Introduction
As a major structural component of the vessel wall,
vascular smooth muscle cells (VSMCs) provide vasoactivity by
contracting and relaxing, regulating extracellular matrix (ECM)
turnover and providing mechanical stability. Physiologically, VSMCs
display a characteristic contractile (differentiated) phenotype
exhibited as a minimal rate of proliferation, and balance the
production and degradation of ECM components in a constant
equilibrium. However, VSMCs exhibit remarkable plasticity and are
able to switch from a contractile phenotype to a less natural
synthetic (proliferative) phenotype in response to various stimuli.
During this process, termed phenotypic switching or phenotypic
modulation, VSMCs lose their contractile apparatus (myofilaments)
and exhibit vigorous proliferation and increased synthesis of ECM
components (1–4).
Previous studies have reported that hypoxia can
cause phenotypic switching of VSMCs by multiple mediators via
different signaling pathways (5,6).
Although the molecular mechanisms underlying hypoxia-induced
phenotypic switching of VSMCs remain unclear, various microRNAs
(miRNAs) have been identified as crucial post-transcriptional
modulators that regulate the phenotype of VSMCs. For example, miRNA
(miR)-142-3p is understood to be a key regulator of the
transforming growth factor β (TGF-β)-mediated contractile phenotype
of VSMCs, targeting Dedicator of cytokinesis 6 to inhibit cell
migration (7); miR-96 combined
with Tribbles-like protein 3 participates in the regulation of the
VSMC contractile phenotype via the bone morphogenic protein 4
signaling pathway (8).
Additionally, various studies have reported that specific miRNAs,
including miR-21 and miR-130a, regulate the behavior of hypoxic
VSMCs according to their phenotype (9,10).
However, whether and how miR-26b participates in regulating
hypoxia-induced phenotypic switching of VSMCs remains unknown,
although miR-26b has been confirmed as an important regulator of
different cellular processes (11–13).
In the present study, the expression level of
miRNA-26b-5p in VSMCs exposed to low oxygen was detected, and the
correlation of this change with the mRNA levels of the specific
VSMC biomarkers, desmin, H-caldesmon and smoothelin, was analyzed.
A miR-26b-5p agomir was then used, and its effect on cell
morphology, collagen Iα expression, and the protein expression
levels of Smad2, 3, and 4, and phosphorylated (p)-Smad2 and 3 in
hypoxic VSMCs were determined. Additionally, expression changes of
cytoplasmic Smad4 in normoxic and hypoxic VSMCs transfected with
the miR-26b-5p agomir were examined, and the potential binding
sites of miR-26b-5p in the Smad4 sequence were analyzed. The data
reported in the current study suggest that miR-26b-5p regulates
hypoxia-induced phenotypic switching of VSMCs via the TGF-β/Smad4
signaling pathway.
Materials and methods
Animals
In the current study, a total of 45 male mice (age,
8–12 weeks) were purchased from the Animal Centre of the Second
Military Medical University (Shanghai, China). All animals received
care in compliance with the Guide for the Care and Use of
Laboratory Animals, prepared by the Institute of Laboratory Animal
Resources, National Research Council (Washington, DC, USA). Animals
were housed at room temperature, with free access to food and
water, and were maintained in a 12 h light/dark cycle. Prior to the
experiments, mice were acclimated to laboratory conditions for at
least 7 days. The current study was approved by the Medical Ethics
Committee of Gongli Hospital (Shanghai, China).
Cell culture
Mice at 8–12 weeks of age were sacrificed by
CO2 overexposure. Using fine-tipped forceps and spring
scissors, the sheath around the aorta was opened. The connective
fascia and adventitia were then carefully removed, and the aorta
(from the aortic arch to the iliac bifurication) was removed and
placed in a 6-cm culture plate containing Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and Fungizone (cat. no. SV30079.01; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA). The aorta was
subsequently opened with spring scissors and the blood clots were
removed. The aortic intima was then scraped with a scissor blade,
before it was cut into sections (4-mm in length). Each section was
subsequently placed under a plastic cell culture coverslip and
maintained in DMEM supplemented with 20% fetal bovine serum
(HyClone; GE Healthcare Life Sciences) at 37°C, with 5%
CO2. The coverslip was removed after tissue sections had
adhered, and the culture medium was refreshed every 3–4 days. Once
cells had reached ~80% confluence, they were dissociated by adding
0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.). Following
inactivation of trypsin, cells were subcultured at ratio of 1:3.
Cells at passages 3–5 were used in downstream experiments. Each
experiment was repeated at least 3 times with different cell
preparations.
