cells and the linings of
ovary and testes (18). Increased
water permeability observed in Xenopus oocytes, but a failure to
permeate glycerol and urea (18)
suggests that FgAQPs may be more likely to involve osmoregulation
in Fasciola instead of transporting solutes.
Similarly interesting is the distribution of
Toxoplasma gondii (Tg)AQP. In the phylogenetic tree, TgAQP
belongs to the water specific group that presents 47% similarity to
plant tonoplast intrinsic proteins, which is a water specific
channel in theory. Pavlovic-Djuranovic, Schultz and Beitz (19) reported that TgAQP is a bifunctional
channel, permeating both water and glycerol, which suggests that
TgAQP has obtained solute permeability following gene transfer from
plant to an ancestor of Toxoplasma.
The variation of restrictions in aquaporin
proteins from parasites
Aquaporin integrates into a homotetramer in the
plasma membrane and each monomer is an independent functional pore
(20). The central pore formed by
four monomers is said to be a gas channel for CO2 and NO
(21). Arg (ar/R) constriction and
NPA motifs are two major constriction regions that are responsible
for aquaporin selectivity. The ar/R constriction region is the
narrowest part of the water channel, and the residues are Phe56,
His180, Cys189 and Arg195 in human AQP1 and Trp48, Gly191, Phe200
and Arg206 in E. coli GlpF. The substitution of His180,
Cys189 in GlpF by Gly191 and Phe200 may result in the changed size
