Introduction
CD147, a member of the immunoglobulin (Ig)
superfamily (1), is a
transmembrane glycoprotein that is widely expressed in various cell
types and at a high level in human tumors (2,3); its
expression has been reported to be upregulated in a number of
cancer types (4,5). The hepatoma-associated antigen
HAb18G, which was cloned by anti-hepatoma monoclonal antibody (MAb)
HAb18 screening of a human hepatocellular carcinoma cDNA library,
has an identical nucleotide and amino acid sequence to CD147
(6,7). Previous reports suggested that CD147
may be shed from the cell membrane via matrix
metallopeptidase-dependent cleavage, which generates a soluble form
of CD147 that may contain either one N-terminal Ig-like domain or
two Ig-like domains (8,9). Additional studies demonstrated that
full-length CD147 may also be released via microvesicle shedding
(10,11). Soluble CD147 has also been
indicated as a potential marker for the detection of certain types
of cancer (12,13).
ELISA is one of the basic applications of antibodies
that is used to analyze soluble antigens (14); therefore, ELISA may be used to
detect the concentration of soluble CD147 (15). We previously generated a murine
antibody, HAb18, which targeted hepatocellular carcinoma-associated
antigen HAb18G/CD147 (16).
However, a successful sandwich ELISA detection system requires
either MAbs that bind to independent sites on the antigen or
affinity-purified polyclonal antibodies. Antibodies are widely used
for the identification and localization of proteins due to their
ability to bind an antigen with a high degree of affinity and
specificity (17). MAbs have
monospecificity, as they target a single epitope, which results in
reduced cross-reaction (18). By
contrast, polyclonal antibodies exhibit higher sensitivity, as a
number of different epitopes are recognized (17). Owing to the various applications in
which they may be used, antibodies with high specificity and
sensitivity are desired. There are numerous methods used to purify
antibodies, and the choice of purification procedure depends how
the antibodies will be used and on the resources available
(19). IgG may be purified by
ammonium sulfate precipitation, ion-exchange chromatography,
Protein A or Protein G affinity chromatography (20); occasionally, immunoaffinity
chromatography is required to obtain more highly purified products
(19). Currently, the majority of
antibodies against CD147 are purified by Protein A or Protein G
affinity chromatography. However, we have previously found that
anti-CD147 polyclonal antibodies that are purified only by Protein
A or Protein G affinity chromatography do not work well in the
sandwich ELISAs to detect soluble CD147 (data not shown).
The present study produced a rabbit polyclonal
antibody against HAb18G/CD147, which was purified by ammonium
sulfate precipitation followed by antigen-immunoaffinity
chromatography. This polyclonal antibody performed well with MAb
HAb18 in the sandwich ELISA, which was used to detect soluble
CD147.
Materials and methods
Preparation of eukaryotic-expressed
CD147
Chinese hamster ovary (CHO)-derived cell line
CHO-H8F8E10, that stably expresses HAb18GEP-Fc (a recombinant human
protein containing the extracellular portion (EP) and the fragment
crystallizable region (Fc) of HAB18G/CD147, termed hereafter
CD147-Fc), preserved in our laboratory, was cultured in SFM4 medium
(Hyclone; GE Healthcare Life Sciences; Logan, UT, USA) at 37°C. The
recombinant eukaryotic expression vector pcDNA5/HAb18G-Fc, which
contains the extracellular Ig-like domains of HAb18G with human Fc
fragment in the C-terminal domain, was produced and large-scale
cell culture was accomplished. Culture suspensions were collected
and separated by tangential flow microfiltration (Sartorious AG,
Göttingen, Germany). CD147-Fc recombinant protein was purified by
Protein A chromatography with GE HiTrap rProtein A (GE Healthcare
Life Sciences), according to the manufacturer's instructions. The
Fc fragment was cleaved by Human Rhinovirus (HRV) 3C Protease (Sino
Biological, Inc., Beijing, China). Briefly, CD147-Fc recombinant
protein in cleavage buffer (Sino Biological, Inc.) was mixed with
the recombinant HRV 3C protease in cleavage buffer at a mass ratio
of 100:1 and incubated at 4°C overnight. The mixture was purified
by Ni2+ affinity chromatography with GE HisTrap (GE
Healthcare Life Sciences), followed by Protein A chromatography
with GE HiTrap rProtein A, according to the manufacturer's
instructions, in order to remove the residual Fc fragments and HRV
3C protease which also have a polyhistidine tag. Fractions were
desalted and concentrated by an ultrafiltration device (Merck KGaA
Darmstadt, Germany). The concentration of purified CD147 was
determined by the Bicinchoninic Acid (BCA) assay (Thermo Fisher
Scientific, Inc. Waltham, MA, USA). Samples were subsequently
analyzed by 12% SDS-PAGE.
