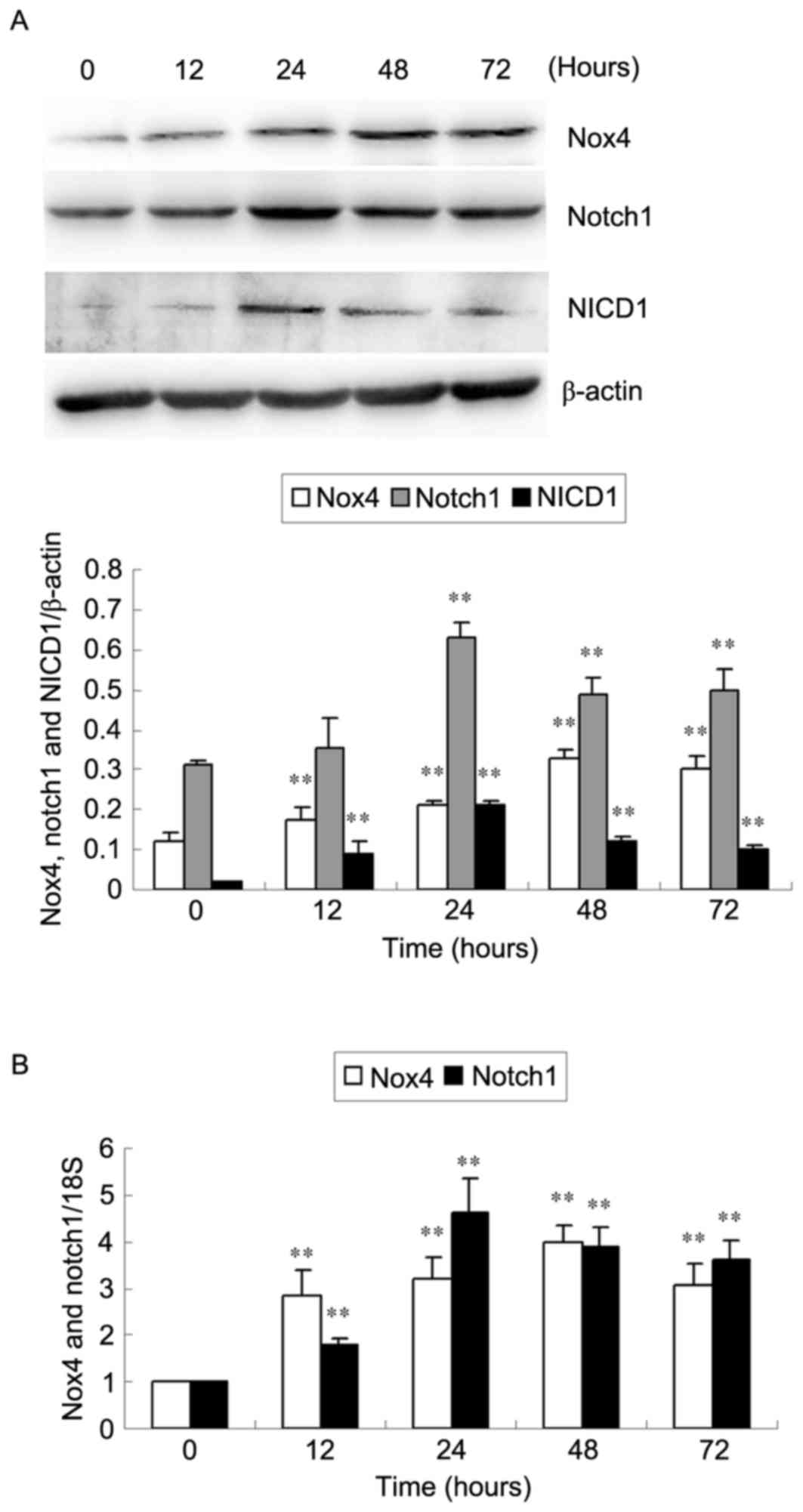
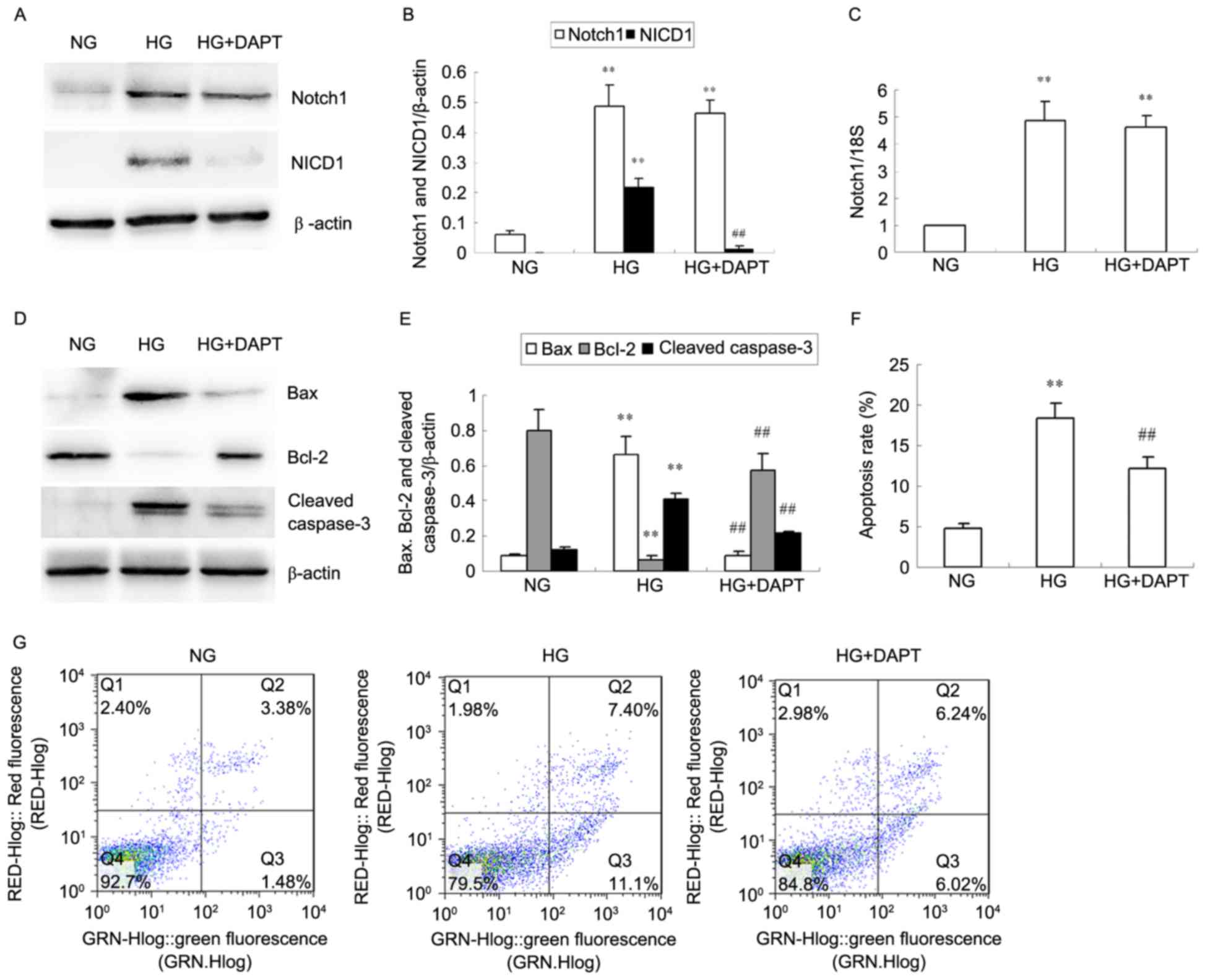
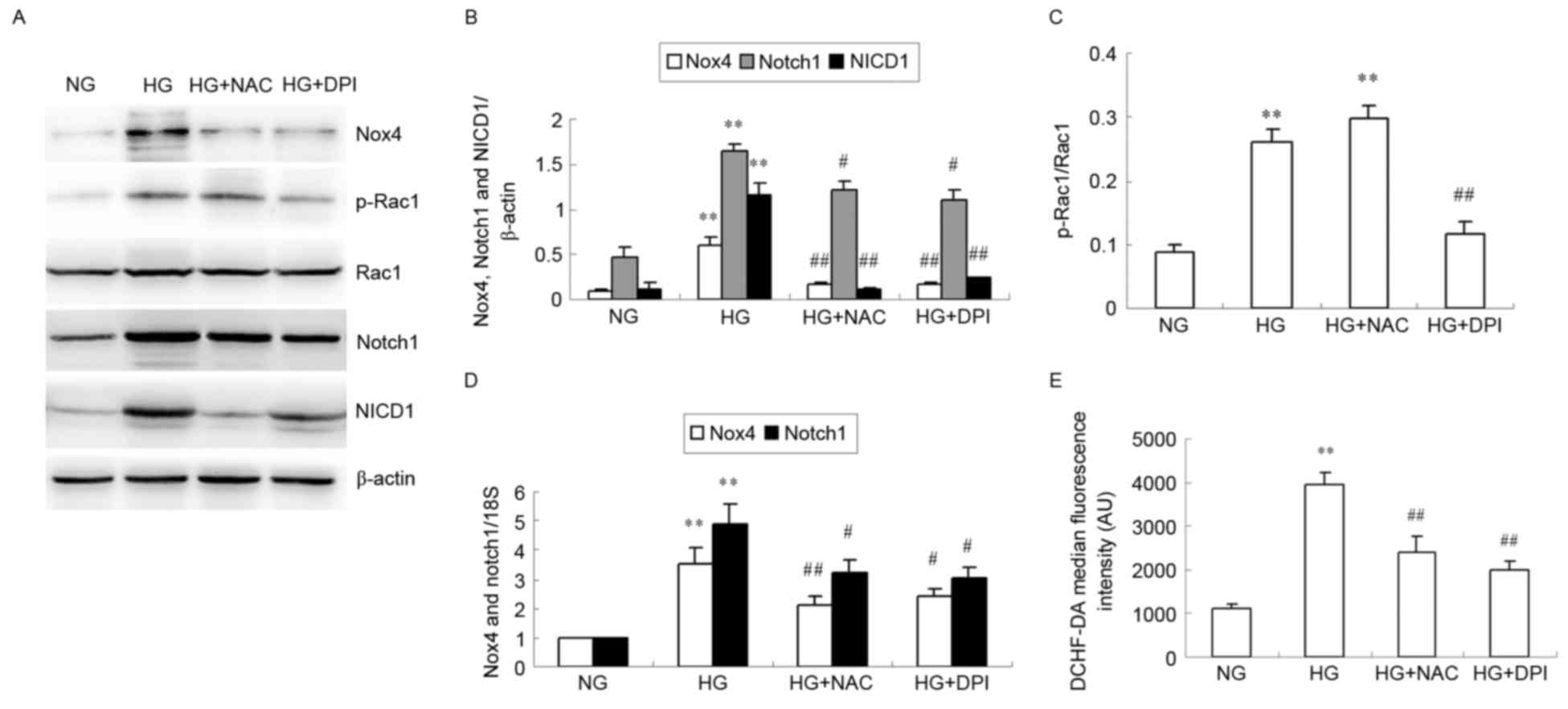
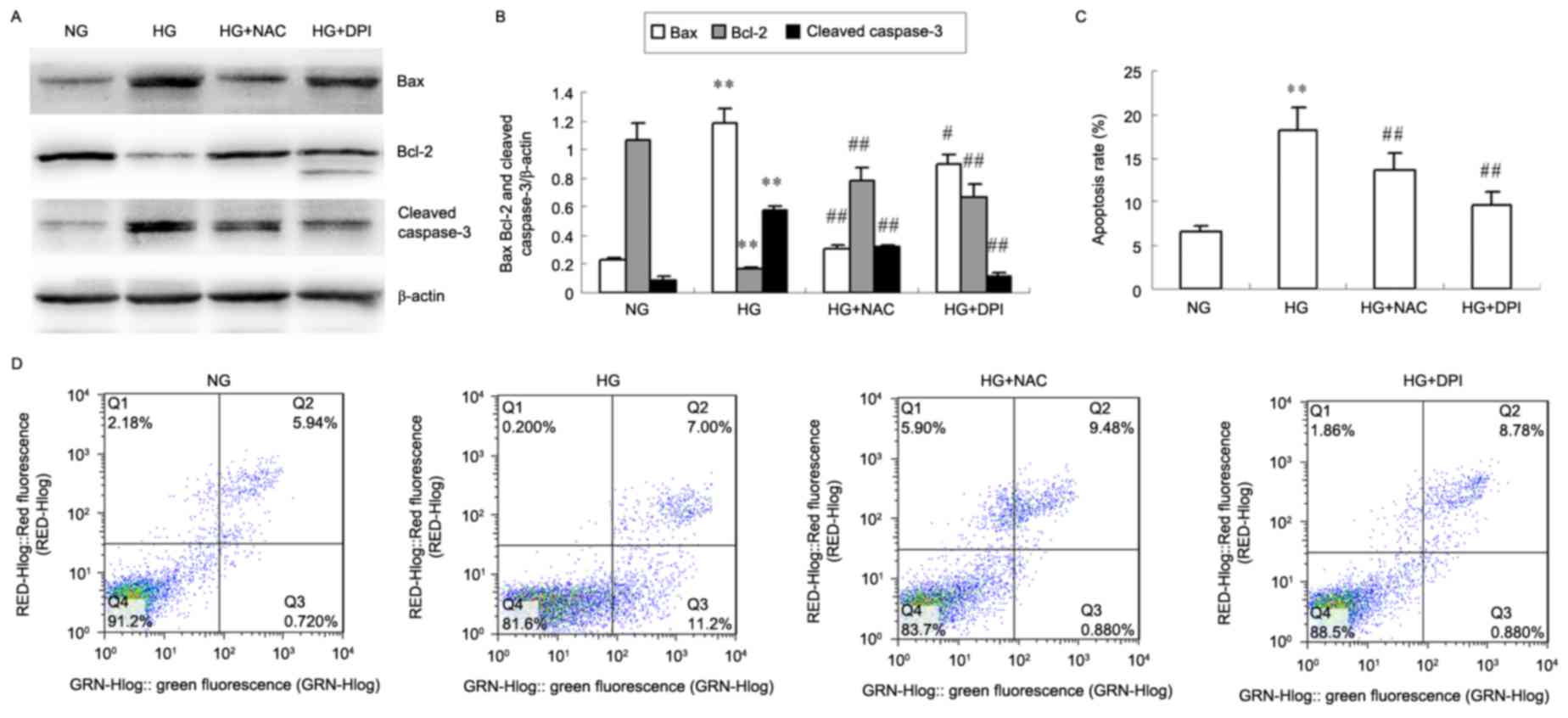
|
1
|
Shah A, Xia L, Masson EA, Gui C, Momen A,
Shikatani EA, Husain M, Quaggin S, John R and Fantus IG:
Thioredoxin-interacting protein deficiency protects against
diabetic nephropathy. J Am Soc Nephrol. 26:2963–2977. 2015.
View Article : Google Scholar :
|
|
2
|
Al-Kafaji G, Sabry MA and Skrypnyk C:
Time-course effect of high-glucose-induced reactive oxygen species
