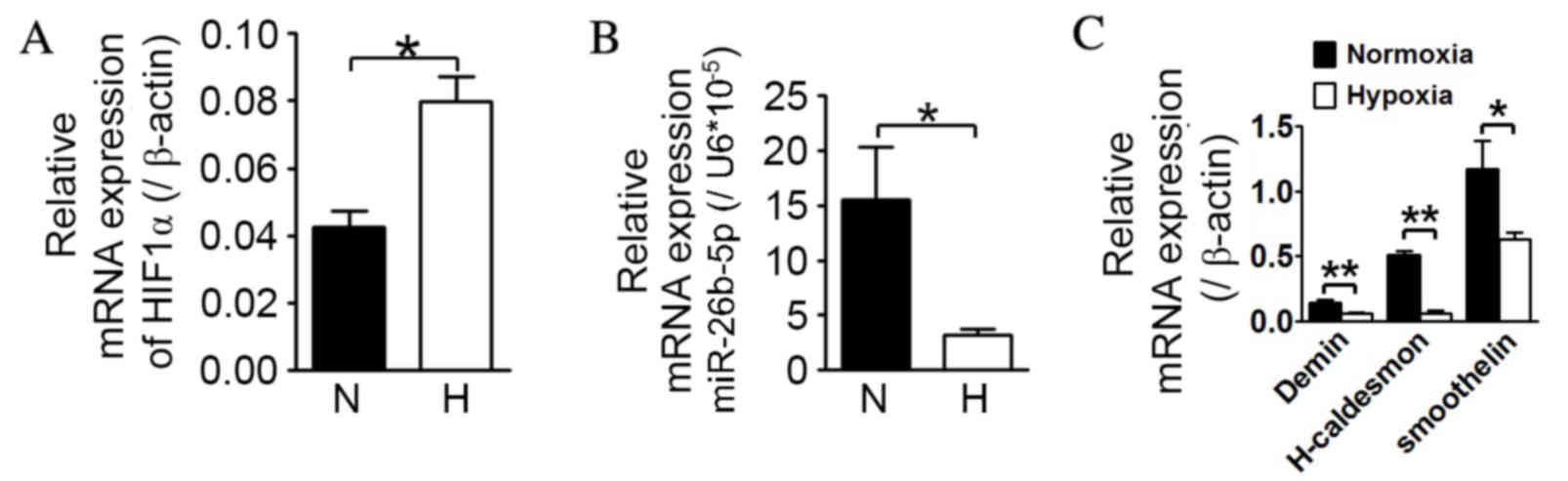
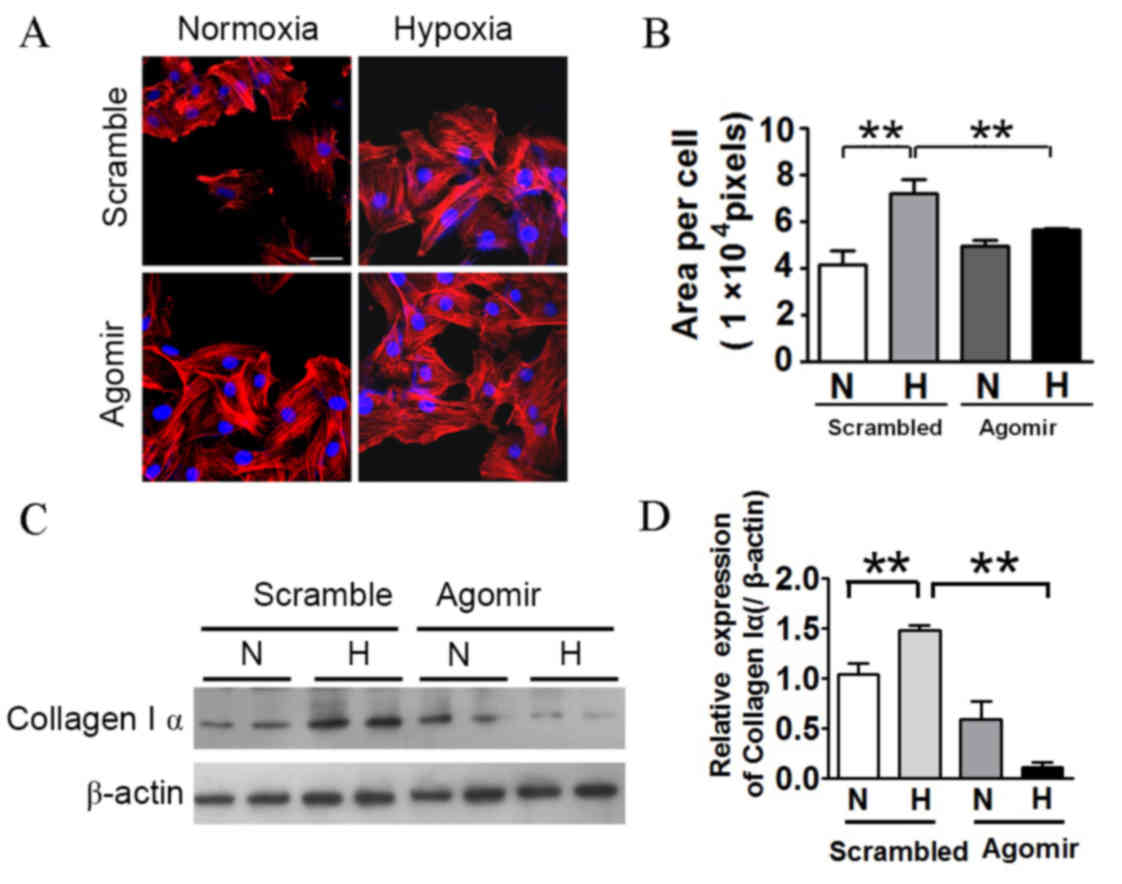
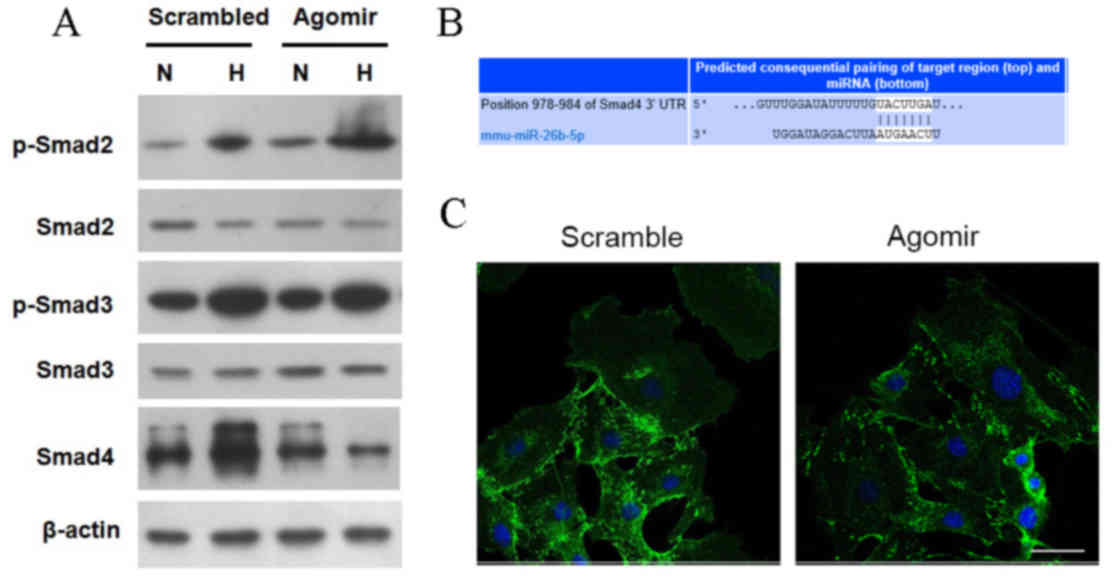
|
1
|
Alexander MR and Owens GK: Epigenetic
control of smooth muscle cell differentiation and phenotypic
switching in vascular development and disease. Annu Rev Physiol.
