Introduction
Angiotensin II (Ang II) is an octapeptide hormone
best known for its role in the maintenance of blood pressure and
water-electrolyte balance, while the most commonly-known function
of relaxin 2 (RLN2) is its role in pregnancy and parturition.
However, many other features of these peptide hormones have been
noted at the tissue level. During the last few decades, an
increasing number of studies have implicated Ang II and relaxin 2
as well as other peptide hormones in cancer initiation, progression
and metastasis. For example, strong expression has been observed of
relaxin 2, the relaxin receptor RXFP1/LGR7, Ang II and angiotensin
receptor type 1 in tumor tissue compared to normal prostate or
breast tissue (1–4). Recent studies of the authors have
analyzed the interaction between the rennin-angiotensin system
(RAS) and the relaxin family peptide system (RFPS), and their
effects on various aspects of prostate cancer development. The
findings suggest that the two investigated systems are functionally
linked and have an impact on cell growth and proliferation by their
partially overlapping signal transduction pathways. It has been
reported that Ang II and relaxin 2 can serve an important role in
increasing the aggressiveness of prostate tumors by upregulating
BIRC5 expression and gelatinase A and B secretion. In
addition, previous results of the authors suggest that both peptide
hormones are implicated in the transition from the
androgen-dependent to the androgen-independent phenotype in
prostate cancer by modulation of the expression of androgen
receptors (AR) (5,6).
The present study demonstrates that Ang II and
relaxin 2 alter the mRNA expression of NF-κB family members in
normal and cancer prostate cell lines. The NF-κB family consists of
transcription factors [nuclear factor-κB subunit 1 (NF-κB1) and
nuclear factor-κB subunit 2 (NF-κB2), REL proto-oncogene nuclear
factor-κB subunit (REL), RELA nuclear factor-κB subunit (RelA),
RELB proto-oncogene nuclear factor-κB subunit (RelB)] that serve
critical roles in cell proliferation and differentiation,
regulation of survival and apoptosis. However, the biological
significance of NF-κB activation in carcinoma tissues remains
unclear. Several researchers have reported constitutive activation
of NF-κB in tumors such as ovarian cancer, breast cancer,
pancreatic cancer and prostate cancer. Over-expression of NF-κB in
the nucleus of prostate cancer cells is associated with
chemoresistance, more aggressive cancer phenotypes and metastatic
spread. In addition, studies have indicated that activation of
NF-κB signaling promotes castrate-resistant growth of prostate
tumors (7–10).
These studies are a continuation of earlier research
(5,6) intended to elucidate the mechanisms of
tumorigenesis and prostate cancer progression associated with RAS
and RFPS.
Materials and methods
Reagents
Ang II (H-1705) and relaxin 2 (H-6784) were obtained
from Bachem (Bubendorf, Switzerland). The octapeptide Ang II is a
major biologically active component of the RAS. Relaxin 2, the
6-kDa heterodimeric polypeptide hormone, is a member of the RFPS.
The pleiotropic effects of both peptides are determined by the
signaling pathway profile activated in target cells. For all
experiments, peptides were used at a final concentration of
10−8 M. This concentration was selected on the basis of
earlier research work (5,6). Unless otherwise specified, the medium
and other culture supplements were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA).
Cell lines and culture conditions
Prostate cancer cell lines (LNCaP, DU-145, PC3) were
obtained from the American Type Culture Collection (Manassas, VA,
USA), while immortalized, normal prostate epithelial PNT1A cells
were obtained from the European Collection of Authenticated Cell
Cultures (Salisbury, UK). All 4 stable cell lines were
authenticated by short-tandem repeat DNA profiling by the LGC
Standards Cell Line Authentication Service (Koeln, Germany).
The LNCaP cells, identified in a relatively slow
growing and weakly tumorigenic prostate cancer, were isolated from
a supraclavicular lymph node metastasis. The PC3 cells were
characterized by low diversity and high invasiveness (stage IV) and
were established from bone metastases. The androgen-independent
DU-145 cells were derived from brain metastases, and represent an
intermediate model between the LNCaP and PC3 cells (11). The PNT1A cells were established by
immortalization of normal adult prostatic epithelial cells by
transfection with a plasmid containing the SV40 genome with a
defective replication origin. This stable cell line represents a
good model to study of initial steps leading to transformation of
the prostate gland (12). The
cells were grown in a classical two-dimensional (2D) cell culture
system using plates or flasks and Advanced RPMI-1640 medium
supplemented with 5% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate
and antibiotics. The incubator was maintained at an optimal
temperature (37°C), humidity (95%) and other conditions, such as
the carbon dioxide (5% CO2) of the atmosphere inside
needed to grow human cells. Cells were harvested with 0.25%
trypsin/EDTA. The cells were passaged at least twice after thawing
from liquid nitrogen. Further experiments used cells with a passage
number between 15 and 35.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The prostate cells were exposed to Ang II, relaxin 2
or a combination of both for 48 h. Total RNA was extracted from the
cells using TRIzol reagent (Thermo Fisher Scientific, Inc.) and
purified with the standard phenol: chloroform method. The
concentration of recovered RNA and its purity was determined by
BioDrop µLITE: A UV/Vis spectrophotometer designed for micro-volume
measurements (Isogen Life Science B.V., Utrecht, Netherlands). mRNA
expression was evaluated by RT-qPCR, using a LightCycler 480
real-time PCR system (Roche Diagnostics, Basel, Switzerland) and
EvaGreen PCR master mix (IMMUNIQ, Zory, Poland). Reverse
transcriptase synthesis of cDNA was performed on a 10 µg total mass
of RNA in a final volume of 50 µl using an oligo(dT) 15 Primer
Random Hexamer primer and ImProm-II™ Reverse
Transcription System (Promega Corporation, Madison, WI, USA), as
described previously (13).