and the increased hydrophobicity, which allow a larger molecule,
such as glycerol, to pass through (22,23).
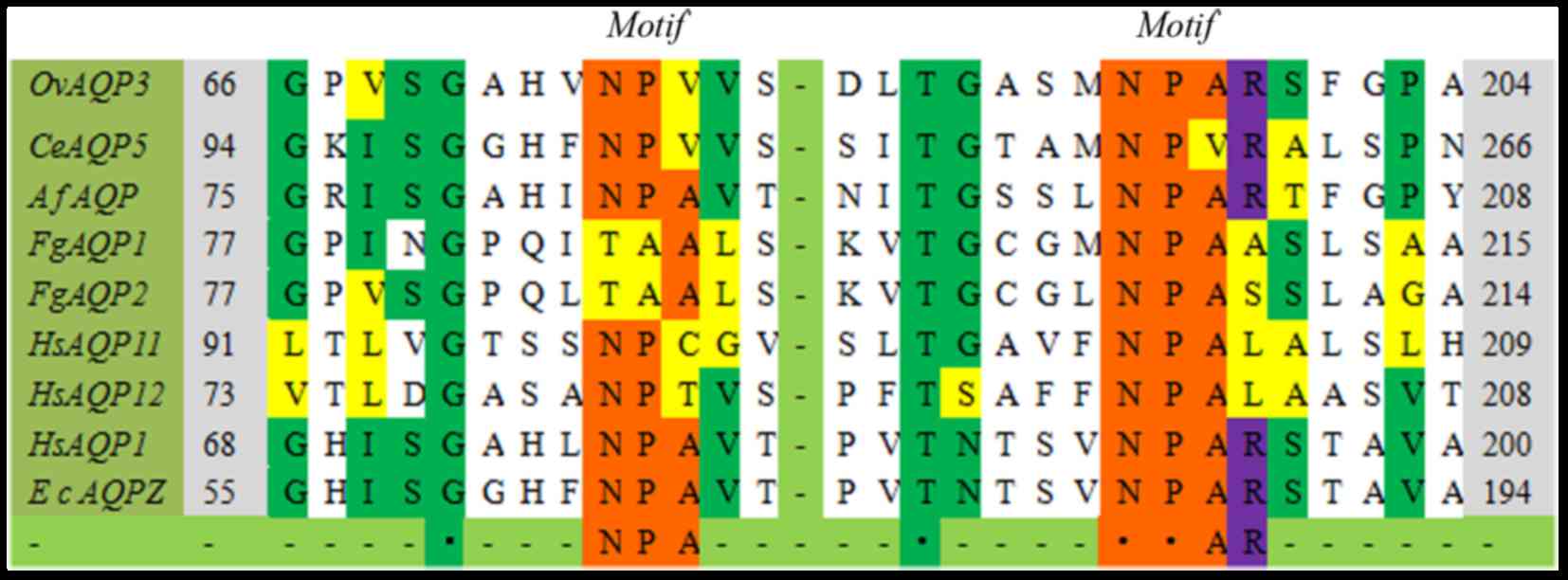
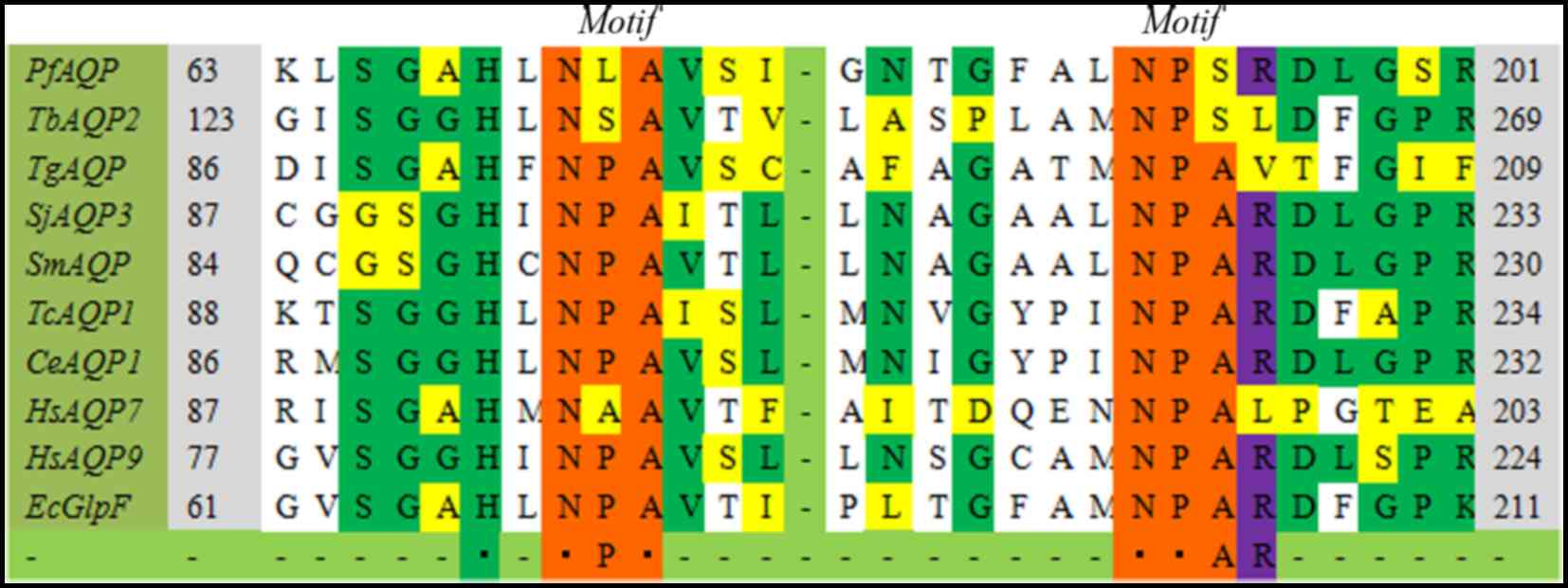
Here, the ClustalW analysis demonstrated that the
most remarkable point lies in the highly conserved arginine residue
(Figs. 2 and 3) following the second NPA box, except in
4 AQP sequences (TbAQP2, TgAQP, FgAQP1,2,
HsAQP11,12). Arginine is a polar molecule with positive
charges, and serves a vital role in the transport selectivity and
expulsion of proton (23). The
removal of the positive charge AQP1-R195V or AQP1-H180A/R195V would
result in proton leak (23). In
TgAQP, the Arg204 residue mutates into valine, a similar mutation
to the plant TIP subfamily (19).
The unusual selectivity filter replaces Arg264 with Leu264 in
TbAQP2 and displays the ability to allow the passage of
large molecules like melarsoprol and pentamidine through the
channel (24). As a result,
TbAQP2 may transport larger solutes compared with
TbAQP3, and may be responsible for it susceptibility of
melarsoprol and pentamidine (24).
In addition, the cysteine residue serves a significant role in the
ar/R restriction. Cys in AQPs is a vital residue reacting to
mercurial compounds. HsAQP1 (C189) and HsAQP9
(C213) can be inhibited by mercuric chloride (25,26).
It can be suggested that cysteines at position 204, 203 and 220 in
FgAQP1, FgAQP2 and OvAQP3,
respectively, may also be mercury-sensitive sites and could be
blocked by mercury ions.