Immunization of rabbits with
CD147
New Zealand white rabbits (n=2; weight 2.4 and 2.7
kg; age, 7 months; housed at 12 h light/dark cycles at 20–26°C and
with free access to food and water) were used in the present study
and handled in the Animal Center of the Fourth Military Medical
University (Xi'an, China). All animal procedures were performed
according to the University's Institutional Animal Care and Use
Committee. The rabbits were given a hypodermic injection of
recombinant purified CD147 protein (800 µg), mixed with Complete
Freund's Adjuvant (Sigma-Aldrich; Merck KGaA) in a 1:1 ratio. After
3 weeks, the rabbits were boosted 2 times with CD147 (400 µg) mixed
with incomplete Freund's adjuvant (1:1) at 2-week intervals. Prior
to each injection, blood samples were obtained from the marginal
vein of the rabbit ear, centrifuged at 2,000 × g for 10 min at 4°C
and the sera was used to determine antibody titer by ELISA.
Antiserum titer determination by
ELISA
The titer of antiserum was determined by indirect
ELISA. For each well of the 96-well plate, 1 µg of CD147 was
diluted in 100 µl of 0.1 M sodium bicarbonate solution pH 9.6 and
incubated overnight at 4°C. The plates were washed 3 times with PBS
+ Tween-20 (PBST; 0.05% Tween-20), and then blocked with 1% bovine
serum albumin (BSA; MP Biomedicals, LLC, Santa Ana, CA, USA) for 1
h at 37°C. The plates were washed again 3 times with PBST and
incubated with 100 µl rabbit antisera against CD147 at 6 different
dilutions between 1:16,000 and 1:512,000 for 1 h at 37°C.
Non-immune serum was used as a negative control. Following 3 washes
with PBST, plates were incubated with 100 µl diluted horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:10,000
dilution; cat. no. 31460; Pierce; Thermo Fisher Scientific, Inc.)
for 30 min at 37°C. The reaction was developed by adding 100 µl
3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich; Merck KGaA) for
12 min at room temperature. Finally, 200 M sulfuric acid was added
to stop the reaction, and the absorbance was determined at 450 nm
using a BioTeck Epoch microplate reader. The experiments were
repeated two times.
Purification of rabbit anti-CD147
IgG
The polyclonal antibodies against CD147 were
purified from the rabbit immune sera by ammonium sulfate
precipitation followed by antigen-immunoaffinity chromatography.
Briefly, rabbit blood was collected from the carotid artery
following anesthesia with 40 mg/kg pentobarbital sodium and prior
to sacrifice. Approximately 100 ml blood was collected from each
rabbit and ~30 ml immunized rabbit serum was collected by
centrifugation at 1,000 × g for 10 min at 4°C. Serum proteins were
then precipitated in 50% ammonium sulfate at 4°C overnight and
centrifuged at 2,000 × g for 20 min at 4°C. The pellet was
subsequently dissolved in PBS (pH 7.4). To improve specificity, the
polyclonal antibodies were purified by antigen-immunoaffinity
chromatography (CNBr-activated sepharose 4B; GE Healthcare Life
Sciences), according to the manufacturer's protocol. The purity and
reactivity of the anti-CD147 polyclonal antibodies were analyzed by
10% SDS-PAGE [as described previously (21)] and indirect ELISA, respectively.
