Introduction
Diabetic nephropathy (DN) is a frequently occurring
type of progressive kidney disease that develops in the diabetic
population. Reactive oxygen species (ROS) have been revealed as
important signaling molecules that mediate renal injury in patients
with diabetes (1). High glucose
(HG) increases intracellular ROS levels in renal cells and
contributes to the development and progression of diabetic renal
injury (2,3). Under normal physiological conditions,
low levels of ROS are produced by the nicotinamide adenine
dinucleotide phosphate-oxidase (NADPH) oxidase (Nox) family as
byproducts of the mitochondrial electron transport chain, and are
important in the regulation of various cellular functions including
inflammatory gene expression, proliferation and apoptosis (4). The Nox family are membrane bound
enzymatic complexes and structural homologues of phagocytic Nox
(gp91phox/Nox2) and are categorized as follows:
Nox1-Nox5 and Dual oxidase Duox proteins 1 and 2 (5). All Nox subunits have been reported to
bind ≥1 regulatory subunits, including p22phox which is
localized in the membrane, cytosolic submits p40phox,
p47phox, p67phox and Ras-related C3 botulinum
toxin substrate (Rac) GTPases (5).
Various studies have demonstrated that the translocation and
binding of p47phox to Nox and the participation of Rac
are key steps in the activation of Nox and the generation of ROS
(6). Nox4 is a critical
ROS-generating complex, expressed in the kidney (5,6).
It has previously been demonstrated that the Notch
pathway is involved in the occurrence of DN and various other
kidney diseases (7). In mammals,
the Notch pathway consists of four receptors (Notch1-4) and five
ligands, termed Jagged1, Jagged2, Delta-like ligand (Dll) 1, Dll3
and Dll4. The interaction of these ligands with the Notch receptors
results in receptor conformational changes, subsequent γ-secretase
mediated proteolysis and release of Notch intracellular domain
(NICD), which on transfer to the nucleus, activates gene
transcription of Hes family basic helix-loop-helix (BHLH)
transcription factor (Hes) 1 and Hes related family BHLH
transcription factor with YRPW motif 1 (8). The Notch signaling pathway is
important in differential gene expression and influences cell
differentiation, proliferation and apoptosis (9,10).
Yan et al (11) demonstrated that transforming growth
factor-β (TGF-β) triggers apoptosis in human cultured endothelial
cells, an effect dependent on the overexpression of Nox4 and
production of ROS via modulation of p38 and Notch pathways.
However, it has not yet been revealed whether Nox4 regulates renal
tubular epithelial cell apoptosis via the Notch pathway in DN.
Previous studies have demonstrated that HG affects normal physical
metabolism and function of tubular cells, inducing cell apoptosis
(12). In the present study, the
HG-induced human renal proximal tubular cell line (HKC) was
selected to detect and evaluate the functional activity of Nox4,
the Notch signaling pathway and cell apoptotic rate under HG
conditions. In addition, Nox4 and the Notch pathway were chemically
inhibited in order to further explore the mechanism of tubular cell
apoptosis.
Materials and methods
Cell culture
HKC cells were obtained from the cell resource
center at Peking Union Medical College (Beijing, China) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C in an environment containing 5% CO2. HKC cells were
grown to 70% confluence and synchronized in serum-free DMEM for 24
h, then stimulated with normal glucose (NG; 5.5 mmol/l D-Glucose;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), HG (30 mmol/l
D-Glucose), HG plus γ-secretase inhibitor (DAPT; 1 µmol/l), HG plus
N-acetylcysteine (NAC; 5 mmol/l) or HG plus diphenylene iodonium
(DPI; 5 µmol/l) all from Sigma-Aldrich; Merck KGaA, at the
indicated time points.
Western blotting
The cells were washed with phosphate-buffered saline
(PBS) and lysed for 40 min at 4°C with lysis buffer (20 mmol/l HCl,
2.5 mmol/l EDTA, 10% glycerol, 0.1% SDS, 1% Triton X-100, 1% sodium
deoxycholate, 10 mmol/l sodium pyrophosphate, 50 mmol/l NaF, 1
mmol/l sodium vanadate, 1 mmol/l phenylmethylsulfonyl fluoride).