The sequences used in the current work indicated
that the functional residues were corresponding to the Froger's
prediction method (27) at
P1–5 positions, although limited aquaporin sequences
have been used (40 aquaporin sequences in total, 14 for GlpF
cluster, 26 for AQP cluster). In addition, the authors previously
identified some more conserved residues (data not shown), but their
roles are still unclear and need to be confirmed later in
detail.
The variation of the NPA motifs in aquaporin
of parasites
NPA motifs are characteristics of MIP family
members, comprising Asn-Pro-Ala (11). The Asn residue can be considered as
the secondary proton filter pointing towards the inner channel, of
which the side chains serve as hydrogen-bond donors, forming new
hydrogen bonds with travelling water molecules (28).
As presented in the aligned sequences, NPA boxes
(Figs. 2 and 3) are highly conserved in MIP family
members, despite of a few variations. The variations were primarily
at the second or third position in the first NPA motif, except in
FgAQP1–2. The NPA motif in FgAQP1–2 comprises
TAA motif (Thr-Ala-Ala), and the similar formation is demonstrated
in the Burkholderia coenocepacia SPA at a corresponding
position (29). In fewer cases,
including PfAQP, TbAQP2, CeAQP5 and
HsAQP7, both of the two NPA motifs become distinct in a
single aquaporin sequence (30,31).
These mutations alter the bulk and polarity of amino acid residues,
which leads to the changed hydrophilic property and the
physiological roles. Replacement of Thr by Asn at the first TAA
motif of FgAQP1 made its water flux increase two-fold when
expressed in Xenopus laevis oocytes, however, this
alteration does not alter the impermeability of urea and glycerol
(18). In Trypanosoma
brucei, TbAQP2 contains two non-canonical NSA/NPS motifs that
are scarcely expressed. Perhaps the protein is an organelle
aquaporin and is expressed only under given stress conditions
(30).
The altered motifs, however, do not always alter its
transport properties (31). In
Plasmodium falciparum, the classical NPA sequences were
substituted with the uncommon NLA and NPS sequences in B and E
loops, respectively. After the divergent motifs are converted to
canonical NPA motifs, the swelling rates of the mutative protein to
water and glycerol are equal with wild type PfAQP (31). An identical variation at the first
NPA (changed into NPV) motif was identified in OvAQP3
and CsAQP1 (32). The
OvAQP3 was predicted to be a similar advanced structure
to mammalian AQP1, the specific water channel. However,
OvAQP3 can transport glycerol and urea, but not only
water molecules, which suggests that sequence-based aquaporin
classification do not completely reveal its real function in
parasites (32).
Potential drug targets in parasites:
Aquaporins
Aquaporins can regulate multiple significant
physiological roles in living organisms, which means that they are
regarded as prime candidates for pharmacological intervention in
human diseases (33). The numbers
of aquaporin-encoding genes are varied in parasites, for instance,
there are five genes in Leishmania major and one gene in
P. falciparum (31,34). AQPs are desired as novel targets
for antiparasitic drugs (35,36).
Aquaglyceroporins isolated from Leishmania
major (Lm) AQP1 and Schistosoma mansoni (Sm)AQP are
physiological water channels. They can act as conduit for
parasite-killing drugs and increase the accumulation of metalloids,
such as As(III) and Sb(III) in L. major and Potassium
Antimonyl Tartrate in S. mansoni (36,37).
A previous study demonstrated that, following silencing SmAQP using
specific siRNA, the treated Schistosomula exhibits more resistance
to PAT (36). Proteomic analysis
of the Schistosome tegument membranes presented a single AQP
homologue (38), which exhibits
31–36% sequence identity with human AQP 3,7,9 and 10. In addition,
SmAQP may also act as a channel for glycolytic end-products to
avoid the accumulation of toxins (39). The Arg residue located inside the
vestibule of the channel may also severely affect the metalloid
sensitivity (40). For instance,
alteration of Arg230 in LmAQP1 to Ala or Lys presented a negligible
low level transport of metalloid in cells, and therefore appeared
more resistant to As(III) and Sb(III) than cells expressing wild
type LmAQP1 (41). The side chain
of the Ala residue at the position of 163 in LmAQP1 may serve a
role in drug resistance because of a steric hindrance effect
(42). The Thr164 substituted by
Cys in LmAQP1 altered the mercurial sensitivity that can be blocked
by HgCl2 (42). These
studies suggested that aquaporin in parasites possess transport
property for drugs and, therefore, may have potential access to be
a viable candidate for therapeutic targets.