The indirect ELISA was performed similar as the antiserum titer
ELISA described above, with the purified polyclonal antibodies used
in 6 serial dilutions from 40 to 0.625 ng/ml.
Preparation of recombinant CD147
protein for antibody purification
As large amounts of coupling antigen are required
for antigen-immunoaffinity chromatography, a prokaryotic expression
system for CD147 was prepared. The extracellular domain of
HAb18G/CD147 was amplified by polymerase chain reaction (PCR) using
the expression plasmid pBluescript KS(+)/HAb18G as the template for
the amplification. The primers used encompassed the entire
transcript with SphI and MluI cloning sites added to
the forward (CCC AAG CTT ATG GCG GCT GCG CTG TTC GTG CTG) and
reverse (CGC GGA TCC TCA GGA AGA GTT CCT GGC GGA) primers,
respectively. PCR products were purified using the Wizard PCR preps
kit (Promega Corporation, Madison, WI, USA). Following restriction
endonuclease digestion (SphI and MluI; New England
Biolabs, Inc., Ipswich, MA, USA), the fragment was inserted into
the pGEX-6p-1 prokaryotic expression vector (GE Healthcare Life
Sciences), which has a C-terminal glutathione S-transferase (GST)
tag. Positive Escherichia coli BL21 (GE Healthcare Life
Sciences) clones containing recombinant plasmid pGEX-6P-1/HAb18GEP
were selected by growth on ampicillin-containing agar plates. A
single colony of transformed E. coli was selected and
cultured overnight at 37°C in Luria-Bertani (LB) medium (Thermo
Fisher Scientific, Inc.) supplemented with 100 µg/ml ampicillin.
The culture mixture was transferred to fresh LB medium (1:100
dilution) containing 100 µg/ml ampicillin and incubated at 37°C
with continuous shaking until the absorbance at 600 nm reached
0.6–0.8. Expression conditions were optimized, and expression of
the CD147-GST fusion proteins was induced by addition of 1 mM
isopropyl β-D-1-thiogalactopyranoside at 16°C for 5 h. Following
induction for 5 h, the cells were harvested by centrifugation at
4,000 × g for 20 min at 4°C. The supernatant was discarded and the
pellet was resuspended in PBS (pH 7.4), and lysed by sonication on
ice with 4-sec pulses at high intensity and a 7-sec cooling period
between each burst for 120 cycles. The suspension was centrifuged
at 12,000 × g for 50 min at 4°C to remove insoluble debris. The
resultant supernatant was subsequently added to a GSTrap column (GE
Healthcare Life Sciences) pre-equilibrated with PBS and the AKTA
program was performed according to the GSTrap protocol. The
flow-through was collected for SDS-PAGE analysis, as
aforementioned, and the column was washed with PBS (pH 7.4).
Finally, the bound protein was eluted with elution buffer (reduced
glutathione; Amresco, LLC, Solon, OH, USA) and analyzed by 10%
SDS-PAGE and western blot analysis. Fractions were desalted and
concentrated by an ultrafiltration device with PBS (pH 7.4). The
concentration of CD147-GST was determined by BCA assay.
SDS-PAGE and western blot
analysis
Eukaryotic-expressed CD147 was separated by 12%
non-reduced SDS-PAGE; prokaryotic-expressed CD147 and purified
polyclonal antibodies were both separated by 10% non-reduced
SDS-PAGE. All the samples were analyzed for protein concentration
by BCA assay (Thermo Fisher Scientific, Inc.), and 10 µg was loaded
in each lane. Coomassie brilliant blue R250 (Sigma-Aldrich; Merck
KGaA) was used to stain the gels. Quantification of gel staining
was performed with GeneSnap software, version 4.0 (SynGene,
Frederick, MD, USA). All the purification experiments described
above were analyzed by coomassie brilliant blue staining, with the
exception of the purified prokaryotic-expressed CD147 that was also
analyzed by western blot analysis. Briefly, samples were
transferred to a polyvinylidene fluoride microporous membrane
(Merck KGaA) and probed with primary antibody HAb18 (22) overnight at 4°C and secondary
horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody
(1:3,000 dilution; cat. no. A16072; Thermo Fisher Scientific, Inc.)
for 50 min at room temperature. Signals were visualized by Western
Blotting Detection Reagents (cat. no. 29100; Engreen Biosystem Co.,
Ltd., Beijing, China), using a Kodak 4000MM Image Station (Kodak;
Rochester, NY, USA) and the Carestream Molecular Imaging Software,
version 5.4.2 (Caresteam, Rochaster, NY, USA).