Amplification reactions were performed in a final volume of 20 µl,
containing 2 µl cDNA. Detection temperature was above
non-specific/primer-dimer melting temperature. Primer sequences
were designed using PrimerQuest Tool (www.idtdna.com/primerquest/home/index) or Primer3
Input (http://bioinfo.ut.ee/primer3-0.4.0/) and checked for
specificity using BLAST (https://blast.ncbi.nlm.nih.gov/). All primers were
designed to be intron-spanning to avoid amplifying genomic DNA.
Detection temperature was above unspecific/primer-dimer melting
temperature. Sequences of primers, annealing and detection
temperatures are presented in Table
I. H3F3A and RPLPO were included as housekeeping gene controls
to correct for identical amount of cDNA in all qPCR reactions. The
Universal Human Reference RNA (Stratagene; Agilent Technologies,
Inc., Santa Clara, CA, USA), composed of total RNA from 10 human
cell lines, was used as a calibrator. The primers and reaction
conditions are presented in Table
I. All the reactions were run in duplicate, including
no-template controls. The relative gene expression level was
calculated according to the Roche algorithm (14).
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers and reaction conditions. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers and reaction conditions.
| Gene name | Gene primer sequence
(5′-3′) | Annealing temperature
(°C) | Detection temperature
(°C) |
|---|
| H3F3A | Forward:
5-AGGACTTTAAAACAGATCTGCGCTTCCAGAG-3′ | 65 | 72 |
|
| Reverse:
5-ACCAGATAGGCCTCACTTGCCTCCTGC-3′ |
|
|
| RPLPO | Forward: 5-
ACGGATTACACCTTCCCACTTGCTAAAAGGTC-3′ | 65 | 72 |
|
| Reverse: 5-
AGCCACAAAGGCAGATGGATCAGCCAAG-3′ |
|
|
| NFKB1 | Forward:
5′-GTGGTGCCTCACTGCTAACT-3′ | 58 | 72 |
|
| Reverse:
5′-GGATGCACTTCAGCTTCTGT-3′ |
|
|
| NFKB2 | Forward:
5′-TAGCCACAGAGATGGAGGAG-3′ | 60 | 72 |
|
| Reverse:
5′-CCGAGTCGCTATCAGAGGTA-3′ |
|
|
| REL | Forward:
5′-AAAGACTGCAGAGACGGCTA-3′ | 58 | 72 |
|
| Reverse:
5′-CTCACCACATTGAGGTCACA-3′ |
|
|
| RELA | Forward:
5′-GCACAGATACCACCAAGACC-3′ | 58 | 72 |
|
| Reverse:
5′-TCAGCCTCATAGAAGCCATC-3′ |
|
|
| RELB | Forward:
5′-CATTGAGCGGAAGATTCAAC-3′ | 56 | 72 |
|
| Reverse:
5′-GCAGCTCTGATGTGTTTGTG-3′ |
|
|
| AR | Forward:
5′-AAGGCTATGAATGTCAGCCCA-3′ | 60 | 72 |
|
| Reverse:
5′-CATTGAGGCTAGAGAGCAAGGC-3′ |
|
|
Statistical analysis
The results are presented as mean ± standard error
of the mean of at least three independent samples. The measurements
were analyzed using one-way analysis of variance with Dunnett's
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Influence of Ang II and relaxin 2 on
mRNA expression of members of the NF-kB transcription factor gene
family in prostate cancer cell lines
The results of the RT-qPCR identified relative NFKB1
expression to be almost double the expression in the PC3
(1.05±0.19; Fig. 2A, right panel)
and DU-145 (1.05±0.16; Fig. 2A,
left panel) cell lines than in LNCaP cells (0.55±0.13; Fig. 1A). Similarly, androgen-dependent
LNCaP prostate cancer cells (6.61±0.92; Fig. 1B) presented lower NFKB2 mRNA
expression than androgen-independent lines (10.11±1.63 for DU-145
and 49.00±6.10 for PC3; Fig. 2B,
right panel). Furthermore, NFKB1 gene expression is
downregulated by both investigated peptide hormones in prostate
cancer cells. However, NFKB2 mRNA expression was dependent upon
both the line and the peptide (Figs.
1B and 2B). RELA expression
was comparable in LNCaP (0.66±0.11; Fig. 1C) and PC3 (0.54±0.98; Fig. 2C, right panel) cell lines but was
twice as high in DU-145 (1.47±0.40; Fig. 2C, left panel). While relaxin 2
induces alterations in RELA expression only in PC3 and DU-145
androgen-independent prostate cancer cell lines, Ang II only
increases RELA expression in LNCaP and PC3 cells, not in DU-145
(Figs. 1C and 2C). The highest RELB expression was
observed in PC3 cells (9.81±2.35; Fig.
1D) and lowest in LNCaP cells (0.35±0.10; Fig. 2D). RELB expression did not
significantly change in response to Ang II and RLN2 in any of the
cell lines tested (P>0.05; Fig.
2D, both panels). REL expression in LNCaP cells (0.68±0.10;
Fig. 1E) was less than half that
observed in androgen-independent cells (DU-145, 2.03±0.41; PC3,
1.68±0.45; Fig. 2E). Ang II
increases REL mRNA expression in LNCaP and DU-145 cells, but no
changes were identified in PC3 cells in any of the cases
examined.