Cells were cultured to 90% confluence in 6
cm2 culture dishes and then subcultured at a ratio of
1:2. VSMCs (5–10×106 cells) were then subjected to
normoxic or hypoxic conditions. For normoxic conditions, mouse
VSMCs (mVSMCs) were incubated at 37°C with 5% CO2
humidified atmosphere. For hypoxia, mVSMCs were cultured in
normoxic conditions for ≥24 h until adherent, and then cultured at
37°C for 3 h in a humidified hypoxic chamber supplemented with 1%
O2, 94% N2 and 5% CO2.
Transient transfection of miR-26b-5p
agomir
The micrON™ miR-26b-5p agomir and micrON™miRNA
agomir control (scramble) were purchased from Guangzhou Ribobio
Co., Ltd. (Guangzhou, China) and used to treat mVSMCs using
riboFECT CP reagent (RiboBio Co., Ltd.) in accordance with the
manufacturer's instructions.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis of mRNA levels
According to the manufacturer's instructions, the
total RNA was extracted using TRIzol Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Following quantification of RNA samples
using a NanoDrop spectrophotometer (Thermo Fisher Scientific. Inc.,
Wilmington, DE, USA), 1 µg RNA was used to generate cDNA by RT
reaction using PrimeScript Reverse Transcriptase kit (Takara Bio.
Inc., Otsu, Japan) according to the manufacturer's instructions.
The PCR thermal cycling parameters consisted of 1 cycle for 5 min
at 95°C, followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec,
and 72°C for 30 sec, and a final extension step at 72°C for 5 min.
A dissociation curve was obtained for each PCR product. qPCR was
performed on an ABI PRISM 7000 Sequence Detection System using SYBR
Premix Ex Taq™ II kit (Takara Bio. Inc.) with specific primers for
hypoxia inducible factor (HIF)-1α, desmin, H-caldesmon, smoothelin
and β-actin. Each sample was analyzed in triplicate and target gene
expression levels were normalized to β-actin mRNA levels. The fold
change in target gene expression was calculated using the
2−∆∆Cq method (14).
The primer sequences were as follows: HIF-1α forward,
5′-GATGAGGCTTACCATCAGCT-3′, and reverse,
5′-ATGTCACCATCATCTGTGAG-3′; desmin forward,
5′-GTTTCAGACTTGACTCAGGCAG-3′, and reverse,
5′-TCTCGCAGGTGTAGGACTGG-3′; H-caldesmon forward,
5′-ATGGTAGAGGAGAAAACACCAGA-3′, and reverse,
5′-CCATCCCCTTCTATTTTGGACTC-3′; smoothelin forward,
5′-GAGCGGCAAGACAACAAGGA-3′, and reverse, 5′-CAGTCTCCCTGCCAATCGT-3′;
β-actin forward 5′-CAACCGTGAAAAGATGACCC-3′, and reverse,
5′-GTCTCCGGAGTCCATCACAA-3′.
RNA extraction and RT-qPCR analysis of
miRNA levels
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed at 42°C for 60 min followed by 70°C for 10 min. PCR was
performed using a Bulge-Loop™ miRNA RT-qPCR kit (catalogue no.
R11067.1; Ribobio, Co., Ltd.). Thermal cycling conditions consisted
of 1 cycle for 10 min at 95°C followed by 40 cycles at 95°C for 2
sec, 60°C for 20 sec, and 70°C for 10 sec. A dissociation curve was
obtained for each PCR product. The primers used for the detection
of miR-26b-5p and control U6 small nuclear RNA were designed and
produced by Guangzhou Ribobio Co., Ltd. Each sample was analyzed in
triplicate and target gene expression levels were normalized to U6
mRNA levels. The fold change in target gene expression was
calculated using the 2−ΔΔCq method.
Western blotting
Cells were harvested and lysed with
radioimmunoprecipitation assay lysis buffer to extract the total
protein. Protein concentration was measured by the
micro-bicinchoninic acid assay, and 100 µg protein per lane was
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on a 12% gel, then transferred onto polyvinylidene
difluoride membranes (Beyotime Institute of Biotechnology, Haimen,
China). Non-specific binding sites were blocked by immersing the
membrane in tris-buffered saline (TBS) solution containing 5%
non-fat milk for 1 h at room temperature and agitating at 50 rpm.
The membranes were then washed twice for 2 min in TBS solution.