Subsequently, the homogenate was centrifuged at 14,000 × g for 20
min at 4°C. The protein concentration was measured using a Bradford
assay. Equal amounts of extracted protein samples (40 µg) were
separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes. The membranes were blocked with 5% dry milk for
1 h at 37°C and incubated with rabbit anti-Nox4 (14347–1-AP; 1:400
dilution; ProteinTech Group, Inc., Chicago, IL, USA), anti-Notch1
(20687–1-AP; 1:1,000 dilution; ProteinTech Group, Inc.), anti-Notch
intracellular domain 1 (NICD1; 4147; 1:1,000 dilution; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-phosphorylated
(p)-Rac1 (2461; 1:1,000 dilution; Cell Signaling Technology, Inc.),
anti-Rac1 (36,742; 1:2,000 dilution; Signalway Antibody LLC,
College Park, Maryland, USA), anti-B-cell lymphoma 2 apoptosis
regulator (Bcl-2; 4223; 1:1,000 dilution; Cell Signaling
Technology, Inc.), anti-cleaved caspase-3 (9664; 1:1,000 dilution;
Cell Signaling Technology, Inc.), anti-Bcl-2 associated protein X
apoptosis regulator (Bax; 2772; 1:1,000 dilution; Cell Signaling
Technology, Inc.) and anti-β-actin (sc-130656; 1:1,000 dilution;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) polyclonal
antibodies at 4°C overnight. The membranes were then washed and
incubated with a goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibody (SA00001-2; 1:5,000
dilution; ProteinTech Group, Inc.) for 2 h at room temperature. The
labeled bands were quantified using a UVP Image Station Lab works
4.5 (UVP Inc., Upland, CA, USA) and compared with β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed using oligo (dT) primer (Sangon Biotech Co., Ltd.,
Shanghai, China) in the presence of the avian myeloblastosis virus
reverse transcriptase (Takara Biotechnology Co., Ltd., Dalian,
China), to produce cDNA. cDNA was amplified on the 7900HT Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with SYBR Premix EX Taq™ kit (Takara Biotechnology
Co., Ltd.) at default thermal cycling conditions: 2 min at 50°C, 10
min at 95°C and then 40 cycles of 15 sec at 95°C for denaturation
and 1 min at 60°C for annealing and extension. Results were
quantified using the relative standard curve method of
analysis/ΔCq method of analysis (13), relative to 18S rRNA. The
oligonucleotide primer sequences were as follows: 18S, forward
5′-CGCCGCTAGAGGTGAAATTC-3′ and reverse 5′-CCAGTCGGCATCGTTTATGG-3′;
Nox4, forward 5′-GTTGGGGCTAGGATTGTGTCT-3′ and reverse
5′-TCGGCACATGGGTAAAAGGA-3′; and Notch1, forward
5′-CTAAGATCTCCTGAGGGCTTCAAAGTGTC-3′ and reverse
5′-GCGAATTCCTTGAAGGCCTCCGGAT-3′.
Intracellular ROS detection
Total ROS levels were detected using the
fluorescence probe 5-(and 6) chloromethyl-2,
7-dichlorodihydrofluorescein diacetate (CM-DCHF-DA; Invitrogen;
Thermo Fisher Scientific, Inc.). HKC cells were seeded into 6-well
plates at a density of 1×106 cells/ml and incubated
under different experimental conditions for 24 h. The cells were
washed with PBS three times, trypsinized and centrifuged at 300 × g
for 5 min at 4°C. The cells were then resuspended and incubated in
pre-warmed PBS with 10 µM DCHF-DA at 37°C for 30 min in the dark.
Subsequently, cells were washed with PBS three times to remove the
free DCFH-DA and fixed with 1% paraformaldehyde for 10 min at 4°C.
The levels of intracellular ROS were quantified using a flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with FlowJo
software version 7.6 (FlowJo LLC, Ashland, OR, USA).
Annexin V/propidium iodide (PI)
staining assay
Apoptotic rates in the differing groups were
detected using an Annexin V/PI staining kit (MultiSciences Biotech
Co., Ltd., Hangzhou, China) according to the manufacturer's
protocol. HKC cells were incubated under different experimental
conditions for 24 h, and then washed with PBS three times,
trypsinized and centrifuged at 300 × g for 5 min at 4°C. Cells were
resuspended in 1X binding buffer, incubated with Annexin V-FITC and
PI at room temperature for 5 min in the dark. Following fixation
with 1% paraformaldehyde for 10 min at 4°C, HKC cells analyzed
using a flow cytometer (Epics-XLII; Beckman Coulter, Inc., Brea,
CA, USA) with FlowJo software version 7.6 (FlowJo LLC).
Statistical analysis
Data are presented as the mean + standard deviation
of at least three independent experiments. All data were analyzed
using SPSS software version 15.0 (SPSS, Inc., Chicago, IL, USA).