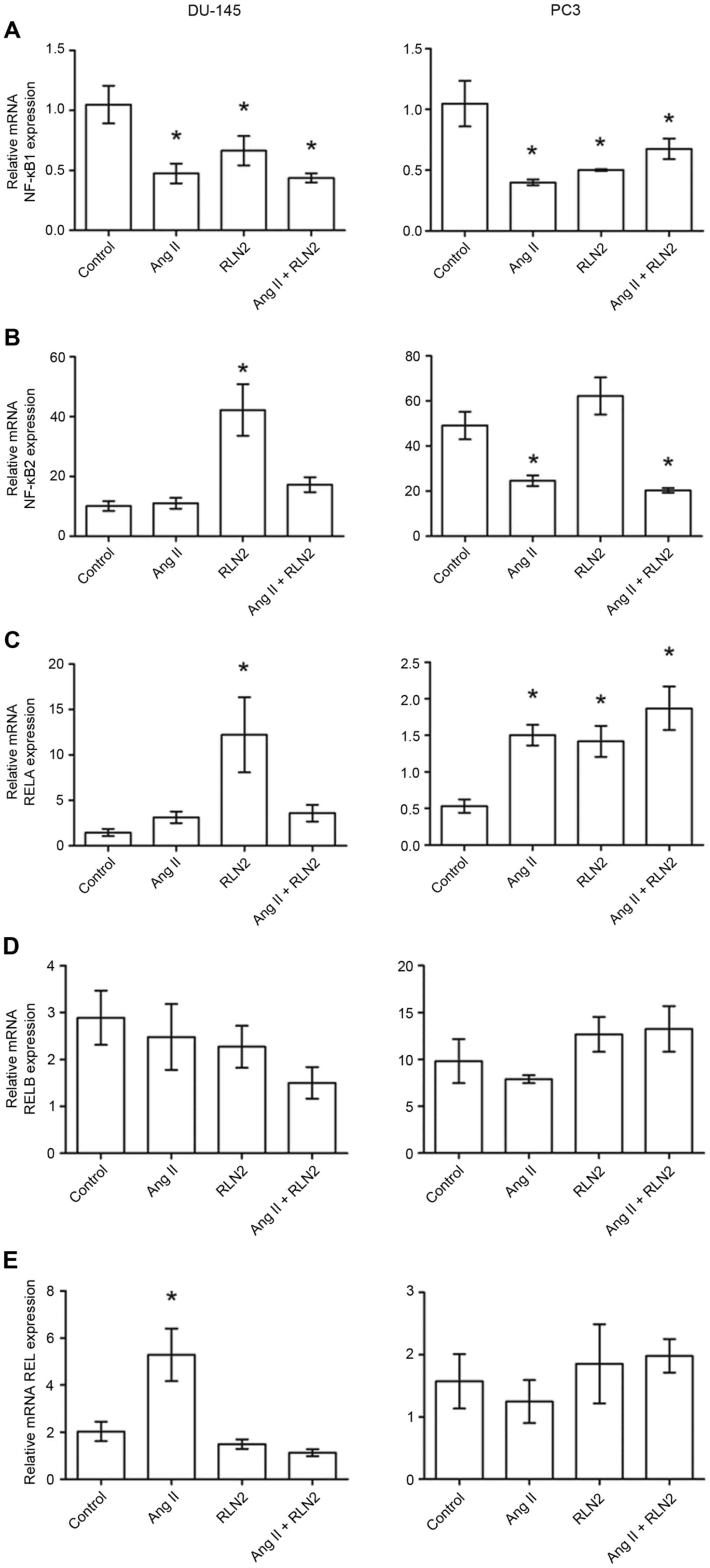 | Figure 2.The changes in mRNA expression of (A)
NFKB1, (B) NFKB2, (C) RELA, (D) RELB and (E) REL genes in
androgen-independent prostate cancer cell lines DU-145 and PC3
following exposure to peptide hormones (Ang II, RLN2, Ang II +
RLN2). Relative gene expression was calculated based on the Roche
guidebook according to a previously published algorithm. H3F3A and
RPLPO were used as endogenous controls and Universal Human
Reference RNA was used as a calibrator. Data are presented as the
mean ± standard error of the mean (n≥3). *P<0.05 vs. control.
NFKB1, nuclear factor-κB subunit 1; NFKB2, nuclear factor-κB
subunit 2; REL, REL proto-oncogene nuclear factor-κB subunit; RELA,
RELA proto-oncogene nuclear factor-κB subunit; RELB, RELB
proto-oncogene nuclear factor-κB subunit; Ang II, angiotensin II;
RLN2, relaxin 2. |
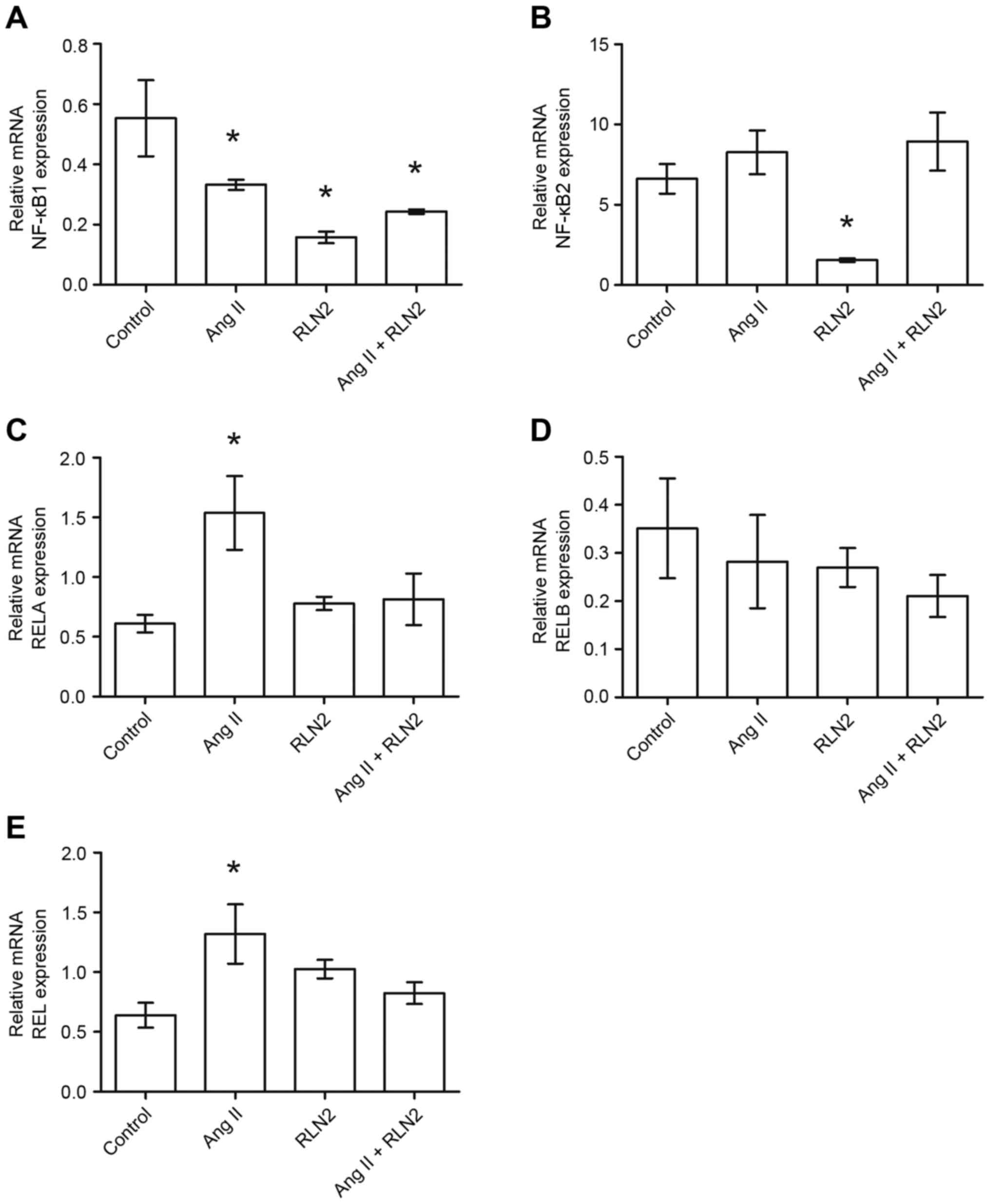 | Figure 1.The changes in mRNA expression of (A)
NFKB1, (B) NFKB2, (C) RELA, (D) RELB and (E) REL genes in the
androgen-dependent prostate cancer cell line LNCaP following
exposure to peptide hormones (Ang II, RLN2, Ang II + RLN2).
Relative gene expression was calculated based on the Roche
guidebook according to a previously published algorithm. H3F3A and
RPLPO were used as endogenous controls and Universal Human
Reference RNA was used as a calibrator. Data are presented as the
mean ± standard error of the mean (n≥3). *P<0.05 vs. control.
NFKB1, nuclear factor-κB subunit 1; NFKB2, nuclear factor-κB