Immune complexes were formed by incubating the membranes overnight
at 4°C with primary antibodies against collagen Iα (polyclonal
rabbit anti-mouse; dilution, 1:1,000; cat. no. ab34710; Abcam,
Cambridge, UK), as well as β-actin (monoclonal rabbit anti-mouse;
dilution, 1:1,000; cat. no. 8457), Smad2 (monoclonal rabbit
anti-mouse; dilution, 1:100; cat. no. 5339) and p-Smad2 (polyclonal
rabbit anti-mouse; dilution, 1:1,000; cat. no. 3101), Smad3 and
p-Smad3 (monoclonal rabbit anti-mouse; dilution, 1:1,000; cat nos.
9523 and 9520, respectively), Smad4 (monoclonal rabbit anti-mouse;
dilution, 1:1,000; cat. no. 38,454; all from Cell Signaling
Technology, Inc., Danvers, MA, USA). Blots were then washed and
incubated for 1 h with horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (1:2,000; cat. no. SA00001-2;
ProteinTech, Rosemont, IL, USA). Subsequently, immunoreactive
protein bands were analyzed using the Pierce ECL Western Blotting
Substrate (cat. no. 32106; Pierce Biotechnology, Inc., Rockford,
IL, USA), and quantified using ImageJ software (version 1.44p;
National Institutes of Health, Bethesda, MD, USA).
Immunocytofluorescence assay
The culture medium was removed and the differently
treated cells were fixed in 4% paraformaldehyde for 15 min at room
temperature before they were washed three times in TBS.
Subsequently, cells were blocked with 5% bovine serum albumin
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) diluted in
TBS for 1 h at room temperature on a shaker. The blocking solution
was then removed, and a primary antibody against Smad4 (polyclonal
rabbit anti-mouse; dilution, 1:1,00; cat. no. ab208804; Abcam)
diluted in TBS was added to appropriate wells and incubated at 4°C
overnight. The cells were washed with TBS and then incubated with
an Alexa Fluor®-labeled polyclonal goat anti-rabbit
secondary antibody (2 µg/ml; cat. no. A-11008; Invitrogen; Thermo
Fisher Scientific, Inc.) for 3 h at room temperature. Slides were
subsequently washed, air dried and mounted on coverslips with DAPI
(1 mg/ml; Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
D1306). The samples were mounted on glass slides with
ProLong® Gold Antifade Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and visualized using an inverted
fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany). A
rhodamine phalloidin (Cytoskeleton Inc., Denver, CO, USA) probe was
used to label F-actin. The cell area was calculated as: Total
area/nuclear number.
miRNA target analysis
A potential miRNA-26b-5p target sequence in the
Smad4 3′-untranslated region (3′-UTR) was analyzed using
TargetScanMouse 7.1 software (www.targetscan.org/mmu_71). In brief, the mouse
‘Smad4’ gene and miRNA ‘miR-26b-5p’ sequence names were entered and
submitted. The predicted Smad4 3′UTR-miR-26b-5p target binding
regions were then shown.
Statistical analysis
The results presented are the average of at least
three experiments and reported as the mean ± standard deviation.
Statistical analyses were performed with one-way analysis of
variance or Student's t-test using SPSS 11.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
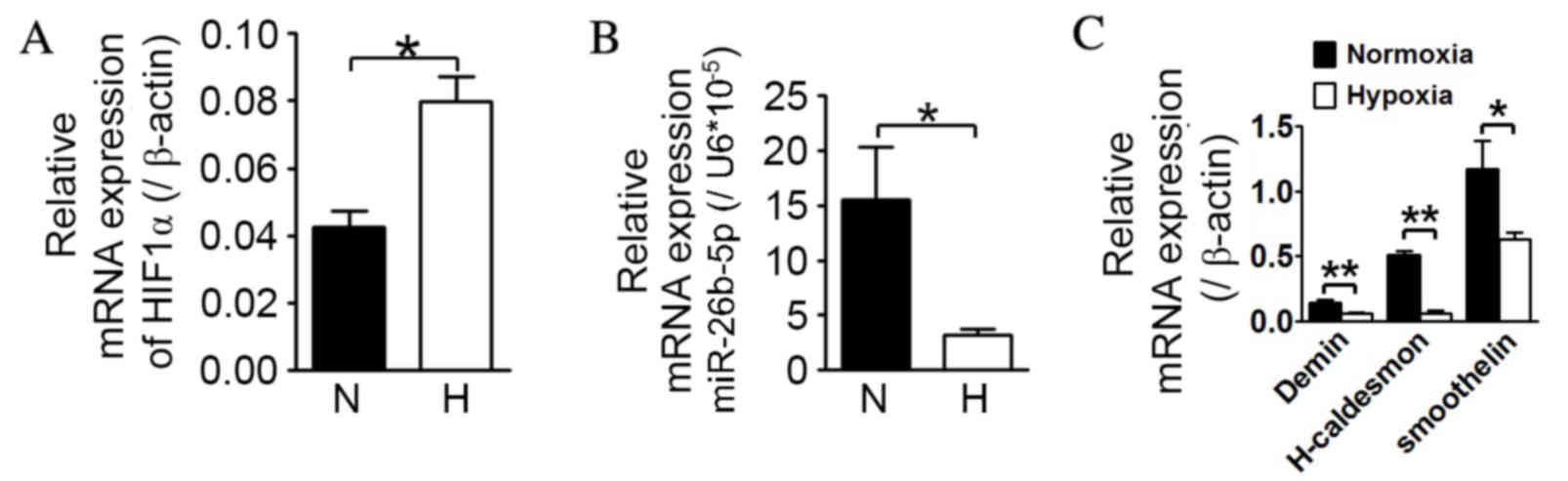
Hypoxia causes significant
downregulation in miR-26b-5p expression and decreases the mRNA
levels of contractile mVSMC biomarkers
As demonstrated in Fig.