Establishment of sandwich ELISA
A monoclonal antibody against the extracellular
domain of HAb18G/CD147, which was previously produced in our
laboratory and designated HAb18 (22), was diluted in sodium bicarbonate
solution (pH 9.6) at the concentration of 10 µg/ml (100 µl/well)
and used to coat 96-well plates. The plates were incubated at 4°C
overnight, washed 3 times with PBST and blocked with 1% BSA (200
µl/well) for 1 h at 37°C. Subsequently, samples to be tested, or
the highly purified CD147 protein (eukaryotic expression of the
extracellular domain of HAb18G/CD147) were added to the individual
wells and incubated at 37°C for 1 h. Purified CD147 was used as a
standard, serially diluted in 1% BSA/PBS (1,000, 500, 250, 125,
62.5, and 31.25 pg/ml), of which 100 µl was added to individual
wells. After washing 3 times with PBST, anti-CD147 polyclonal
antibodies, which were purified by different methods (including
ammonium sulfate precipitation, antigen-immunoaffinity
choromatography with ammonium sulfate precipitation and protein A
chromatography with ammonium sulfate), or a commercial antibody
against CD147 (cat. no. orb42082; Biorbyt Ltd., Cambridge, UK) were
added (100 µl/well) and incubated at 37°C for 1 h. After 3 washes
with PBST, HRP-conjugated goat anti-rabbit IgG was added (100
µl/well) and the plate was incubated at 37°C for 30 min. After
washing 3 times with PBST, TMB substrate was added to the wells
(100 µl/well) and was measured at 450 nm using a BioTeck Epoch
microplate reader. This test was repeated 15 times.
Results
Characterization of the CD147 antigen
for immunization
As the first step in the production of the
polyclonal antibodies against CD147, a CD147 protein was prepared.
To obtain antibodies that resemble those produced in the human
body, the eukaryotic-expressed CD147 was chosen as the immunogen.
The recombinant expression vector pCDNA5/HAb18G-Fc contains
extracellular domains of HAb18G and its C-terminal domain has an Fc
fragment. The Fc fragment was cleaved by HRV 3C protease to avoid
the production of antibodies against the Fc fragment. The Fc
fragment and HRV 3C protease have polyhistidine tags, therefore,
the mixture was purified by Ni2+ affinity chromatography
followed by Protein A chromatography to obtain high-purity CD147
protein. The purified, eukaryotic-expressed CD147 was analyzed by
12% SDS-PAGE under reducing conditions (Fig. 1). The recombinant human CD147-Fc
protein consists of 422 amino acids and has a calculated molecular
mass of 46.8 kDa; however, as a result of glycosylation, the
recombinant protein migrates at ~58–65 kDa (Fig. 1, lane 1). The recombinant protein
CD147-Fc has been degraded to some degree and exhibits dimer
formation. The molecular mass of the cleaved extracellular domains
of CD147 is ~30–40 kDa and the molecular mass of the HRV 3C
protease is ~21 kDa (Fig. 1, lane
2). Following purification by Ni2+ affinity
chromatography, a certain amount of recombinant CD147-Fc protein
and HRV 3C protease remained (Fig.