74:13–40. 2012. View Article : Google Scholar
|
|
2
|
Chamley-Campbell J, Campbell GR and Ross
R: The smooth muscle cell in culture. Physiol Rev. 59:1–61.
1979.
|
|
3
|
Thyberg J, Hedin U, Sjölund M, Palmberg L
and Bottger BA: Regulation of differentiated properties and
proliferation of arterial smooth muscle cells. Arteriosclerosis.
10:966–90. 1990. View Article : Google Scholar
|
|
4
|
Nikkari ST, Rantala I, Pystynen P and
Nikkari T: Characterization of the phenotype of smooth muscle cells
in human fetal aorta on the basis of ultrastructure,
immunofluorescence, and the composition of cytoskeletal and
cytocontractile proteins. Atherosclerosis. 74:33–40. 1988.
View Article : Google Scholar
|
|
5
|
Yin H, Li Q, Qian G, Wang Y, Li Y, Wu G
and Wang G: Rab1 GTPase regulates phenotypic modulation of
pulmonary artery smooth muscle cells by mediating the transport of
angiotensin II type 1 receptor under hypoxia. Int J Biochem Cell
Biol. 43:401–408. 2011. View Article : Google Scholar
|
|
6
|
Jie W, Guo J, Shen Z, Wang X, Zheng S,
Wang G and Ao Q: Contribution of myocardin in the hypoxia-induced
phenotypic switching of rat pulmonary arterial smooth muscle cells.
Exp Mol Pathol. 89:301–306. 2010. View Article : Google Scholar
|
|
7
|
Kim K, Yang DK, Kim S and Kang H:
miR-142-3p is a regulator of the TGFβ-mediated vascular smooth
muscle cell phenotype. J Cell Biochem. 116:2325–2333. 2015.
View Article : Google Scholar
|
|
8
|
Kim S, Hata A and Kang H: Down-regulation
of miR-96 by bone morphogenetic protein signaling is critical for
vascular smooth muscle cell phenotype modulation. J Cell Biochem.
115:889–895. 2014. View Article : Google Scholar :
|
|
9
|
Sarkar J, Gou D, Turaka P, Viktorova E,
Ramchandran R and Raj JU: MicroRNA-21 plays a role in
hypoxia-mediated pulmonary artery smooth muscle cell proliferation
and migration. Am J Physiol Lung Cell Mol Physiol. 299:L861–L871.
2010. View Article : Google Scholar :
|
|
10
|
Brock M, Haider TJ, Vogel J, Gassmann M,
Speich R, Trenkmann M, Ulrich S, Kohler M and Huber LC: The
hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell
proliferation by directly targeting CDKN1A. Int J Biochem Cell
Biol. 61:129–37. 2015. View Article : Google Scholar
|
|
11
|
Yuan B, Yu WY, Dai LS, Gao Y, Ding Y, Yu
XF, Chen J and Zhang JB: Expression of microRNA-26b and
identification of its target gene EphA2 in pituitary tissues in
Yanbian cattle. Mol Med Rep. 12:5753–5761. 2015.
|
|
12
|
Xu G, Ji C, Song G, Shi C, Shen Y, Chen L,
Yang L, Zhao Y and Guo X: Obesity-associated microRNA-26b regulates
the proliferation of human preadipocytes via arrest of the G1/S
transition. Mol Med Rep. 12:3648–3654. 2015.
|
|
13
|
Lamberti M, Capasso R, Lombardi A, Di
Domenico M, Fiorelli A, Feola A, Perna AF, Santini M, Caraglia M
and Ingrosso D: Two different Serum MiRNA signatures correlate with
the clinical outcome and histological subtype in pleural malignant
mesothelioma Patients. PLoS One. 10:e01353312015. View Article : Google Scholar :
|
|
14
|
Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S,
Wang Q, Yan Y, Kang C, Jin S, et al: miR-146b-5p inhibits glioma
migration and invasion by targeting MMP16. Cancer Lett.