1, compared with normoxia, mVSMCs exposed to low oxygen
displayed a statistically significant upregulation in HIF-1α mRNA
levels (P=0.0018; Fig. 1A), and a
significant downregulation in miR-26b-5p expression (P=0.0275;
Fig. 1B). Additionally,
significant downregulation of desmin, H-caldesmon and smoothelin
mRNA expression levels was detectable in response to hypoxia in
mVSMCs compared with the levels in normoxic conditions (Desmin,
P=0.0010; H-caldesmon, P=0.0052; smoothelin, P=0.0456; Fig. 1C).
miR-26b-5p agomir reverses changes in
cell area and collagen Iα expression in hypoxic mVSMCs
To further investigate the importance of miR-26b-5p
in hypoxia-induced phenotypic switching of mVSMCs, the miR-26b-5p
agomir and scramble were transfected into mVSMCs cultured in
normoxic and hypoxic conditions. The cell area analysis (Fig. 2A and B) demonstrated that mVSMCs
transfected with scramble exhibited an area increase in hypoxic
mVSMCs compared with those in normoxic conditions (P=0.0075).
However, hypoxic mVSMCs transfected with miR-26b-5p agomir were
smaller compared with hypoxic mVSMCs transfected with scramble
(P=0.0051). Western blot analysis demonstrated a similar effect on
collagen Iα expression (Fig. 2C).
Hypoxia was associated with an upregulation in collagen Iα protein
expression when compared with normoxia (P=0.0006). However,
expression of collagen Iα in hypoxic mVSMCs transfected with
miR-26b-5p agomir was significantly downregulated compared with
that of hypoxic mVSMCs transfected with agomir (P=0.00009; Fig 2D).
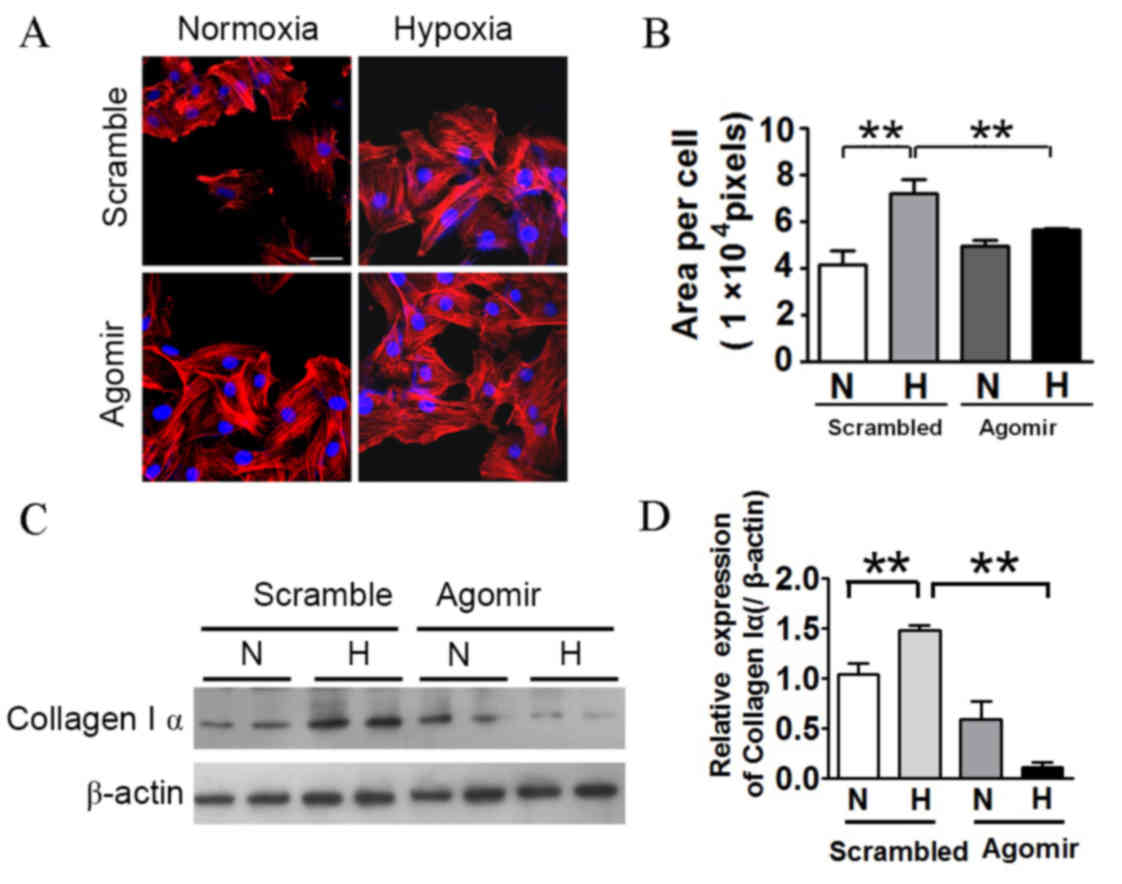 | Figure 2.The miR26b-5p agomir reverses changes
in cell area and collagen Iα expression in hypoxic mVSMCs. (A) Cell
area of mVSMCs transfected with scramble or miR-26b-5p agomir in
normoxic and hypoxic conditions. Red fluorescence, F-actin; blue