Differences between groups were analyzed using one-way analysis of
variance followed by a post hoc Bonferroni's test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
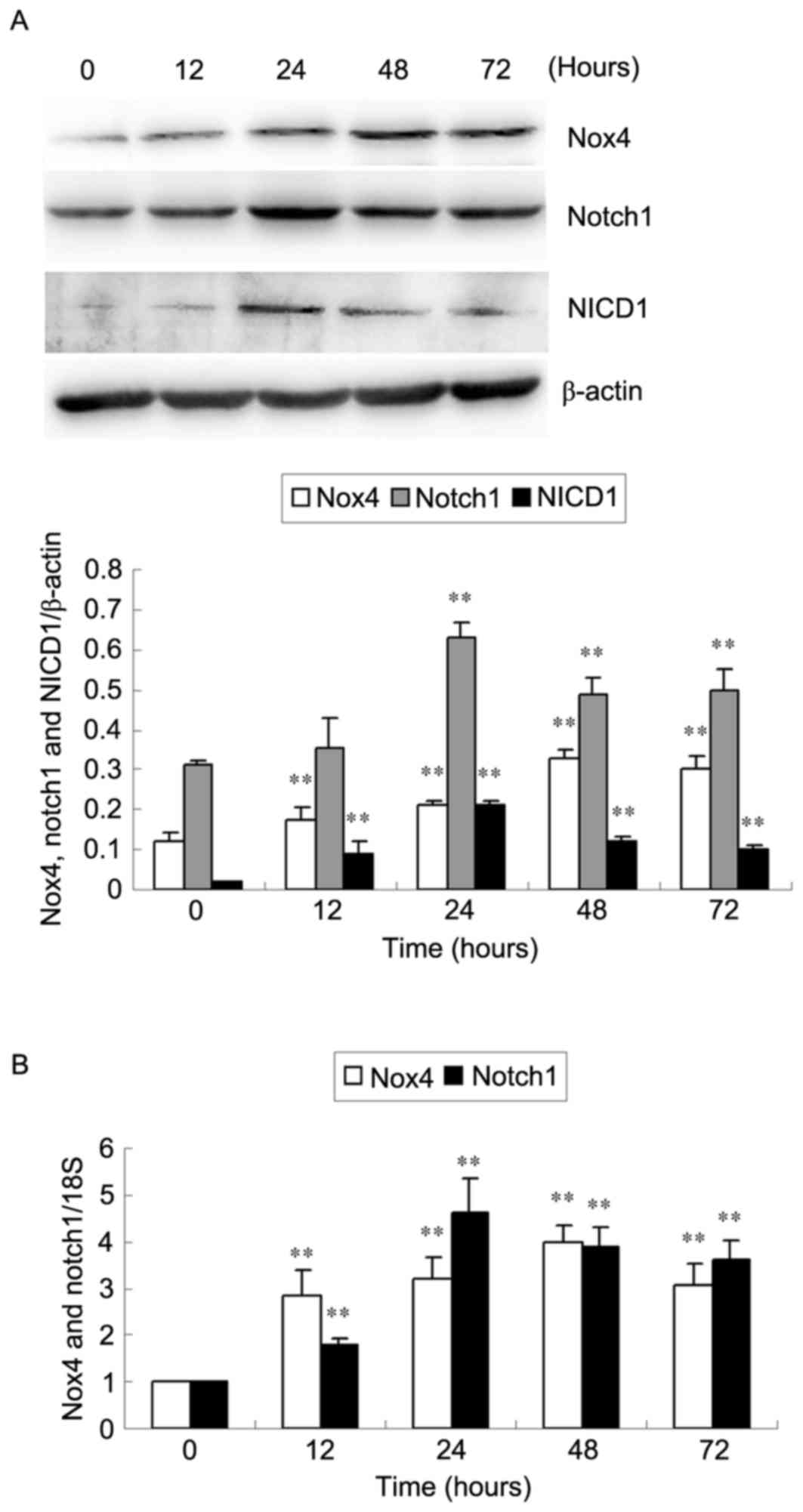
HG induces Nox4 and Notch pathway
expression in HKC cells
Western blotting and RT-qPCR analyses were used to
examine HG-induced Nox4 and Notch1 protein and mRNA expression
levels in HKC cells (Fig. 1). Nox4
protein and mRNA expression increased as early as 12 h, reached a
peak at 48 h and decreased at 72 h (P<0.01; Fig. 1). Notch1 protein and mRNA
expression significantly increased at 24 h, then decreased at 48
and 72 h (P<0.01; Fig 1). NICD1
protein expression levels reached a peak at 24 h compared with 0 h
following stimulation with HG (P<0.01; Fig. 1A). Furthermore, no significant
differences in Nox4, Notch1 and NICD1 expression were observed in
HKC cells cultured under NG conditions among different time points
(all P>0.05; data not shown).
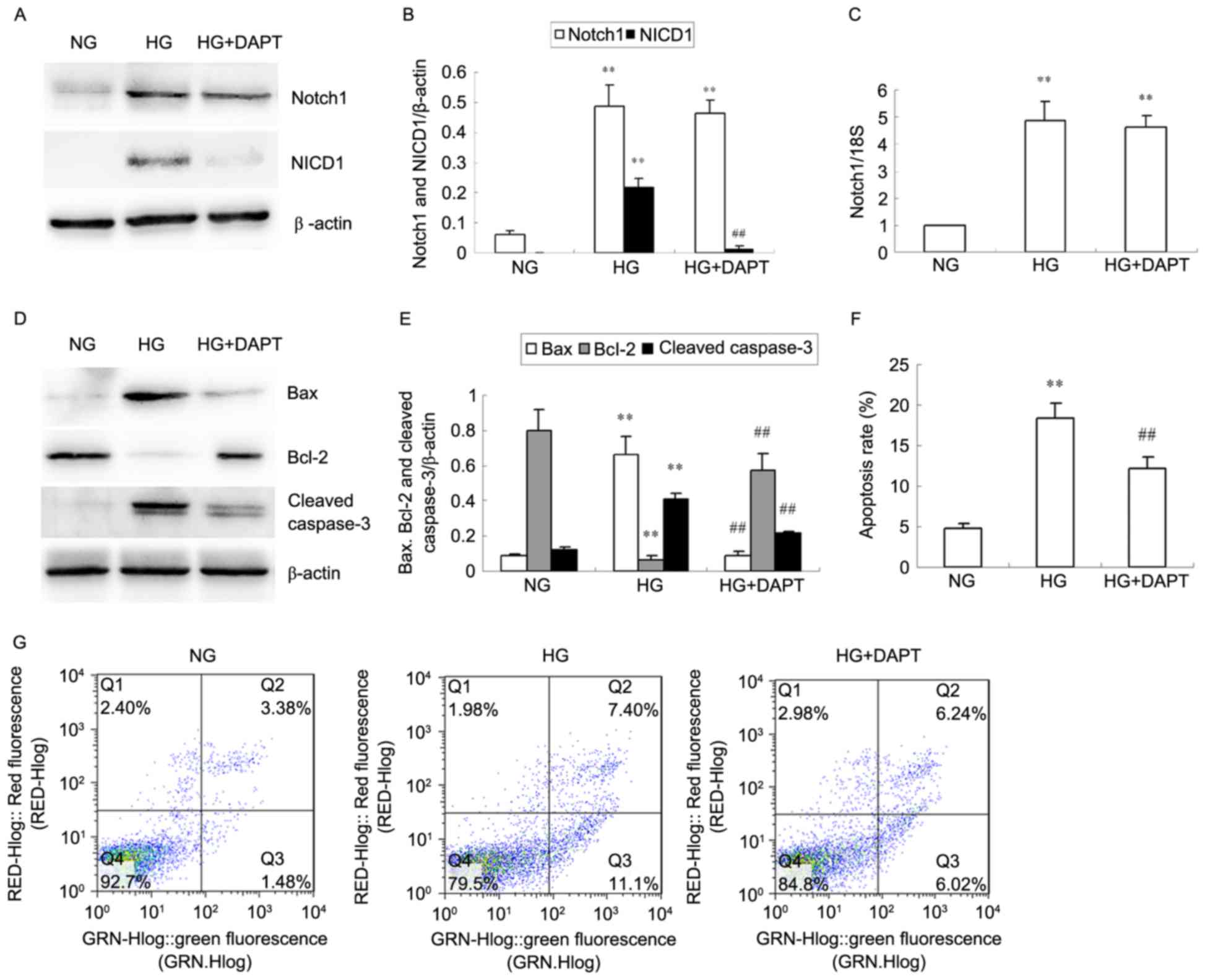
DAPT inhibits Notch pathway expression
and cell apoptosis in HG-induced HKC cells
A significant increase in NICD1 protein expression
was observed in HKC cells stimulated with HG for 24 h when compared
with NG and this was then decreased with addition of DAPT
(P<0.01; Fig. 2A and B). Notch1
protein (Fig. 2A and B) and mRNA
(Fig. 2C) expression was also
significantly increased in HKC cells stimulated with HG for 24 h
compared with NG (P<0.01; Fig.
2A-C), however, DAPT did not inhibit Notch1 protein and mRNA
overexpression induced by HG (P>0.05; Fig. 2A-C). HG significantly increased Bax
and cleaved caspase-3 protein levels in HKC cells compared with NG,
and simultaneously decreased Bcl-2 protein expression (P<0.01;