on mitochondrial biogenesis and function in human renal mesangial
cells. Cell Biol Int. 40:36–48. 2016. View Article : Google Scholar
|
|
3
|
Hou Y, Wu M, Wei J, Ren Y, Du C, Wu H, Li
Y and Shi Y: CD36 is involved in high glucose-induced epithelial to
mesenchymal transition in renal tubular epithelial cells. Biochem
Biophys Res Commun. 468:281–286. 2015. View Article : Google Scholar
|
|
4
|
Brown DI and Griendling KK: Nox proteins
in signal transduction. Free Radic Biol Med. 47:1239–1253. 2009.
View Article : Google Scholar :
|
|
5
|
Holterman CE, Read NC and Kennedy CR: Nox
and renal disease. Clin Sci (Lond). 128:465–481. 2015. View Article : Google Scholar
|
|
6
|
Krause KH: Tissue distribution and
putative physiological function of NOX family NADPH oxidases. Jpn J
Infect Dis. 57:S28–S29. 2004.
|
|
7
|
Juillerat-Jeanneret L, Flohr A, Schneider
M, Walter I, Wyss JC, Kumar R, Golshayan D and Aebi JD: Targeted
γ-secretase inhibition to control the Notch pathway in renal
diseases. J Med Chem. 58:8097–8109. 2015. View Article : Google Scholar
|
|
8
|
Mertens PR, Raffetseder U and Rauen T:
Notch receptors: A new target in glomerular diseases. Nephrol Dial
Transplant. 23:2743–2745. 2008. View Article : Google Scholar
|
|
9
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar
|
|
10
|
McCright B: Notch signaling in kidney
development. Curr Opin Nephrol Hypertens. 12:5–10. 2003. View Article : Google Scholar
|
|
11
|
Yan F, Wang Y, Wu X, Peshavariya HM,
Dusting GJ, Zhang M and Jiang F: Nox4 and redox signaling mediate
TGF-β-induced endothelial cell apoptosis and phenotypic switch.