fluorescence, cell nucleus. Scale=50 µm. (B) Quantification of cell
area. Cell area of hypoxic mVSMCs transfected with scramble was
greater in hypoxic cells compared with normoxic mVSMCs transfected
with scramble. However, miR-26b-5p agomir caused a decrease in cell
area in hypoxic mVSMCs compared with scramble, and was comparable
with those from normoxic mVSMCs transfected with scramble or
miR-26b-5p agomir. (C and D) Hypoxia led to upregulation of
collagen Iα expression, which was suppressed by miR-26b-5p agomir.
Values presented as mean ± standard deviation. **P<0.01,
comparison indicated by brackets. miR, microRNA; mVSMCs, mouse
vascular smooth muscle cells; N, normoxia; H, hypoxia. |
miR-26b-5p agomir suppresses Smad4
expression in hypoxic mVSMCs
Western blotting demonstrated that the expression
levels of Smad2 and Smad3 proteins in hypoxic and normoxic mVSMCs
transfected with agomir or scramble were comparable (Fig. 3A). However, hypoxia resulted in
upregulation of p-Smad2 and p-Smad3 proteins in
scramble-transfected mVSMCs, and the effect was not altered by
miR-26b-5p agomir. Furthermore, the expression level of Smad4 in
hypoxic mVSMCs transfected with scramble was increased compared
with normoxic mVSMCs transfected with scramble. However,
transfection with miR-26b-5p agomir resulted in reduced expression
of Smad4 in hypoxic mVSMCs compared with normoxic mVSMCs.
Furthermore, miRNA target analysis identified the miR-26b-5p target
sequence in the Smad4 3′UTR as UACUUGA between 1,030–1,040 bp
(Fig. 3B). Additionally,
immunofluorescent staining demonstrated that immunoreactivity of
Smad4 in the cytoplasm of normoxic mVSMCs transfected with scramble
was more extensive and strongly distributed compared with hypoxic
mVSMCs transfected with agomir (Fig.
3C).