subunit 2; REL, REL proto-oncogene nuclear factor-κB subunit; RELA,
RELA proto-oncogene nuclear factor-κB subunit; RELB, RELB
proto-oncogene nuclear factor-κB subunit; Ang II, angiotensin II;
RLN2, relaxin 2. |
Influence of Ang II and relaxin 2 on
mRNA expression of members of the NF-kB transcription factor gene
family in the prostatic epithelial cell line
Of all cell lines tested, normal human prostate
epithelial cells (PNT1A) demonstrated the highest expression of
RELA (1.72±0.33; Fig. 3C), and
neither Ang II nor relaxin 2 induced changes in RELA expression in
PNT1A cells (P>0.05; Fig. 3C).
In addition, RELB was expressed at a high level in PNT1A cells
(2.88±0.35; Fig. 3D) and was
observed to be up regulated by both investigated hormones (all
P<0.05; Fig. 3D). RELB
expression was more than three times higher for Ang II and four
times higher for relaxin 2. The expression of NFKB1 (0.69±0.15;
Fig. 3A) and REL (1.40±0.08;
Fig. 3E) was comparable to the
LNCaP cells but lower than in DU-145 and PC3 cell lines. Only
relaxin 2 was demonstrated to influence the expression of both REL
and NFKB1 in PNT1A, with the expression of NFKB1 being increased
two-fold following incubation with relaxin 2, when compared with
the control (P<0.05; Fig. 3A).
Expression of REL increased 2.5 times, when compared with the
control (P<0.05; Fig. 3E).
NFKB2 expression was observed in the normal prostate cell line
(8.83±2.28; Fig. 3B), but the mRNA
level remained constant regardless of treatment (P>0.05;
Fig. 3B).