Fig. 2D and E). Treatment with
DAPT reversed the alterations in Bax, Bcl-2 and cleaved caspase-3
protein levels in HG-induced HKC cells (P<0.01; Fig. 2D and E). Furthermore, the increased
apoptosis rate in HG HKC cells compared with NG cells (P<0.01;
Fig. 2F and G) was reduced by
treated with DAPT, as demonstrated by flow cytometry (P<0.01 vs.
HG; Fig. 2F and G).
NAC and DPI inhibit Nox4 and Notch
pathway expression and ROS generation in HG-induced HKC cells
Western blotting was used to examine HG-induced
protein expression of Nox4, p-Rac1, Rac1, Notch1 and NICD1 in HKC
cells after 24 h (Fig. 3A-C). HG
significantly increased Nox4, Notch1 and NICD1 protein expression
levels compared with NG (P<0.01; Fig. 3A and B), but this increase was
inhibited by NAC and DPI (P<0.05 or P<0.01; Fig. 3A and B). HG significantly increased
the ratio of p-Rac1/Rac1 compared with NG (P<0.01; Fig. 3A and C), but while this effect was
inhibited by DPI (P<0.01 vs. HG; Fig. 3A and C), no effect was observed
with NAC (P>0.05 vs. HG; Fig. 3A
and C). No alteration of total Rac1 expression was observed in
the cultured HKC cells of different groups (Fig. 3A). RT-qPCR analysis revealed that
mRNA expression of Nox4 and Notch1 in the HG-induced HKC cells
significantly increased compared with NG (P<0.01; Fig. 3D). Nox4 and Notch1 mRNA levels
significantly decreased compared with HG in cells cultured with NAC
or DPI in HG culture medium (P<0.05 or P<0.01; Fig. 3D). Intracellular ROS levels were
observed to be increased in the HG group compared with the NG group
(P<0.01; Fig. 3E), and this
HG-induced ROS production was significantly suppressed by addition
of NAC or DPI (P<0.01 vs. HG; Fig.
3E).