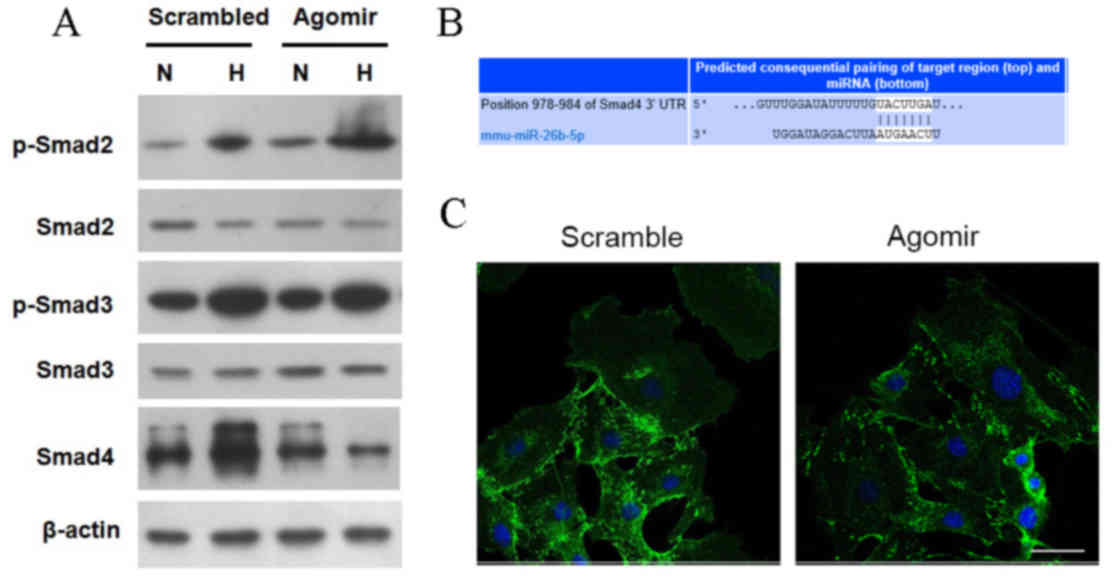 | Figure 3.miR-26b-5p-regulated hypoxia induces
phenotypic switching of mVSMCs by targeting Smad4. (A) Protein
expression levels of Smad2, Smad3, p-Smad2, and p-Smad3 and Smad4
were detected in normoxic and hypoxic mVSMCs transfected with
scramble or miR-26b-5p agomir. (B) Binding site of miR-26b-5p on
Smad4 3′UTR was identified as UACUUGA at position 978–984. (C)
Transfection with miR-26b-5p agomir resulted in weaker
immunoreactivity of Smad4 in hypoxic mVSMCs. Green fluorescence,
Smad4; blue fluorescence, cell nucleus. Scale bar=50 µm. miR,
microRNA; mVSMCs, mouse vascular smooth muscle cells; UTR,
untranslated region; N, normoxia; H, hypoxia. |
Discussion
Hypoxia is a common characteristic of various
pathological diseases, including hypoxic pulmonary hypertension. It
is accepted that cells respond to reduced oxygen availability
through changes in gene expression that are mediated by HIFs, which
are composed of an oxygen-regulated α subunit (HIF-1α, HIF-2α or
HIF-3α) and a constitutively expressed β subunit (15,16).
HIF-1α is distributed extensively in different cell types,
therefore increased expression and activity of HIF-1α is considered
to be an indicator of hypoxia. In the present study, mVSMCs exposed
to low oxygen demonstrated a significant upregulation in HIF-1α
mRNA levels compared with mVSMCs exposed to normoxia, suggesting
that the in vitro hypoxic model used was reliable. In
accordance with previous reports, the current study demonstrated
that hypoxia stimuli resulted in downregulation in the mRNA levels
of desmin, H-caldesmon and smoothelin, which are considered to be
specific biomarkers of the contractile phenotype of VSMCs (17–19),
indicating that hypoxic mVSMCs had lost their differentiated
phenotype. Taken together, these findings suggested that miR-26b-5p
participates in phenotypic switching of hypoxic mVSMCs.
In order to confirm the aforementioned hypothesis,
miR-26b-5p agomir was used in further experiments. The current
study demonstrated that hypoxic mVSMCs transfected with scramble
were larger than those cultured in normoxic conditions, and
exhibited increased expression of collagen Iα protein, indicating
increased synthesis of ECM components, which is characteristic of
phenotypic switching of VSMCs from a contractile to synthetic
phenotype. However, miR-26b-5p agomir reversed the changes in cell
area and collagen Iα expression in hypoxic mVSMCs, resulting in
cell size and collagen Iα expression comparable with that in
normoxic mVSMCs transfected with agomir and normoxic mVSMCs
transfected with scramble. The findings of the present study
suggested that miR-26b-5p participates in the phenotypic switching
of VSMCs caused by hypoxia.
Smad proteins are intracellular proteins that
transduce extracellular TGF-β signals to the nucleus where they
activate downstream gene transcription to control cellular
functions, including in VSMCs (20,21).