Conclusions and perspectives
Since the discovery of the first aquaporin, the
protein has attracted considerable interests in the development of
new pharmacological agents for the treatment of parasitological
diseases, due to their potential structure and functions. In
general, aquaporins are conserved between species during the
evolutionary process, especially at the transmembrane regions and
functional sites (e.g., NPA and ar/R). In parasitic aquaporins,
variations seem to have differential effects for different
aquaporin, but not always altering the selectivity of the
aquaporin. As the common component of the parasite-host interface,
aquaporins possess great advantages of being new chemotherapeutic
targets. However, few helminthic aquaporins have been well
studied.
Some compounds, such as mercuric chloride, can act
as aquaporin inhibitors, interdicting the ability of the protein to
transport solutes (43). Some
aquaporin modulators are promising agents for the treatment of
human disorders (33).
Furthermore, the elucidation of 3D structures and more
crystallographic information of aquaporins will increase our
understanding of drug design. However, much requires elucidation in
order to develop the potential applications of parasitic
aquaporins. The development of mammalian aquaporins would be
provided references for the studies on parasitic aquaporins. In
summary, aquaporins are an ancient protein with the ability of
transportation of water molecules and nutrients, osmoregulation,
invasion and drug resistance. More studies on parasitic aquaporins
are required.
Acknowledgements
Project support was provided by the Science Fund for
Creative Research Groups of Gansu Province (grant no.
1210RJIA006).
References
|
1
|
Mehlhorn H: Encyclopedia of parasitology
I. 3rd. Springer; 2008, View Article : Google Scholar
|
|
2
|
Ramasamy R: Zoonotic malaria-global
overview and research and policy needs. Front Public Health.
2:1232014. View Article : Google Scholar :
|
|
3
|
Halonen SK and Weiss LM: Toxoplasmosis.
Handb Clin Neurol. 114:125–145. 2013. View Article : Google Scholar :
|
|
4
|
Pace D: Leishmaniasis. J Infect. 69 Suppl
1:S10–S18. 2014. View Article : Google Scholar
|
|
5
|
Robinson MW and Dalton JP: Zoonotic
helminth infections with particular emphasis on fasciolosis and
other trematodiases. Philos Trans R Soc Lond B Biol Sci.
364:2763–2776. 2009. View Article : Google Scholar :
|
|
6
|
Kirk K: Channels and transporters as drug
targets in the Plasmodium-infected erythrocyte. Acta Trop.
89:285–298. 2004. View Article : Google Scholar
|
|
7
|
Sibley LD: How apicomplexan parasites move
in and out of cells. Curr Opin Biotechnol. 21:592–598. 2010.
View Article : Google Scholar :
|
|
8
|
Gomes D, Agasse A, Thiébaud P, Delrot S,
Gerós H and Chaumont F: Aquaporins are multifunctional water and
solute transporters highly divergent in living organisms. Biochim
Biophys Acta. 1788:1213–1228. 2009. View Article : Google Scholar
|
|
9
|
Shiels A: Focus on molecules: Major
intrinsic protein. Exp Eye Res. 101:107–108. 2012. View Article : Google Scholar
|
|
10
|
Castro-Borges W, Simpson DM, Dowle A,
Curwen RS, Thomas-Oates J, Beynon RJ and Wilson RA: Abundance of
tegument surface proteins in the human blood fluke Schistosoma
mansoni determined by QconCAT proteomics. J Proteomics.
74:1519–1533. 2011. View Article : Google Scholar
|
|
11
|
Fujiyoshi Y, Mitsuoka K, de Groot BL,
Philippsen A, Grubmüller H, Agre P and Engel A: Structure and
function of water channels. Curr Opin Struct Biol. 12:509–515.
2002. View Article : Google Scholar
|
|
12
|
Sui H, Han BG, Lee JK, Walian P and Jap
BK: Structural basis of water-specific transport through the AQP1
water channel. Nature. 414:872–878. 2001. View Article : Google Scholar
|
|
13
|
Campbell EM, Ball A, Hoppler S and Bowman
AS: Invertebrate aquaporin: A review. J Comp Physiol B.