1, lane 3). However, following Protein A chromatography, CD147
was of high purity and there were no residual Fc fragments or HRV
3C protease.
 | Figure 1.SDS-PAGE analysis of the CD147 antigen
prior to rabbit immunization. Eukaryotic-expressed recombinant
protein CD147-Fc was treated with HRV 3C protease for 16 h, and the
mixture was purified by Ni2+ affinity chromatography
followed by Protein A chromatography. SDS-PAGE analysis
demonstrated that CD147 was of high purity and indicated that there
was no residual Fc fragment or 3C protease. Lane 1, recombinant
eukaryotic-expressed CD147-Fc, which has a dimer formation and has
been partially degraded; Lane 2, the recombinant CD147-Fc protein
was treated with HRV 3C protease for 16 h, resulting in a mixture
of CD147-Fc, CD147, Fc fragments and HRV 3C protease; Lane 3,
mixture purified by Ni2+ affinity chromatography, which
is not very powerful, and a certain amount of recombinant protein
and HRV 3C protease remain; Lane 4, CD147 immunogen purified by
Protein A chromatography, no recombinant protein or HRV 3C protease
was detected; Lane M, protein molecular weight marker. HRV, human
rhinovirus. |
Antiserum titer analysis by ELISA
Rabbits were immunized with the purified eukaryotic
CD147 immunogen, and the titer of the antiserum was detected by
indirect ELISA. A total of 6 dilutions, between 1:16,000 and
1:512,000, of the antiserum were reacted with an equal amount of
the recombinant CD147 protein. The antibody titer is defined as the
highest dilution of serum at which the optical density 450 (OD450)
ratio (OD450 of post-immunization serum/OD450 of pre-immunization
serum) is >2:1. The antibody titer was demonstrated to be
~1:512,000 (Fig. 2). Generating a
specific antibody preparation from low titer antiserum is
difficult, and the production of a high-titer antiserum is a basic
requirement for high-quality antibodies. The CD147 antiserum titer
produced in the present study was high, which indicated that a
strong response has been generated, and thus, the antibodies could
be purified.
Characterization of the prokaryotic
recombinant CD147 protein for antigen-immunoaffinity
chromatography
Antigen-immunoaffinity chromatography requires large
amounts of CD147 protein to purify the CD147 antibodies. Therefore,
a prokaryotic expression system was used to make CD147 protein
instead of the eukaryotic vector, as CD147 proteins may be
expressed in large amounts in a short time and has increased
stability compared with eukaryotic-expressed CD147. The
extracellular domain sequence of the CD147 gene was cloned in an
expression plasmid with a C-terminal GST tag, and the construct was
transformed into E. coli BL21 competent cells. The GST tag
was used to purify the recombinant CD147 with a GSTrap affinity
column. The purified CD147-GST fusion protein, whose expected size
is ~44 kDa, was analyzed by 10% SDS-PAGE and confirmed by western
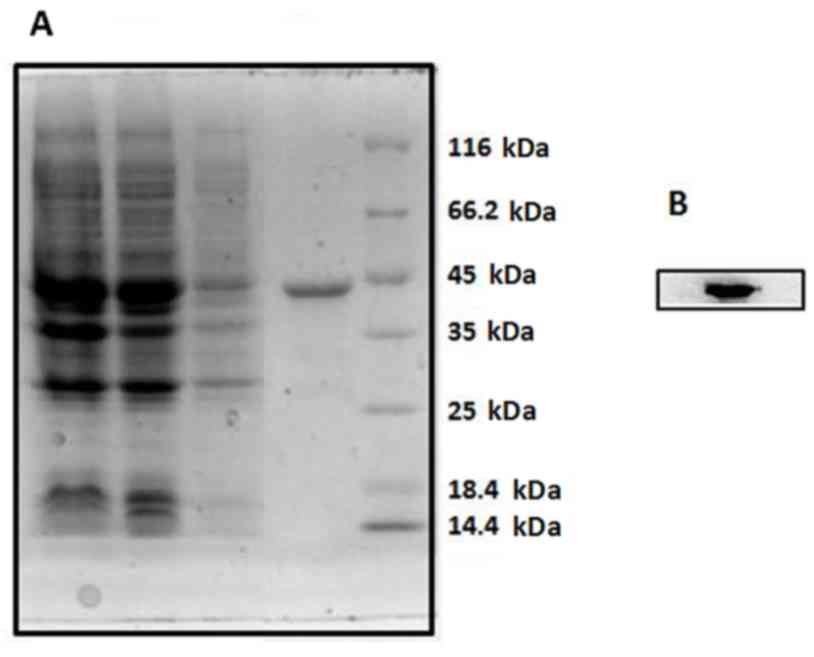
blot analysis with anti-CD147 MAb HAb18 (Fig. 3). SDS-PAGE and western blot
analysis demonstrated that purification of CD147 was of high purity
and confirmed the presence of a corresponding band for expressed
CD147-GST protein. This high-quality purified CD147 protein is
required to produce high-quality purified antibodies.