339:260–269. 2013. View Article : Google Scholar
|
|
15
|
Prabhakar NR and Semenza GL: Adaptive and
maladaptive cardiorespiratory responses to continuous and
intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2.
Physiol Rev. 92:967–1003. 2012. View Article : Google Scholar :
|
|
16
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar :
|
|
17
|
Haeberle JR: Thin-filament linked
regulation of smooth muscle myosin. J Muscle Res Cell Motil.
20:363–370. 1999. View Article : Google Scholar
|
|
18
|
Morgan KG and Gangopadhyay SS: Invited
review: Cross-bridge regulation by thin filament-associated
proteins. J Appl Physiol. 91:953–962. 2001.
|
|
19
|
Niessen P, Rensen S, Van Deursen J, De Man
J, De Laet A, Vanderwinden JM, Wedel T, Baker D, Doevendans P,
Hofker M, et al: Smoothelin-a is essential for functional
intestinal smooth muscle contractility in mice. Gastroenterology.
129:1592–1601. 2005. View Article : Google Scholar
|
|
20
|
Tang Y, Urs S, Boucher J, Bernaiche T,
Venkatesh D, Spicer DB, Vary CP and Liaw L: Notch and transforming
growth factor-beta (TGFbeta) signaling pathways cooperatively
regulate vascular smooth muscle cell differentiation. J Biol Chem.
285:17556–17563. 2010. View Article : Google Scholar :
|
|
21
|
Rodríguez-Vita J, Sánchez-Galán E,
Santamaría B, Sánchez-López E, Rodrigues-Díez R, Blanco-Colio LM,
Egido J, Ortiz A and Ruiz-Ortega M: Essential role of TGF-beta/Smad
pathway on statin dependent vascular smooth muscle cell regulation.
PLoS One. 3:e39592008. View Article : Google Scholar :
|
|
22
|
Yang G and Yang X: Smad4-mediated TGF-beta
signaling in tumorigenesis. Int J Biol Sci. 6:1–8. 2010. View Article : Google Scholar :
|
|
23
|
Stone JD, Holt AW, Vuncannon JR, Brault JJ
and Tulis DA: AMP-activated protein kinase inhibits transforming
growth factor-β-mediated vascular smooth muscle cell growth:
Implications for a Smad-3-dependent mechanism. Am J Physiol Heart
Circ Physiol. 309:H1251–H1259. 2015. View Article : Google Scholar :
|
|
24
|
Martin-Garrido A, Williams HC, Lee M,
Seidel-Rogol B, Ci X, Dong JT, Lassègue B, Martín AS and Griendling
KK: Transforming growth factor β inhibits platelet derived growth
factor-induced vascular smooth muscle cell proliferation via
Akt-independent, Smad-mediated cyclin D1 downregulation. PLoS One.
8:e796572013. View Article : Google Scholar :
|
|
25
|
Huang D, Wang Y, Wang L, Zhang F, Deng S,
Wang R, Zhang Y and Huang K: Poly(ADP-ribose) polymerase 1 is
indispensable for transforming growth factor-β Induced Smad3
activation in vascular smooth muscle cell. PLoS One. 6:e271232011.
View Article : Google Scholar :
|
|
26
|
Mao X, Debenedittis P, Sun Y, Chen J, Yuan
K, Jiao K and Chen Y: Vascular smooth muscle cell Smad4 gene is
important for mouse vascular development. Arterioscler Thromb Vasc
Biol. 32:2171–2177. 2012. View Article : Google Scholar :
|