178:935–955. 2008. View Article : Google Scholar
|
|
14
|
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R,
Agre P and Rosen BP: Arsenite transport by mammalian
aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA. 99:pp.
6053–6058. 2002; View Article : Google Scholar :
|
|
15
|
Yakata K, Hiroaki Y, Ishibashi K, Sohara
E, Sasaki S, Mitsuoka K and Fujiyoshi Y: Aquaporin-11 containing a
divergent NPA motif has normal water channel activity. Biochim
Biophys Acta. 1768:688–693. 2006. View Article : Google Scholar
|
|
16
|
Itoh T, Rai T, Kuwahara M, Ko SB, Uchida
S, Sasaki S and Ishibashi K: Identification of a novel aquaporin,
AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res
Commun. 330:832–838. 2005. View Article : Google Scholar
|
|
17
|
Yue C, Cao H, Wang L, Zhou Y, Hao X, Zeng
J, Wang X and Yang Y: Molecular cloning and expression analysis of
tea plant aquaporin (AQP) gene family. Plant Physiol Biochem.
83:65–76. 2014. View Article : Google Scholar
|
|
18
|
Geadkaew A, von Bülow J, Beitz E, Grams
SV, Viyanant V and Grams R: Functional analysis of novel aquaporins
from Fasciola gigantica. Mol Biochem Parasitol. 175:144–153. 2011.
View Article : Google Scholar
|
|
19
|
Pavlovic-Djuranovic S, Schultz JE and
Beitz E: A single aquaporin gene encodes a water/glycerol/urea
facilitator in Toxoplasma gondii with similarity to plant tonoplast
intrinsic proteins. FEBS Lett. 555:500–504. 2003. View Article : Google Scholar
|
|
20
|
Murata K, Mitsuoka K, Hirai T, Walz T,
Agre P, Heymann JB, Engel A and Fujiyoshi Y: Structural
determinants of water permeation through aquaporin-1. Nature.
407:599–605. 2000. View
Article : Google Scholar
|
|
21
|
Herrera M and Garvin JL: Aquaporins as gas
channels. Pflugers Arch. 462:623–630. 2011. View Article : Google Scholar
|
|
22
|
Stroud RM, Savage D, Miercke LJ, Lee JK,
Khademi S and Harries W: Selectivity and conductance among the
glycerol and water conducting aquaporin family of channels. FEBS
Lett. 555:79–84. 2003. View Article : Google Scholar
|
|
23
|
Beitz E, Wu B, Holm LM, Schultz JE and
Zeuthen T: Point mutations in the aromatic/arginine region in
aquaporin 1 allow passage of urea, glycerol, ammonia, and protons.
Proc Natl Acad Sci USA. 103:pp. 269–274. 2006; View Article : Google Scholar :
|
|
24
|
Baker N, Glover L, Munday JC, Andrés D
Aguinaga, Barrett MP, de Koning HP and Horn D: Aquaglyceroporin 2
controls susceptibility to melarsoprol and pentamidine in African
trypanosomes. Proc Natl Acad Sci USA. 109:pp. 10996–1001. 2012;
View Article : Google Scholar :
|
|
25
|
Preston GM, Jung JS, Guggino WB and Agre
P: The mercury-sensitive residue at cysteine 189 in the CHIP28
water channel. J Biol Chem. 268:17–20. 1993.
|
|
26
|
Ishibashi K, Kuwahara M, Gu Y, Tanaka Y,
Marumo F and Sasaki S: Cloning and functional expression of a new
aquaporin (AQP9) abundantly expressed in the peripheral leukocytes
permeable to water and urea, but not to glycerol. Biochem Biophys
Res Commun. 244:268–274. 1998. View Article : Google Scholar
|
|
27
|
Froger A, Tallur B, Thomas D and
Delamarche C: Prediction of functional residues in water channels
and related proteins. Protein Sci. 7:1458–1468. 1998. View Article : Google Scholar :
|
|
28
|
Chakrabarti N, Tajkhorshid E, Roux B and
Pomès R: Molecular basis of proton blockage in aquaporins.