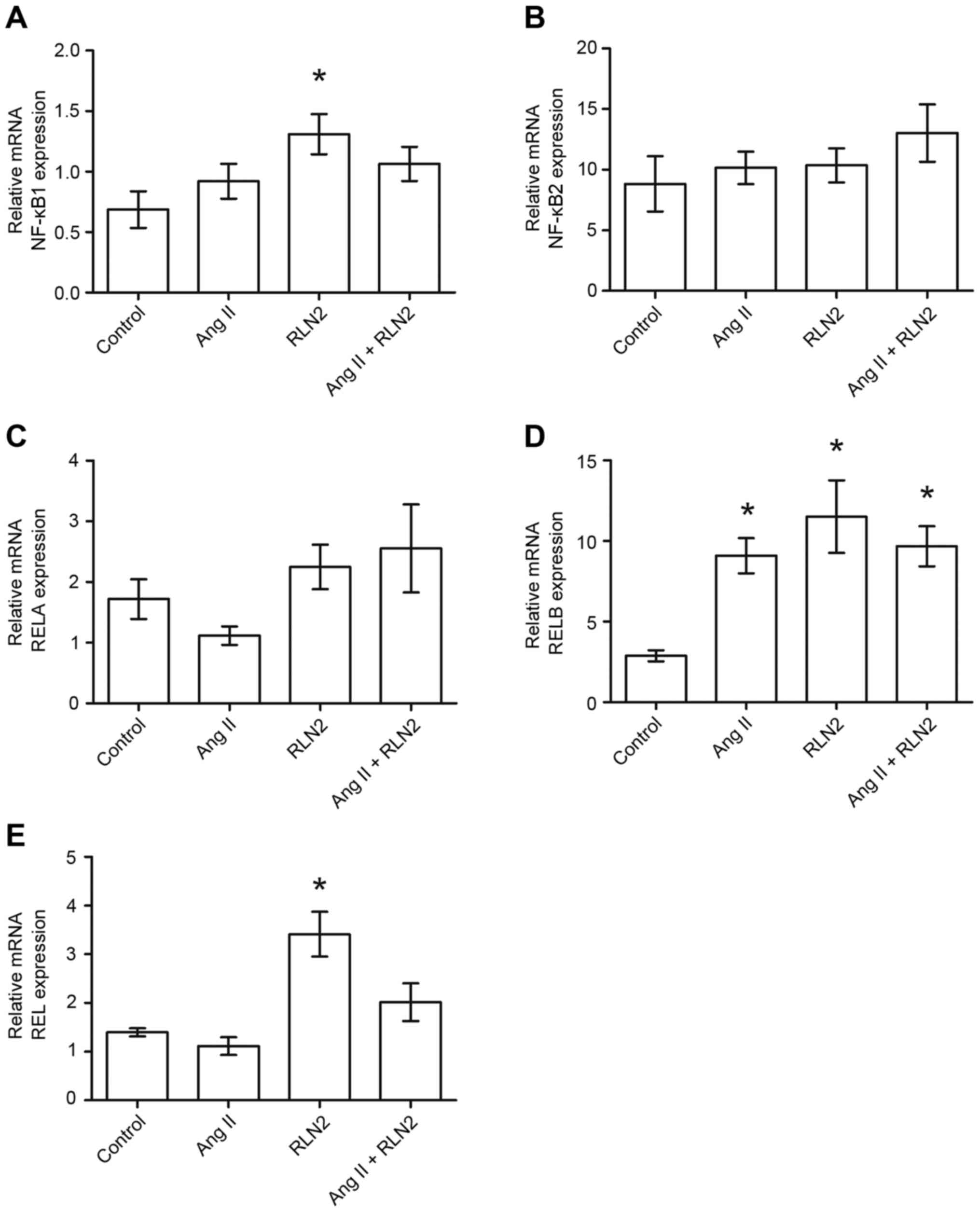 | Figure 3.The changes in mRNA expression of (A)
NFKB1, (B) NFKB2, (C) RELA, (D) RELB and (E) REL genes in
non-cancerous prostate epithelial cells (PNT1A) following exposure
to peptide hormones (Ang II, RLN2, Ang II + RLN2). Relative gene
expression was calculated based on the Roche guidebook according to
a previously published algorithm. H3F3A and RPLPO were used as
endogenous controls and Universal Human Reference RNA was used as a
calibrator. Data are presented as the mean ± standard error of the
mean (n ≥3). *P<0.05 vs. control. NFKB1, nuclear factor-κB
subunit 1; NFKB2, nuclear factor-κB subunit 2; REL, REL
proto-oncogene nuclear factor-κB subunit; RELA, RELA proto-oncogene
nuclear factor-κB subunit; RELB, RELB proto-oncogene nuclear
factor-κB subunit; Ang II, angiotensin II; RLN2, relaxin 2. |
Discussion
Members of the NF-κB family are important regulators
of the signal transduction pathway, and the serve essential roles
in a variety of physiological and pathological processes. NF-κB
activation is known to be associated with inflammation-associated
tumor promotion, progression and metastasis in prostate cancer
(7–10). The present study compares the
changes in NFKB1, NFKB2, RELA, RELB and REL gene expression
following exposure to Ang II and relaxin 2 in prostate
non-cancerous epithelial (PNT1A), androgen-dependent (LNCaP) and
androgen-independent (DU-145, PC3) prostate cancer cell lines. As
these cell lines represent various steps of prostate tumor
development from disease promotion and early-stage cancer to
hormone-refractory disease, their aggressiveness and hormonal
status are different (10,11).
Androgen-independent cell lines, such as DU-145 or
PC-3, constitutively express high levels of NF-kB, while
androgen-dependent cell lines, for example LNCaP and normal human
prostate epithelial cells, demonstrate only low NF-kB gene
activation (9,15). These results are in line with those
obtained in the authors studies for NFKB1 and NFKB2. RT-qPCR
indicated that the relative expression of both NFKB genes was the
highest for PC3 cells and the lowest for LNCaP cells. Similarly,
Suh et al (16) noted that
only prostate cell lines with low NF-kB activity express endogenous
AR, and suggest that the AR and NF-κB are inversely related; the
loss of AR is accompanied by an increase in NF-κB activity. It
seems that constitutive activation of NF-kB may contribute to
compensatory cellular changes allowing cell survival and growth in
the absence of AR activation (16). Earlier findings of the authors
indicate that AR levels are >30 times higher in LNCaP cells than
in PC3 cells (6). Alimirah et
al (17) report that AR mRNA
levels were ~50% lower in DU-145 cells than in LNCaP cells, and
that AR expression is much lower in PC3 cells than in both DU-145
and LNCaP cells (17).
No differences in NFKB1 level were observed between
the androgen-independent cell lines, regardless of AR expression.
However, a significantly higher level of NFKB2 gene expression was
observed in the PC3 cell line than in the less aggressive DU-145. A
similar situation was found in PNT1A cells; the prostatic
epithelial cell line and the LNCaP cells demonstrated comparable
NFKB1 expression, and Bidaux et al (18) report that AR expression is far
lower in PNT1A than LNCaP. In this case, NFKB2 mRNA expression was
also higher in the non-cancerous than the LNCaP cell line.
Therefore, the authors speculate that the level of NFKB2 mRNA, but
not NFKB1, in prostate cells may be closely linked to AR
expression.