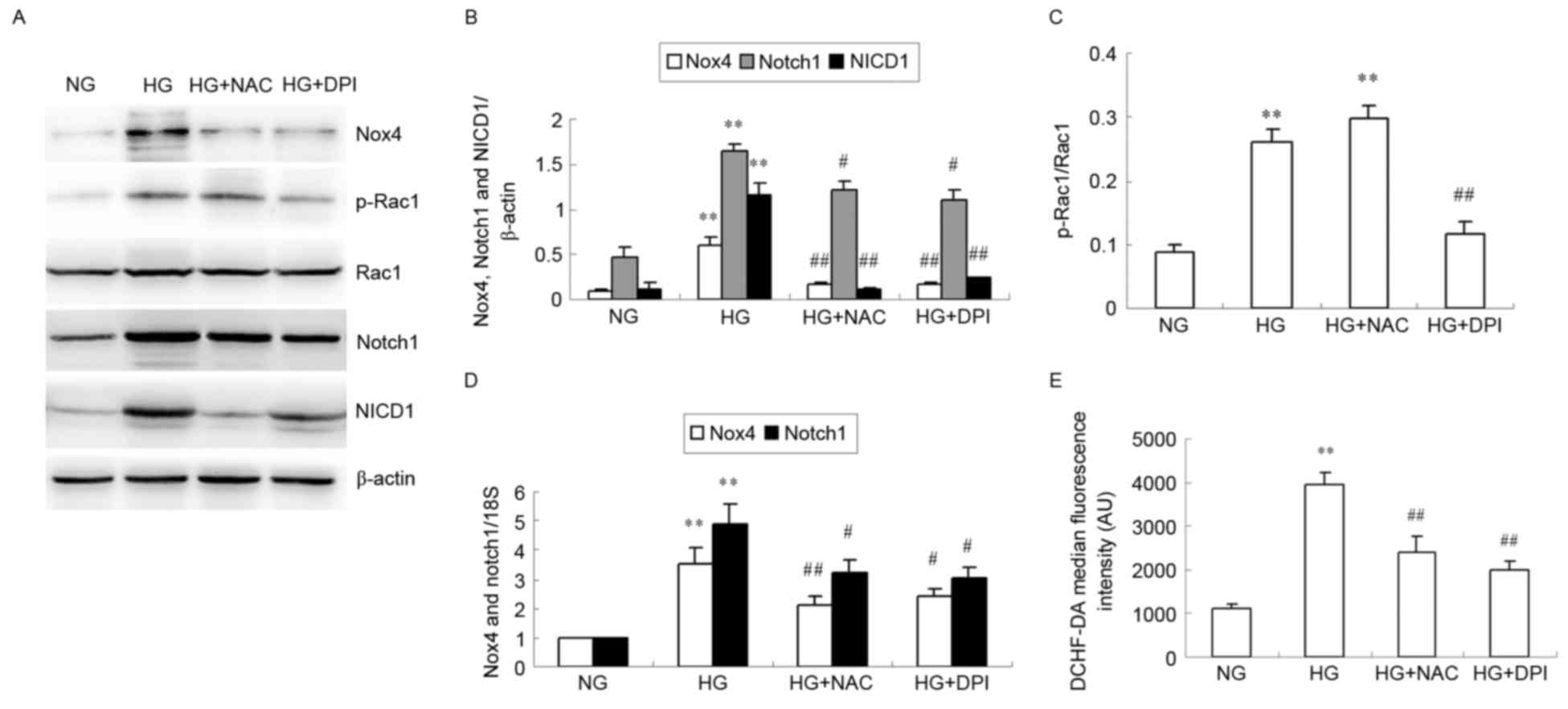 | Figure 3.Effects of NAC and DPI on HG-induced
Nox4 and Notch pathway expression and ROS generation in HKC cells.
HKC cells were incubated with NG, HG, HG + NAC or HG + DPI for 24
h. (A) Representative image of Nox4, p-Rac1, Rac1, Notch1 and NICD1
protein expression levels analyzed by western blotting.
Quantification of (B) Nox4, Notch1, NICD1 relative to β-actin and
(C) relative p-Rac1/Rac1 expression levels. (D) Nox4 and Notch1
mRNA expression was analyzed by reverse transcription-quantitative
polymerase chain reaction relative to 18S rRNA. (E) Quantitative
analysis of DCHF-DA fluorescence intensity using flow cytometry.
Data were expressed as the mean + standard deviation. **P<0.01
vs. NG. #P<0.05 and ##P<0.01 vs. HG.