Cell Death Dis. 5:e10102014. View Article : Google Scholar :
|
|
12
|
Dang J, Jia R, Tu Y, Xiao S and Ding G:
Erythropoietin prevents reactive oxygen species generation and
renal tubular cell apoptosis at high glucose level. Biomed
Pharmacother. 64:681–685. 2010. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Bondi CD, Manickam N, Lee DY, Block K,
Gorin Y, Abboud HE and Barnes JL: NAD(P)H oxidase mediates
TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc
Nephrol. 21:93–102. 2010. View Article : Google Scholar :
|
|
15
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar :
|
|
16
|
Eid AA, Gorin Y, Fagg BM, Maalouf R,
Barnes JL, Block K and Abboud HE: Mechanisms of podocyte injury in
diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes.
58:1201–1211. 2009. View Article : Google Scholar :
|
|
17
|
Han HJ, Lee YJ, Park SH, Lee JH and Taub
M: High glucose-induced oxidative stress inhibits Na+/glucose
cotransporter activity in renal proximal tubule cells. Am J Physiol
Renal Physiol. 288:F988–F996. 2005. View Article : Google Scholar
|
|
18
|
Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW,
Ihm CG and Moon JY: Angiotensin II-induced mitochondrial Nox4 is a
major endogenous source of oxidative stress in kidney tubular
cells. PLoS One. 7:e397392012. View Article : Google Scholar :
|
|
19
|
Cheng HT, Kim M, Valerius MT, Surendran K,
Schuster-Gossler K, Gossler A, McMahon AP and Kopan R: Notch2, but
not Notch1, is required for proximal fate acquisition in the
mammalian nephron. Development. 134:801–811. 2007. View Article : Google Scholar :
|
|
20
|
Cheng HT and Kopan R: The role of Notch
signaling in specification of podocyte and proximal tubules within
the developing mouse kidney. Kidney Int. 68:1951–1952. 2005.
View Article : Google Scholar
|
|
21
|
Cummins TD, Mendenhall MD, Lowry MN, Korte
EA, Barati MT, Khundmiri SJ, Salyer SA, Klein JB and Powell DW:
Elongin C is a mediator of Notch4 activity in human renal tubule
cells. Biochim Biophys Acta. 1814:1748–1757. 2011. View Article : Google Scholar :
|
|
22
|
Graziani I, Eliasz S, De Marco MA, Chen Y,
Pass HI, De May RM, Strack PR, Miele L and Bocchetta M: Opposite
effects of Notch-1 and Notch-2 on mesothelioma cell survival under
hypoxia are exerted through the Akt pathway. Cancer Res.
68:9678–9685. 2008. View Article : Google Scholar
|
|
23
|
Guo H, Lu Y, Wang J, Liu X, Keller ET, Liu
Q, Zhou Q and Zhang J: Targeting the Notch signaling pathway in
cancer therapeutics. Thorac Cancer. 5:473–486. 2014. View Article : Google Scholar :
|
|
24
|
Ding X, Zhu F, Li T, Zhou Q, Hou FF and
Nie J: Numb protects renal proximal tubular cells from puromycin
aminonucleoside-induced apoptosis through inhibiting Notch
signaling pathway. Int J Biol Sci. 7:269–278. 2011. View Article : Google Scholar :
|
|
25
|
Huang R, Zhou Q, Veeraragoo P, Yu H and
Xiao Z: Notch2/Hes-1 pathway plays an important role in renal
ischemia and reperfusion injury-associated inflammation and
apoptosis and the γ-secretase inhibitor DAPT has anephroprotective
effect. Ren Fail. 33:207–216. 2011. View Article : Google Scholar
|
|
26
|
Meng S, Su Z, Liu Z, Wang N and Wang Z:
Rac1 contributes to cerebral ischemia reperfusion-induced injury in
mice by regulation of Notch2. Neuroscience. 306:100–114. 2015.
View Article : Google Scholar
|
|
27
|
Habib SL: Diabetes and renal tubular cell
apoptosis. World J Diabetes. 4:27–30. 2013. View Article : Google Scholar :
|