Structure. 12:65–74. 2004. View Article : Google Scholar
|
|
29
|
Wree D, Wu B, Zeuthen T and Beitz E:
Requirement for asparagine in the aquaporin NPA sequence signature
motifs for cation exclusion. FEBS J. 278:740–748. 2011. View Article : Google Scholar
|
|
30
|
Uzcategui NL, Szallies A,
Pavlovic-Djuranovic S, Palmada M, Figarella K, Boehmer C, Lang F,
Beitz E and Duszenko M: Cloning, heterologous expression, and
characterization of three aquaglyceroporins from Trypanosoma
brucei. J Biol Chem. 279:42669–42676. 2004. View Article : Google Scholar
|
|
31
|
Hansen M, Kun JF, Schultz JE and Beitz E:
A Single, bi-functional aquaglyceroporin in blood-stage Plasmodium
falciparum malaria parasites. J Biol Chem. 277:4874–4882. 2002.
View Article : Google Scholar
|
|
32
|
Geadkaew A, von Bülow J, Beitz E, Tesana
S, Grams S Vichasri and Grams R: Bi-functionality of Opisthorchis
viverrini aquaporins. Biochimie. 108:149–159. 2015. View Article : Google Scholar
|
|
33
|
Huber VJ, Tsujita M and Nakada T:
Aquaporins in drug discovery and pharmacotherapy. Mol Aspects Med.
33:691–703. 2012. View Article : Google Scholar
|
|
34
|
Beitz E: Aquaporin Water and solute
channels from malaria parasites and other pathogenic protozoa.
ChemMedChem. 1:587–592. 2006. View Article : Google Scholar
|
|
35
|
Munday JC, Eze AA, Baker N, Glover L,
Clucas C, Andrés D Aguinaga, Natto MJ, Teka IA, McDonald J, Lee RS,
et al: Trypanosoma brucei aquaglyceroporin 2 is a high-affinity
transporter for pentamidine and melaminophenyl arsenic drugs and
the main genetic determinant of resistance to these drugs. J
Antimicrob Chemother. 69:651–663. 2014. View Article : Google Scholar
|
|
36
|
Faghiri Z and Skelly PJ: The role of
tegumental aquaporin from the human parasitic worm, Schistosoma
mansoni, in osmoregulation and drug uptake. FASEB J. 23:2780–2789.
2009. View Article : Google Scholar :
|
|
37
|
Gourbal B, Sonuc N, Bhattacharjee H,
Legare D, Sundar S, Ouellette M, Rosen BP and Mukhopadhyay R: Drug
uptake and modulation of drug resistance in Leishmania by an
aquaglyceroporin. J Biol Chem. 279:31010–31017. 2004. View Article : Google Scholar
|
|
38
|
Braschi S, Curwen RS, Ashton PD,
Verjovski-Almeida S and Wilson A: The tegument surface membranes of
the human blood parasite Schistoma mansoni: A proteomic analysis
after differential extraction. Proteomics. 6:1471–1482. 2006.
View Article : Google Scholar
|
|
39
|
Faghiri Z, Camargo SM, Huggel K, Forster
IC, Ndegwa D, Verrey F and Skelly PJ: The tegument of the human
parasitic worm Schistosoma mansoni as an excretory organ: The
surface aqp SmAQP is a lactate transporter. Plos One. 5:e104512010.
View Article : Google Scholar :
|
|
40
|
Mitani-Ueno N, Yamaji N, Zhao FJ and Ma
JF: The aromatic/arginine selectivity filter of NIP aquaporins
plays a critical role in substrate selectivity for silicon, boron,
and arsenic. J Exper Bot. 62:4391–4398. 2011. View Article : Google Scholar
|
|
41
|
Figarella K, Uzcategui NL, Zhou Y,
LeFurgey A, Ouellette M, Bhattacharjee H and Mukhopadhyay R:
Biochemical characterization of Leishmania major aquaglyceroporin
LmAQP1: Possible role in volume regulation and osmotaxis. Mol
Microbiol. 65:1006–1017. 2007. View Article : Google Scholar
|
|
42
|
Mukhopadhyay R, Mandal G, Atluri VS,
Figarella K, Uzcategui NL, Zhou Y, Beitz E, Ajees AA and
Bhattacharjee H: The role of alanine 163 in solute permeability of
Leishmania major aquaglyceroporin LmAQP1. Mol Biochem Parasitol.
175:83–90. 2011. View Article : Google Scholar
|
|
43
|
Kuwahara M, Asai T, Sato K, Shinbo I,
Terada Y, Marumo F and Sasaki S: Functional characterization of a
water channel of the nematode Caenorhabditis elegans. Biochim
Biophys Acta. 1517:107–112. 2000. View Article : Google Scholar
|