In the present study, the expression of Smad2, 3 and 4 in mVSMCs
transfected with and without miR-26b-5p agomir were detected to
validate whether TGF-β/Smad signaling participates with miR-26b-5p
to regulate phenotypic switching of hypoxic mVSMCs, and which Smad
isoform mediates this effect. Treatment with the miR-26b-5p agomir
did not affect the expression levels of Smad2 and Smad3, or the
hypoxia-induced upregulation of p-Smad2 and p-Smad3. These
preliminary results suggest that the role of miR-26b-5p in hypoxic
mVSMCs phenotype switching may not be associated with Smad2 and
Smad3. These proteins are involved in TGF-β signaling transduction
(22), and have been previously
reported to be correlated with biological functions of mVSMCs
(23–25) Taken together, the expression
analysis of Smad2, Smad3, p-Smad2 and p-Smad3 in the current study
suggests that Smad2 and Smad3 are not targets of miR-26b-5p during
its regulation of phenotypic switching of hypoxic mVSMCs. However,
the expression of Smad4, a common Smad family member, was altered
in hypoxic mVSMCs when transfected with miR-26b-5p agomir.
miR-26b-5p agomir caused reduced expression of Smad4 in hypoxic
mVSMCs compared with normoxic mVSMCs transfected with scramble,
indicating that Smad4 may be a target of miR-26b-5p. The findings
of the present study suggested that regulation of miR-26b-5p in
hypoxia-induced phenotypic switching of mVSMCs may be mediated by
Smad4. The potential binding site of miR-26b-5p in the Smad4 3′UTR
was identified as UACUUGA at position 978–984.
In conclusion, miR-26b-5p participates in the
regulation of hypoxia-induced phenotypic switching of VSMCs via the
TGF-β/Smad signaling pathway. Specifically, miR-26b-5p targets
Smad4, not Smad2 nor Smad3, during the regulation process. The
results of the current study were in accordance with previous
studies demonstrating that the TGF-β/Smad4 signaling pathway
contributes to VSMCs differentiation and function (26). Thus, miR-26b-5p may be a potential
therapeutic target in diseases associated with hypoxia-induced
phenotypic switching of VSMCs.
References
|
1
|
Alexander MR and Owens GK: Epigenetic
control of smooth muscle cell differentiation and phenotypic
switching in vascular development and disease. Annu Rev Physiol.
74:13–40. 2012. View Article : Google Scholar
|
|
2
|
Chamley-Campbell J, Campbell GR and Ross
R: The smooth muscle cell in culture. Physiol Rev. 59:1–61.
1979.
|
|
3
|
Thyberg J, Hedin U, Sjölund M, Palmberg L
and Bottger BA: Regulation of differentiated properties and
proliferation of arterial smooth muscle cells. Arteriosclerosis.
10:966–90. 1990. View Article : Google Scholar
|
|
4
|
Nikkari ST, Rantala I, Pystynen P and
Nikkari T: Characterization of the phenotype of smooth muscle cells
in human fetal aorta on the basis of ultrastructure,
immunofluorescence, and the composition of cytoskeletal and
cytocontractile proteins. Atherosclerosis. 74:33–40. 1988.
View Article : Google Scholar
|
|
5
|
Yin H, Li Q, Qian G, Wang Y, Li Y, Wu G
and Wang G: Rab1 GTPase regulates phenotypic modulation of
pulmonary artery smooth muscle cells by mediating the transport of
angiotensin II type 1 receptor under hypoxia. Int J Biochem Cell
Biol. 43:401–408. 2011. View Article : Google Scholar
|
|
6
|
Jie W, Guo J, Shen Z, Wang X, Zheng S,
Wang G and Ao Q: Contribution of myocardin in the hypoxia-induced
phenotypic switching of rat pulmonary arterial smooth muscle cells.
Exp Mol Pathol. 89:301–306. 2010. View Article : Google Scholar
|
|
7
|
Kim K, Yang DK, Kim S and Kang H:
miR-142-3p is a regulator of the TGFβ-mediated vascular smooth
muscle cell phenotype. J Cell Biochem. 116:2325–2333. 2015.
View Article : Google Scholar
|
|
8
|
Kim S, Hata A and Kang H: Down-regulation
of miR-96 by bone morphogenetic protein signaling is critical for
vascular smooth muscle cell phenotype modulation. J Cell Biochem.
115:889–895. 2014. View Article : Google Scholar :
|
|
9
|
Sarkar J, Gou D, Turaka P, Viktorova E,
Ramchandran R and Raj JU: MicroRNA-21 plays a role in
hypoxia-mediated pulmonary artery smooth muscle cell proliferation
and migration. Am J Physiol Lung Cell Mol Physiol. 299:L861–L871.