NAC, N-acetylcysteine; DPI, diphenylene iodonium; HG, high glucose;
Nox 4, nicotinamide adenine dinucleotide phosphate-oxidase (NADPH)
oxidase 4; ROS, reactive oxygen species; NG, normal glucose; p,
phosphorylated; Rac 1, Ras-related C3 botulinum toxin substrate 1;
NICD1, Notch intracellular domain 1; DCHF-DA,
dichlorodihydrofluorescein diacetate. |
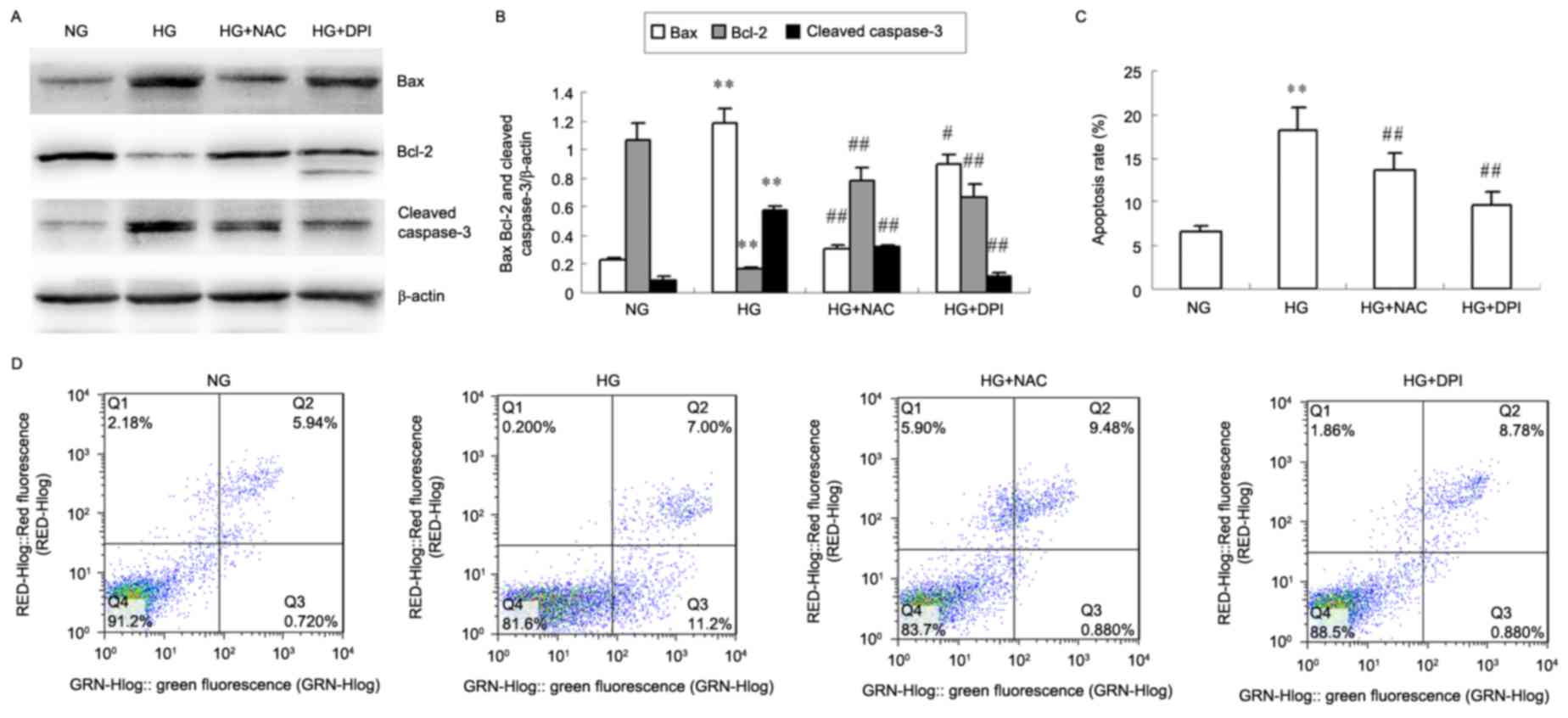
NAC and DPI inhibit HG-induced HKC
cell apoptosis
The protein levels of Bax and cleaved caspase-3
decreased in HKC cells treated with NAC and DPI compared with
levels present in the HG group, whereas Bcl-2 protein levels
increased (P<0.05 or P<0.01; Fig. 4A and B). HG-induced HKC cells
exhibited a significantly increased apoptosis rate after 24 h
compared with the NG cells treated (P<0.01; Fig. 4C and D), whereas NAC and DPI
efficiently inhibited HG-induced HKC cell apoptosis (P<0.01 vs.
HG; Fig. 4C and D).
Discussion
The results of the present study demonstrated that
Nox4 overexpression in HG-induced HKC cells altered Notch pathway
levels and induced cell apoptosis. Nox4 and Notch pathway
inhibitors prevented HG-induced HKC cell apoptosis.
Nox4 is a constitutively active multisubunit enzyme,
which acts as an oxygen sensor and generates various ROS from
molecular oxygen using NADPH as the electron donor. Various studies
have reported that Nox4 is the major source of ROS in kidney
disease including DN, and Nox4-derived ROS are considered to
mediate renal hypertrophy, increase myofibroblast activation and
renal fibrosis (6,14). Additionally, ROS have been revealed
to increase in the presence of HG via activation of NADPH oxidases
(15,16). The present study demonstrated that
Nox4 protein and mRNA expression increased as early as 12 h and
reached a peak at 48 h following stimulation with HG. HG notably
increased the levels of Nox4 and Rac1 phosphorylation, which were
inhibited by the antioxidant NAC and Nox inhibitor DPI.
Hyperglycemia in renal proximal tubule cells may activate Nox and
produce superoxide anion and hydrogen peroxide, which may be
reversed by treatment with DPI, resulting in an increase in TGF-β1
secretion and the activation of the nuclear factor (NF)-κB signal
transduction pathway (17). It has
additionally been demonstrated that Angiotensin II-induced
activation of mitochondrial Nox4 is an important endogenous source
of ROS and is associated with cell survival in kidney tubular cells
(18).