Assessment of the purity and
reactivity of the anti-CD147 polyclonal antibody by SDS-PAGE and
indirect ELISA
A non-reducing SDS-PAGE was used to determine the
purity of rabbit anti-CD147 IgG (120–150 kDa), which was purified
by ammonium sulfate precipitation followed by
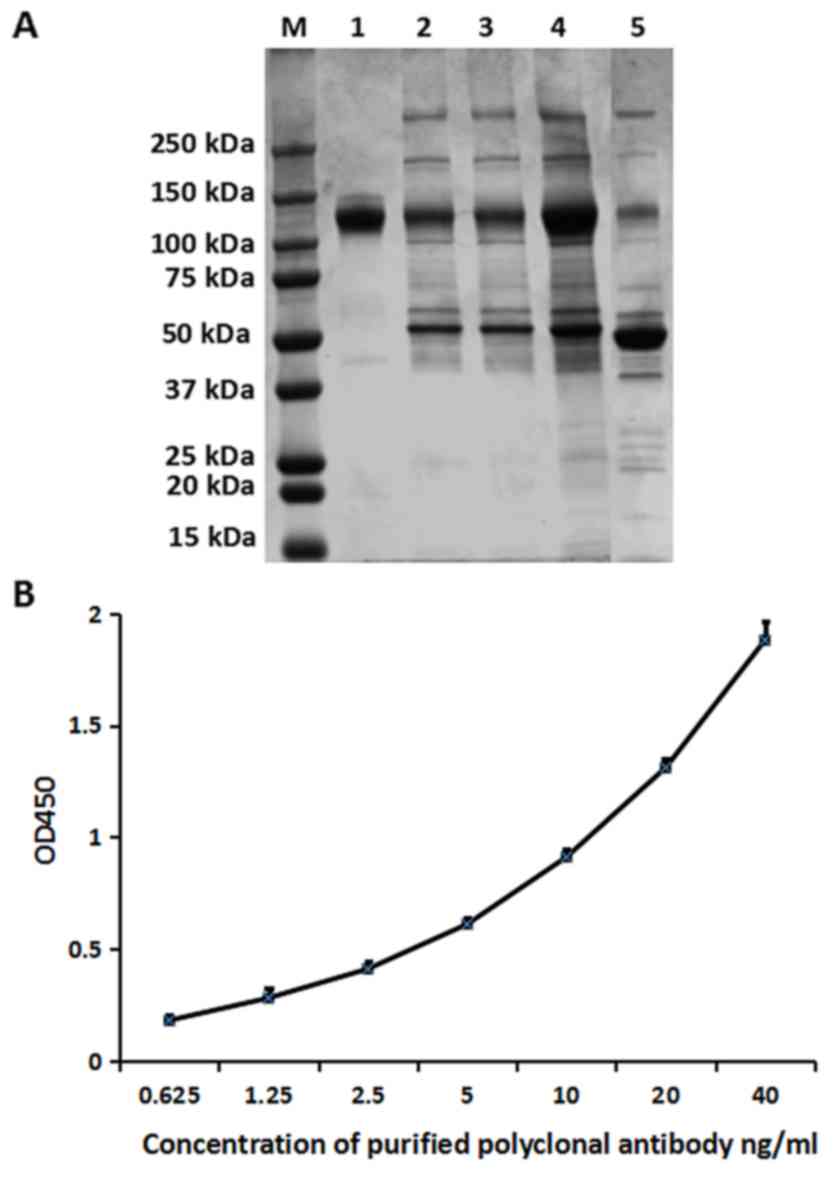
antigen-immunoaffinity chromatography (Fig. 4A, lane 1). Compared with the
antiserum (Fig. 4A, lane 5) and
the antibodies purified only by ammonium sulfate precipitation
(Fig. 4A, lane 4), the
affinity-purified antibodies had a higher purity; the purity of the
rabbit anti-CD147 IgG was ~99%.