The NFKB1 gene maps on chromosome 4q24 and encodes
protein p105/p50, while the NFKB2 gene maps on chromosome 10q24 and
encodes protein p100/p52. Both proteins may promote cell survival
through the induction of target genes, whose products directly or
indirectly regulate the apoptotic machinery in normal and cancerous
cells (10). The noncanonical
NF-κB signaling pathway mediates the activation of p52/RelB,
whereas the canonical pathway mediates the activation of p50/p65.
Previous work has indicated that several interconnections between
classic and alternative NF-κB pathways exist and these may be
essential in various biological processes (19). Earlier studies of the authors note
that the RAS and RFPS influence the growth, division and spread of
prostate cancer cells to some degree via overlapping signal
transduction pathways (6). It is
possible that members of the NF-κB transcription factor gene family
could serve an important role in this case. The current findings
indicated that Ang II and relaxin 2 may modulate the mRNA
expression of nuclear factor κB genes in prostate cells.
One of our most interesting observations is that Ang
II and relaxin 2 significantly decrease the expression of NFKB1
mRNA in all cancer cell lines, yet they increase it in normal
epithelial cells. However, in PNT1A cells, the results were
statistically significant only for relaxin 2. In addition, relaxin
2 radically increased the levels of androgen receptors in this cell
line, reaching levels more than five times above baseline (data not
shown). A dual increase of AR and NFKB1 expression may be required
to promote prostate carcinogenesis. The expression of NFKB2 in
normal epithelial prostate cells is not altered by peptide
hormones.
The mechanism underlying the downregulation of NFKB1
expression by both peptide hormones in prostate cancer cells is not
an obvious one, and further research is needed to clarify it.
Setlur et al (20)
demonstrated that the expression of NFKB1 is upregulated in
localized prostate cancer and hormone-naïve metastasis, but
downregulated in hormone-refractory metastasis. In contrast,
metastatic samples demonstrated greater nuclear localization of
NFKB1, which may be mediated by low levels of the NF-κB inhibitor,
IκBα (20). Nevertheless, all
findings suggested that NFKB1 expression is important for the early
development of prostate cancers and for advanced disease.
Differences in NF-κB activity between androgen-sensitive and
androgen-independent prostate cancer cell lines may contribute to
androgen autonomy (21).
The RELB gene maps on chromosome 19q13.32 and
encodes protein p66, which regulates the migration and invasion
abilities of cancer cells. High constitutive nuclear levels of RelB
have been observed in human prostate cancer specimens with high
Gleason scores (19,22). Furthermore, increased levels of
RelB enhanced prostate cancer cell survival rate after treatments
and contributes to the resistance of PCa cells to radiation
(19).
Josson et al (23) identified that RelB nuclear
localization is significantly higher in the aggressive PC-3
prostate cancer cell line than in the less aggressive LNCaP cell
line. In the present study, the expression of RELB mRNA in prostate
cancer cells was the highest for PC3, indirect for DU-145 and the
lowest for LNCaP. Surprisingly, the levels of RELB mRNA were higher
in the non-tumorigenic PNT1A cell line than in the poorly
tumorigenic androgen-dependent prostate cancer cell line. Hatano
et al (24) report that the
levels of RelB were higher in normal prostate epithelial cells
(PNT2) than in LNCaP cells when cells or cytoplasmic extracts were
used. However, when nucleus extract was used, RelA and RelB
expression was much higher in prostate cancer cells, PC3, DU-145
and LNCaP than in normal prostate epithelial cells (PNT2) (24).
Xu et al (19) observed that LNCaP cell
tumorigenicity was enhanced following RelB overexpression, while
PC-3 cell tumorigenicity was attenuated by RelB knockdown. Wang
et al (25) indicated that
RelB knockdown significantly suppresses the migration and invasion
of DU-145 prostate cancer cells. The authors' previous findings
confirm that both peptide hormones can promote the invasion and
spread of human prostate cells via up-regulation of MMPs (5,6). In
addition, NF-κB can stimulate aggressiveness by regulating the
expression of various matrix metalloproteinases, especially MMP-2
and MMP-9 (7,8). Unexpectedly, Ang II and relaxin 2
significantly enhanced mRNA expression of RELB only in normal
epithelial cells. Therefore, it appears that both peptide hormones
can contribute to the initiation of prostate cancer by the NF-κB
alternative pathway. It is worth noting that the level of RELB mRNA
expression may not reflect the nuclear levels of RelB. Further
studies are required in order to conclude a causal association
between RelB and cancer progression following Ang II or relaxin 2
induction.
The RELA gene maps on chromosome 11q13 and encodes
the protein p65, which regulates the transcription of a wide
variety of genes involved in cell survival, invasion and metastasis
(26). Nuclear expression of RelA
is specific to prostate cancers and related to a poor outcome for
prostate cancer patients, however the RELA gene is not associated
with the Gleason score (26,27).
The level of RELA mRNA presented no relation to the aggressiveness
of the tested prostate cancer cells.
Interestingly, Palvimo et al (28) note that elevated expression of RelA
repressed AR-mediated transactivation in a dose-dependent manner.
Co-immunoprecipitation and protein-protein interaction assays did
not detect any specific association between AR and RelA. However,
it was demonstrated that repression by RelA could not be overcome
by the addition of excess AR. These results suggested that the two
proteins competed for the coactivator(s) present in limiting
amounts in the cell, or that RelA induces the expression of an
unknown repressor of AR (28).
Nelius et al (29) report
that AR (+) PC-3 cells became less tumorigenic on an ambient
testosterone background than AR (−) PC-3. AR expression was
identified to lower the mRNA and protein levels of RelA, and reduce
the activity and nuclear localization of p65. Previous studies of
the authors indicate a decrease in AR expression in PC3 cells
treated with the peptide hormones, but the results were
insignificant. The present findings indicated that, while both
peptide hormones increase mRNA expression of RELA in the
androgen-independent prostate cancer cell line PC3, these
relationships were not observed for the non-cancerous prostate
epithelial cell line. Neither Ang II nor relaxin 2 altered the
level of RELA inPNT1A, regardless of any significant reduction in
AR expression.