The Notch pathway is an evolutionarily conserved
signaling pathway, which participates in a variety of cellular
processes and is important in kidney development (19). Cheng et al (20) observed Notch1 activation in the
comma-shaped and S-shaped bodies during kidney development.
Following inhibition of the Notch pathway, fewer renal epithelial
structures are observed, with a severe deficiency in proximal
tubules and podocytes, accompanied by an increase in intervening,
nonepithelial cells. Notch expression is enhanced in the tubule
cells of fibrotic kidneys from diabetic mice and humans and Notch
interacting proteins have been identified that may be pertinent in
normal and pathological functioning (21). The present study additionally
demonstrated that HG activated the Notch pathway in a
time-dependent manner in HKC cells and the maximum expression of
Notch1 and NICD1 was observed at 24 h. HG increases Notch1
expression and releases NICD1, which travels into the nucleus and
activates the downstream genes in HKC cells (22,23).
DAPT, a Notch pathway inhibitor, suppresses activation of the Notch
pathway by HG, which may inhibit γ-secretase-mediated proteolytic
cleavage of Notch, reducing the release of the NICD from the plasma
membrane into the nucleus (10).
DAPT inhibited NICD1 expression and did not reduce Notch1 protein
and mRNA overexpression in HG-induced HKC cells. DAPT additionally
reduced the ratio of Bax/Bcl-2 and cleaved caspase-3 expression in
HG-induced HKC cells. Flow cytometry demonstrated that HG induced
HKC cell apoptosis, which was subsequently inhibited by treatment
with DAPT. These results indicated that HG induced HKC cell
apoptosis through activation of the Notch pathway. Notch pathway
activity has additionally been revealed to participate in puromycin
aminonucleoside-induced renal proximal tubular cell apoptosis via
caspase-3 (24). The Notch
signaling pathway is important in renal ischemia/reperfusion (I/R),
inducing severe tubular damage and resulting in increased NF-κB,
Bax and tubular cell apoptosis and reduced Bcl-2 expression
(25).
The present study then used the chemical inhibitors
NAC and DPI to investigate if Nox4 mediated HKC cell apoptosis via
regulation of the Notch pathway in HG medium. HG notably increased
levels of Nox4 and Rac1 phosphorylation, which were inhibited by
NAC and DPI. Similarly, NAC and DPI inhibited production of ROS and
activation of the Notch pathway in HG-induced HKC cells. Treatment
with NAC and DPI decreased the ratio of Bax:Bcl-2, reduced cleaved
caspase-3 levels and HKC cell apoptosis following HG stimulation.
Therefore, HG-induced Nox4 overexpression in HKC cells induced cell
apoptosis via activation of the Notch pathway. Similarly, it was
previously demonstrated that Rac1 regulates Notch in mediating
cerebral I/R-induced production of injurious ROS and cell death
in vitro and in vivo (26). HKC cell apoptosis may lead to a
decrease in the number of renal tubular epithelial cells, resulting
in renal interstitial fibrosis and the development of DN (27). Inhibition of Nox4 may therefore
exhibit the potential to treat DN.
In conclusion, the present results indicated that HG
caused the overexpression of Nox4 and Notch signaling molecules in
HKC cells. Nox4 upregulation may serve an important role in
HG-induced HKC cell apoptosis, through the activation of the Notch
pathway. In addition, the present study demonstrated that the
blockade of Nox4 using a chemical inhibitor suppressed HG-induced
HKC cell apoptosis. Since Nox4 is involved in renal tubular
epithelial cell injury in DN, targeting Nox4 may have potential as
an alternative therapeutic strategy for the treatment of patients
with DN. Further studies are required to explore the molecular
mechanisms underlying the involvement of Nox4 in the development of
DN, including the relations between Nox4 and other signaling
pathways, such as the p38 pathway.
Acknowledgements
The present study was supported by the Hebei Natural
Science Foundation of China (grant no. H2014206294).
References
|
1
|
Shah A, Xia L, Masson EA, Gui C, Momen A,
Shikatani EA, Husain M, Quaggin S, John R and Fantus IG:
Thioredoxin-interacting protein deficiency protects against
diabetic nephropathy. J Am Soc Nephrol. 26:2963–2977. 2015.
View Article : Google Scholar :
|
|
2
|
Al-Kafaji G, Sabry MA and Skrypnyk C:
Time-course effect of high-glucose-induced reactive oxygen species
on mitochondrial biogenesis and function in human renal mesangial
cells. Cell Biol Int. 40:36–48. 2016. View Article : Google Scholar
|
|
3
|
Hou Y, Wu M, Wei J, Ren Y, Du C, Wu H, Li
Y and Shi Y: CD36 is involved in high glucose-induced epithelial to
mesenchymal transition in renal tubular epithelial cells. Biochem
Biophys Res Commun. 468:281–286. 2015. View Article : Google Scholar
|
|
4
|
Brown DI and Griendling KK: Nox proteins
in signal transduction. Free Radic Biol Med. 47:1239–1253. 2009.