To determine the reactivity of the purified
antibodies, an indirect ELISA was performed. Antibodies were
purified by ammonium sulfate precipitation followed by
antigen-immunoaffinity chromatography, and different dilutions
(0.625–40 ng/ml) were reacted with an equal amount of CD147
protein. The result demonstrated in Fig. 4B indicated that the purified
antibody had a good affinity for CD147, which indicated that the
purified antibody has a high reactivity.
Establishment of sandwich ELISA
A sandwich ELISA was constructed with the following
components: i) The HAb18 anti-CD147 MAb, previously produced in our
laboratory (22), was used as the
capture antibody; ii) the highly purified eukaryotic CD147 was used
as a standard protein, which was serially diluted to 1000, 500,
250, 125, 62.5, 31.25 pg/ml and iii) the anti-CD147 polyclonal
antibodies were used as the detecting antibody. Detection of the
standard proteins was performed under optimized conditions. The
present study demonstrated that the sandwich ELISA system detected
the concentration of CD147 as low as 31.25 pg/ml (Table I). By contrast, the present study
established other sandwich ELISA systems using various rabbit
anti-CD147 polyclonal antibodies that were purified by methods
other than antigen-immunoaffinity chromatography. The results
demonstrated that only the system comprised of antibodies purified
by antigen-immunoaffinity chromatography was able to detect CD147
protein at concentrations as low as 31.25 pg/ml (Table II).
 | Table I.Sensitivity test results of human
CD147 sandwich ELISA. |
Table I.
Sensitivity test results of human
CD147 sandwich ELISA.
| CD147 standard
concentration (pg/ml) | Average
OD450a ± SD |
|---|
| 1,000 | 1.765±0.021 |
| 500 | 0.884±0.005 |
| 250 | 0.587±0.029 |
| 125 | 0.321±0.020 |
| 62.5 | 0.193±0.005 |
| 31.25 | 0.164±0.009 |
| Blank | 0.069±0.007 |
 | Table II.Comparison of polyclonal antibodies
against CD147 purified by antigen-immunoaffinity chromatography and
other methods of purification. |
Table II.
Comparison of polyclonal antibodies
against CD147 purified by antigen-immunoaffinity chromatography and
other methods of purification.
| Source | Method of
purification | Background of
sandwich ELISA (OD450) | Lowest concentration
of CD147 detected (pg/ml) |
|---|
| Present study |
Antigen-immunoaffinity chromatography |
0.069 | 31.25 |
| Present study | Ammonium sulfate
precipitation alone | 0.17 | 250 |
| Present study | Protein A affinity
chromatography | 0.13 | 250 |
| Purchased from
Biorbyt Ltd., Cambridge, UK | Protein A affinity
chromatography | 0.08 | 125 |
Discussion
The present study obtained high-titer antiserum
against human CD147 and subsequently purified the antiserum by
ammonium sulfate precipitation followed by antigen-immunoaffinity
chromatography. The polyclonal antibodies generated by this
strategy were evaluated by indirect ELISA and proved to be useful
(23). As a high titer of
antiserum is the basis for high-quality antibodies (3), a titer of 1:512,000 determined in the
present study was high enough to produce high quality antibodies.
The purification of immunoglobulins presents certain practical
complications, particularly for polyclonal antibodies (24). There are various types of methods
for the purification of antibodies (4); however, the choice of the
purification method depends on the application of the antibodies
(3). In the present study,
polyclonal antibodies against CD147 were produced for sandwich
ELISA to detect soluble CD147 antigen in serum; therefore,
antigen-immunoaffinity chromatography was used to purify polyclonal
antibodies to obtain highly specificity anti-CD147 antibodies.