The REL gene maps on chromosome 12p12 and encodes
protein p75, which is associated with the malignant progression of
solid tumors such as breast cancer, gastric and pancreatic cancer
(30–32). For example, breast tumors present
increased expression of mRNA for c-Rel, as well as for other NF-kB
family members, compared to non-tumorigenic adjacent tissue
(32). In the present study, Ang
II was indicated to increase the expression of REL gene mRNA in
prostate cancer cells, with the exception of the most aggressive
line (PC3), whereas relaxin 2 only influenced normal prostate
epithelial cells. Interestingly, Mukhopadhyay et al
(33) report that c-Rel, similar
to AR, is a component of the nucleoprotein complex regulating the
androgen-responsive prostate-specific antigen (PSA) promoter.
Moreover, an analysis of the AR and c-Rel protein levels
demonstrated that promoter downregulation was not attributable to
mutual decreases in the quantity of AR or c-Rel. It is worth
remembering that DU-145 and PC-3 do not express PSA.
Based on some of the authors' earlier works and
other recent studies, we postulate that both Ang II and relaxin 2
have an impact on the proliferation or invasion of prostate cells
via canonical and non-canonical NF-kB pathways. The present results
indicated significant differences between the regulation of the
expression of NFKB1, NFKB2, RELA, RELB and REL mRNA in
non-cancerous epithelial, androgen-dependent and
androgen-independent prostate cancer cells by both peptide
hormones. Undoubtedly, further studies are required in order to
examine the interaction between the members of the NF-κB
transcription factor gene family and AR. Nevertheless, it seems
that the RAS and RFPS can serve an important role in the regulation
of both.
Acknowledgements
This work was supported by Ministry of Science and
Higher Education grant NN 403 2081 39 and Medical University of
Lodz grant 503/0-078-04/503-01-001.
References
|
1
|
Domińska K: Relaxin 2-a pregnancy hormone
involved in the process of carcinogenesis. Ginekol Pol. 84:126–130.
2013. View
Article : Google Scholar
|
|
2
|
Domińska K and Lachowicz-Ochedalska A: The
involvement of the renin-angiotensin system (RAS) in
cancerogenesis. Postepy Biochem. 54:294–300. 2008.(In Polish).
|
|
3
|
Wegman-Ostrosky T, Soto-Reyes E,
Vidal-Millán S and Sánchez-Corona J: The renin-angiotensin system
meets the hallmarks of cancer. J Renin angiotensin Aldosterone
Syst. 16:227–233. 2015. View Article : Google Scholar
|
|
4
|
Nair VB, Samuel CS, Separovic F, Hossain
MA and Wade JD: Human relaxin-2: Historical perspectives and role
in cancer biology. Amino Acids. 43:1131–1140. 2012. View Article : Google Scholar
|
|
5
|
Domińska K, Ochędalski T, Kowalska K,
Matysiak-Burzyńska ZE, Płuciennik E and Piastowska-Ciesielska AW: A
common effect of angiotensin II and relaxin 2 on the PNT1A normal
prostate epithelial cell line. J Physiol Biochem. 72:381–392. 2016.
View Article : Google Scholar
|
|
6
|
Domińska K, Ochędalski T, Kowalska K,
Matysiak-Burzyńska ZE, Płuciennik E and Piastowska-Ciesielska AW:
Interaction between angiotensin II and relaxin 2 in the progress of
growth and spread of prostate cancer cells. Int J Oncol.
48:2619–2628. 2016.
|
|
7
|
Jin R, Sterling JA, Edwards JR, DeGraff
DJ, Lee C, Park SI and Matusik RJ: Activation of NF-kappa B
signaling promotes growth of prostate cancer cells in bone. PLoS
One. 8:e609832013. View Article : Google Scholar :
|
|
8
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar :
|
|
9
|
Nguyen DP, Li J, Yadav SS and Tewari AK:
Recent insights into NF-κB signalling pathways and the link between
inflammation and prostate cancer. BJU Int. 114:168–176. 2014.
View Article : Google Scholar
|
|
10
|
Luo JL, Kamata H and Karin M: IKK/NF-κB
signaling: Balancing life and death-a new approach to cancer
therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar :
|
|
11
|
Wu X, Gong S, Roy-Burman P, Lee P and
Culig Z: Current mouse and cell models in prostate cancer research.
Endocr Relat Cancer. 20:R155–R170. 2013. View Article : Google Scholar
|
|
12
|
Avancès C, Georget V, Térouanne B, Orio F,
Cussenot O, Mottet N, Costa P and Sultan C: Human prostatic cell
line PNT1A, a useful tool for studying androgen receptor
transcriptional activity and its differential subnuclear
localization in the presence of androgens and antiandrogens. Mol
Cell Endocrinol. 184:13–24. 2001. View Article : Google Scholar
|
|
13
|
Dominska K, Piastowska-Ciesielska AW,
Pluciennik E, Lachowicz-Ochedalska A and Ochedalski T: A comparison
of the effects of angiotensin IV on androgen-dependent and
androgen-independent prostate cancer cell lines. J Renin
angiotensin Aldosterone Syst. 14:74–81. 2013. View Article : Google Scholar
|
|
14
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression soft-ware tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar :
|
|
15
|
Gasparian AV, Yao YJ, Kowalczyk D, Lyakh
LA, Karseladze A, Slaga TJ and Budunova IV: The role of IKK in
constitutive activation of NF-kappa B transcription factor in
prostate carcinoma cells. J Cell Sci. 115:141–151. 2002.
|
|
16
|
Suh J, Payvandi F, Edelstein LC, Amenta
PS, Zong WX, Gélinas C and Rabson AB: Mechanisms of constitutive
NF-kappa B activation in human prostate cancer cells. Prostate.