View Article : Google Scholar :
|
|
5
|
Holterman CE, Read NC and Kennedy CR: Nox
and renal disease. Clin Sci (Lond). 128:465–481. 2015. View Article : Google Scholar
|
|
6
|
Krause KH: Tissue distribution and
putative physiological function of NOX family NADPH oxidases. Jpn J
Infect Dis. 57:S28–S29. 2004.
|
|
7
|
Juillerat-Jeanneret L, Flohr A, Schneider
M, Walter I, Wyss JC, Kumar R, Golshayan D and Aebi JD: Targeted
γ-secretase inhibition to control the Notch pathway in renal
diseases. J Med Chem. 58:8097–8109. 2015. View Article : Google Scholar
|
|
8
|
Mertens PR, Raffetseder U and Rauen T:
Notch receptors: A new target in glomerular diseases. Nephrol Dial
Transplant. 23:2743–2745. 2008. View Article : Google Scholar
|
|
9
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar
|
|
10
|
McCright B: Notch signaling in kidney
development. Curr Opin Nephrol Hypertens. 12:5–10. 2003. View Article : Google Scholar
|
|
11
|
Yan F, Wang Y, Wu X, Peshavariya HM,
Dusting GJ, Zhang M and Jiang F: Nox4 and redox signaling mediate
TGF-β-induced endothelial cell apoptosis and phenotypic switch.
Cell Death Dis. 5:e10102014. View Article : Google Scholar :
|
|
12
|
Dang J, Jia R, Tu Y, Xiao S and Ding G:
Erythropoietin prevents reactive oxygen species generation and
renal tubular cell apoptosis at high glucose level. Biomed
Pharmacother. 64:681–685. 2010. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Bondi CD, Manickam N, Lee DY, Block K,
Gorin Y, Abboud HE and Barnes JL: NAD(P)H oxidase mediates
TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc
Nephrol. 21:93–102. 2010. View Article : Google Scholar :
|
|
15
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar :
|
|
16
|
Eid AA, Gorin Y, Fagg BM, Maalouf R,
Barnes JL, Block K and Abboud HE: Mechanisms of podocyte injury in
diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes.
58:1201–1211. 2009. View Article : Google Scholar :
|
|
17
|
Han HJ, Lee YJ, Park SH, Lee JH and Taub
M: High glucose-induced oxidative stress inhibits Na+/glucose
cotransporter activity in renal proximal tubule cells. Am J Physiol
Renal Physiol. 288:F988–F996. 2005. View Article : Google Scholar
|
|
18
|
Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW,
Ihm CG and Moon JY: Angiotensin II-induced mitochondrial Nox4 is a
major endogenous source of oxidative stress in kidney tubular
cells. PLoS One. 7:e397392012. View Article : Google Scholar :
|
|
19
|
Cheng HT, Kim M, Valerius MT, Surendran K,
Schuster-Gossler K, Gossler A, McMahon AP and Kopan R: Notch2, but
not Notch1, is required for proximal fate acquisition in the
mammalian nephron. Development. 134:801–811. 2007. View Article : Google Scholar :
|
|
20
|
Cheng HT and Kopan R: The role of Notch
signaling in specification of podocyte and proximal tubules within
the developing mouse kidney. Kidney Int. 68:1951–1952. 2005.
View Article : Google Scholar
|
|
21
|
Cummins TD, Mendenhall MD, Lowry MN, Korte
EA, Barati MT, Khundmiri SJ, Salyer SA, Klein JB and Powell DW:
Elongin C is a mediator of Notch4 activity in human renal tubule
cells. Biochim Biophys Acta. 1814:1748–1757. 2011. View Article : Google Scholar :
|
|
22
|
Graziani I, Eliasz S, De Marco MA, Chen Y,
Pass HI, De May RM, Strack PR, Miele L and Bocchetta M: Opposite
effects of Notch-1 and Notch-2 on mesothelioma cell survival under
hypoxia are exerted through the Akt pathway. Cancer Res.
68:9678–9685. 2008. View Article : Google Scholar
|
|
23
|
Guo H, Lu Y, Wang J, Liu X, Keller ET, Liu
Q, Zhou Q and Zhang J: Targeting the Notch signaling pathway in
cancer therapeutics. Thorac Cancer. 5:473–486. 2014. View Article : Google Scholar :
|
|
24
|
Ding X, Zhu F, Li T, Zhou Q, Hou FF and
Nie J: Numb protects renal proximal tubular cells from puromycin
aminonucleoside-induced apoptosis through inhibiting Notch
signaling pathway. Int J Biol Sci. 7:269–278. 2011. View Article : Google Scholar :
|
|
25
|
Huang R, Zhou Q, Veeraragoo P, Yu H and
Xiao Z: Notch2/Hes-1 pathway plays an important role in renal
ischemia and reperfusion injury-associated inflammation and
apoptosis and the γ-secretase inhibitor DAPT has anephroprotective
effect. Ren Fail. 33:207–216. 2011. View Article : Google Scholar
|
|
26
|
Meng S, Su Z, Liu Z, Wang N and Wang Z:
Rac1 contributes to cerebral ischemia reperfusion-induced injury in
mice by regulation of Notch2. Neuroscience. 306:100–114. 2015.
View Article : Google Scholar
|
|
27
|
Habib SL: Diabetes and renal tubular cell
apoptosis. World J Diabetes. 4:27–30. 2013. View Article : Google Scholar :
|