2010. View Article : Google Scholar :
|
|
10
|
Brock M, Haider TJ, Vogel J, Gassmann M,
Speich R, Trenkmann M, Ulrich S, Kohler M and Huber LC: The
hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell
proliferation by directly targeting CDKN1A. Int J Biochem Cell
Biol. 61:129–37. 2015. View Article : Google Scholar
|
|
11
|
Yuan B, Yu WY, Dai LS, Gao Y, Ding Y, Yu
XF, Chen J and Zhang JB: Expression of microRNA-26b and
identification of its target gene EphA2 in pituitary tissues in
Yanbian cattle. Mol Med Rep. 12:5753–5761. 2015.
|
|
12
|
Xu G, Ji C, Song G, Shi C, Shen Y, Chen L,
Yang L, Zhao Y and Guo X: Obesity-associated microRNA-26b regulates
the proliferation of human preadipocytes via arrest of the G1/S
transition. Mol Med Rep. 12:3648–3654. 2015.
|
|
13
|
Lamberti M, Capasso R, Lombardi A, Di
Domenico M, Fiorelli A, Feola A, Perna AF, Santini M, Caraglia M
and Ingrosso D: Two different Serum MiRNA signatures correlate with
the clinical outcome and histological subtype in pleural malignant
mesothelioma Patients. PLoS One. 10:e01353312015. View Article : Google Scholar :
|
|
14
|
Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S,
Wang Q, Yan Y, Kang C, Jin S, et al: miR-146b-5p inhibits glioma
migration and invasion by targeting MMP16. Cancer Lett.
339:260–269. 2013. View Article : Google Scholar
|
|
15
|
Prabhakar NR and Semenza GL: Adaptive and
maladaptive cardiorespiratory responses to continuous and
intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2.
Physiol Rev. 92:967–1003. 2012. View Article : Google Scholar :
|
|
16
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar :
|
|
17
|
Haeberle JR: Thin-filament linked
regulation of smooth muscle myosin. J Muscle Res Cell Motil.
20:363–370. 1999. View Article : Google Scholar
|
|
18
|
Morgan KG and Gangopadhyay SS: Invited
review: Cross-bridge regulation by thin filament-associated
proteins. J Appl Physiol. 91:953–962. 2001.
|
|
19
|
Niessen P, Rensen S, Van Deursen J, De Man
J, De Laet A, Vanderwinden JM, Wedel T, Baker D, Doevendans P,
Hofker M, et al: Smoothelin-a is essential for functional
intestinal smooth muscle contractility in mice. Gastroenterology.
129:1592–1601. 2005. View Article : Google Scholar
|
|
20
|
Tang Y, Urs S, Boucher J, Bernaiche T,
Venkatesh D, Spicer DB, Vary CP and Liaw L: Notch and transforming
growth factor-beta (TGFbeta) signaling pathways cooperatively
regulate vascular smooth muscle cell differentiation. J Biol Chem.
285:17556–17563. 2010. View Article : Google Scholar :
|
|
21
|
Rodríguez-Vita J, Sánchez-Galán E,
Santamaría B, Sánchez-López E, Rodrigues-Díez R, Blanco-Colio LM,
Egido J, Ortiz A and Ruiz-Ortega M: Essential role of TGF-beta/Smad
pathway on statin dependent vascular smooth muscle cell regulation.
PLoS One. 3:e39592008. View Article : Google Scholar :
|
|
22
|
Yang G and Yang X: Smad4-mediated TGF-beta
signaling in tumorigenesis. Int J Biol Sci. 6:1–8. 2010. View Article : Google Scholar :
|
|
23
|
Stone JD, Holt AW, Vuncannon JR, Brault JJ
and Tulis DA: AMP-activated protein kinase inhibits transforming
growth factor-β-mediated vascular smooth muscle cell growth:
Implications for a Smad-3-dependent mechanism. Am J Physiol Heart
Circ Physiol. 309:H1251–H1259. 2015. View Article : Google Scholar :
|
|
24
|
Martin-Garrido A, Williams HC, Lee M,
Seidel-Rogol B, Ci X, Dong JT, Lassègue B, Martín AS and Griendling
KK: Transforming growth factor β inhibits platelet derived growth
factor-induced vascular smooth muscle cell proliferation via
Akt-independent, Smad-mediated cyclin D1 downregulation. PLoS One.
8:e796572013. View Article : Google Scholar :
|
|
25
|
Huang D, Wang Y, Wang L, Zhang F, Deng S,
Wang R, Zhang Y and Huang K: Poly(ADP-ribose) polymerase 1 is
indispensable for transforming growth factor-β Induced Smad3
activation in vascular smooth muscle cell. PLoS One. 6:e271232011.
View Article : Google Scholar :
|
|
26
|
Mao X, Debenedittis P, Sun Y, Chen J, Yuan
K, Jiao K and Chen Y: Vascular smooth muscle cell Smad4 gene is
important for mouse vascular development. Arterioscler Thromb Vasc
Biol. 32:2171–2177. 2012. View Article : Google Scholar :
|