52:183–200. 2002. View Article : Google Scholar
|
|
17
|
Alimirah F, Chen J, Basrawala Z, Xin H and
Choubey D: DU-145 and PC-3 human prostate cancer cell lines express
androgen receptor: Implications for the androgen receptor functions
and regulation. FEBS Lett. 580:2294–2300. 2006. View Article : Google Scholar
|
|
18
|
Bidaux G, Roudbaraki M, Merle C, Crépin A,
Delcourt P, Slomianny C, Thebault S, Bonnal JL, Benahmed M, Cabon
F, et al: Evidence for specific TRPM8 expression in human prostate
secretory epithelial cells: Functional androgen receptor
requirement. Endocr Relat Cancer. 12:367–382. 2005. View Article : Google Scholar
|
|
19
|
Xu Y, Josson S, Fang F, Oberley TD, St
Clair DK, Wan XS, Sun Y, Bakthavatchalu V, Muthuswamy A and St
Clair WH: RelB enhances prostate cancer growth: Implications for
the role of the nuclear factor-kappaB alternative pathway in
tumorigenicity. Cancer Res. 69:3267–3271. 2009. View Article : Google Scholar :
|
|
20
|
Setlur SR, Royce TE, Sboner A, Mosquera
JM, Demichelis F, Hofer MD, Mertz KD, Gerstein M and Rubin MA:
Integrative microarray analysis of pathways dysregulated in
metastatic prostate cancer. Cancer Res. 67:10296–10303. 2007.
View Article : Google Scholar
|
|
21
|
Altuwaijri S, Lin HK, Chuang KH, Lin WJ,
Yeh S, Hanchett LA, Rahman MM, Kang HY, Tsai MY, Zhang Y, et al:
Interruption of nuclear factor kappaB signaling by the androgen
receptor facilitates 12-O-tetradecanoylphorbolacetate-induced
apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer
Res. 63:7106–7112. 2003.
|
|
22
|
Lessard L, Bégin LR, Gleave ME, Mes-Masson
AM and Saad F: Nuclear localisation of nuclear factor-kappa B
transcription factors in prostate cancer: An immunohistochemical
study. Br J Cancer. 93:1019–1023. 2005. View Article : Google Scholar :
|
|
23
|
Josson S, Xu Y, Fang F, Dhar SK, St Clair
DK and St Clair WH: RelB regulates manganese superoxide dismutase
gene and resistance to ionizing radiation of prostate cancer cells.
Oncogene. 25:1554–1559. 2006. View Article : Google Scholar :
|
|
24
|
Hatano K, Miyamoto Y, Nonomura N and
Kaneda Y: Expression of gangliosides, GD1a, and sialyl
paragloboside is regulated by NF-κB-dependent transcriptional
control of α2,3-sialyltransferase III and VI in human
castration-resistant prostate cancer cells. Int J Cancer.
129:1838–1847. 2011. View Article : Google Scholar
|
|
25
|
Wang J, Yi S, Zhou J, Zhang Y and Guo F:
The NF-κB subunit RelB regulates the migration and invasion
abilities and the radio-sensitivity of prostate cancer cells. Int J
Oncol. 49:381–392. 2016.
|
|
26
|
Domingo-Domenech J, Mellado B, Ferrer B,
Truan D, Codony-Servat J, Sauleda S, Alcover J, Campo E, Gascon P,
Rovira A, et al: Activation of nuclearfactor-kappaB in human
prostate carcinogenesis and association to biochemical relapse. Br
J Cancer. 93:1285–1294. 2005. View Article : Google Scholar :
|
|
27
|
Seo SI, Song SY, Kang MR, Kim MS, Oh JE,
Kim YR, Lee JY, Yoo NJ and Lee SH: Immunohistochemical analysis of
NF-kappaB signaling proteins IKK epsilon, p50/p105, p52/p100 and
RelA in prostate cancers. APMIS. 117:623–628. 2009. View Article : Google Scholar
|
|
28
|
Palvimo JJ, Reinikainen P, Ikonen T,
Kallio PJ, Moilanen A and Jänne OA: Mutual transcriptional
interference between RelA and androgen receptor. J Biol Chem.
271:24151–24156. 1996. View Article : Google Scholar
|
|
29
|
Nelius T, Filleur S, Yemelyanov A,
Budunova I, Shroff E, Mirochnik Y, Aurora A, Veliceasa D, Xiao W,
Wang Z and Volpert OV: Androgen receptor targets NFkappaB and TSP1
to suppress prostate tumor growth in vivo. Int J Cancer.
121:999–1008. 2007. View Article : Google Scholar :
|
|
30
|
Hunter JE, Leslie J and Perkins ND: c-Rel
and its manyroles in cancer: An oldstory with new twists. Br J
Cancer. 114:1–6. 2016. View Article : Google Scholar :
|
|
31
|
Weichert W, Boehm M, Gekeler V, Bahra M,
Langrehr J, Neuhaus P, Denkert C, Imre G, Weller C, Hofmann HP, et
al: Highexpression of RelA/p65 is associated with activation of
nuclear factor-kappaB-dependent signaling in pancreatic cancer and
marks a patient population with poor prognosis. Br J Cancer.
97:523–530. 2007. View Article : Google Scholar :
|
|
32
|
Sarkar DK, Jana D, Patil PS, Chaudhari KS,
Chattopadhyay BK, Chikkala BR, Mandal S and Chowdhary P: Role of
NF-κB as a prognostic marker in breast cancer: A pilot study in
Indian patients. Indian J Surg Oncol. 4:242–247. 2013. View Article : Google Scholar :
|
|
33
|
Mukhopadhyay NK, Ferdinand AS,
Mukhopadhyay L, Cinar B, Lutchman M, Richie JP, Freeman MR and Liu
BC: Unraveling androgen receptor interactomes by an array-based
method: discovery of proto-oncoprotein c-Rel as a negative
regulator of androgen receptor. Exp Cell Res. 312:3782–3795. 2006.
View Article : Google Scholar
|

