Immunoaffinity chromatography uses biologically associated binding
agents and is used to selectively purify or analyze a target
compound (25). In the current
study, polyclonal antibodies against CD147 were purified by
antigen-immunoaffinity chromatography following ammonium sulfate
precipitation. The conditions of the antigen-immunoaffinity
chromatography were optimized, including the buffer type and pH,
and particularly the concentration of the reactors and reaction
time (25,26). Following purification, antibodies
with a purity of ~99% were obtained. In an indirect ELISA against
CD147, the reactivity of the polyclonal antibodies purified by
antigen-immunoaffinity chromatography was demonstrated to be high.
The polyclonal antibody against CD147 and the MAb HAb18 made up the
basic system of the sandwich ELISA for detecting soluble CD147. The
lower limit of the ELISA method used for detecting the CD147
standard was 31.25 pg/ml. By contrast, with antibodies purified by
Protein A or ammonium sulfate precipitation, the sensitivity of the
ELISA using antibodies purified by antigen-immunoaffinity
chromatography was the highest.
The polyclonal antibody purified by
antigen-immunoaffinity chromatography may be a novel tool for
further investigation of soluble CD147 in human serum. The sandwich
ELISA kit described in the present study, which includes the
antigen-immunoaffinity chromatography purified polyclonal antibody,
may be used to detect the presence of CD147 in several types of
cancers to investigate whether soluble CD147 is a biomarker in
certain cancers. Meanwhile, the purification method discussed in
the present study may be applied to the purification of various
other antibodies.
Acknowledgements
The present study was supported by grants from the
National Science and Technology Major Project (grant no.
2012AA02A301).
References
|
1
|
Biswas C: Tumor cell stimulation of
collagenase production by fibroblasts. Biochem Biophys Res Commun.
109:1026–1034. 1982. View Article : Google Scholar
|
|
2
|
Ellis SM, Nabeshima K and Biswas C:
Monoclonal antibody preparation and purification of a tumor cell
collagenase-stimulatory factor. Cancer Res. 49:3385–3391. 1989.
|
|
3
|
Polette M, Gilles C, Marchand V, Lorenzato
M, Toole B, Tournier JM, Zucker S and Birembaut P: Tumor
collagenase stimulatory factor (TCSF) expression and localization
in human lung and breast cancers. J Histochem Cytochem. 45:703–709.
1997. View Article : Google Scholar
|
|
4
|
Tang J, Wu YM, Zhao P, Yang XM, Jiang JL
and Chen ZN: Overexpression of HAb18G/CD147 promotes invasion and
metastasis via alpha3beta1 integrin mediated FAK-paxillin and
FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci. 65:2933–2942. 2008.
View Article : Google Scholar
|
|
5
|
Wu Y, Zhou X and Zheng PS: Involvement of
CD147 isoform-4 in the proliferation of SiHa cells: A possible
molecular mechanism of cervical cancer. Oncol Rep. 26:717–724.
2011.
|
|
6
|
Chen ZN, Yang Z and Mi L: Analysis on the
structure and function of hepatoma transfer-associated factor
HAb18G (in Chinese). J Mol Cell Immunol. 15:341999.
|
|
7
|
Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN
and Chan HC: The involvement of HAb18G/CD147 in regulation of
store-operated calcium entry and metastasis of human hepatoma
cells. J Biol Chem. 276:46870–46877. 2001. View Article : Google Scholar
|
|
8
|
Haug C, Lenz C, Diaz F and Bachem MG:
Oxidized low-density lipoproteins stimulate extracellular matrix
metalloproteinase Inducer (EMMPRIN) release by coronary smooth
muscle cells. Arterioscler Thromb Vasc Biol. 24:1823–1829. 2004.
View Article : Google Scholar
|
|
9
|
Egawa N, Koshikawa N, Tomari T, Nabeshima
K, Isobe T and Seiki M: Membrane type 1 matrix metalloproteinase
(MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix
metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J
Biol Chem. 281:37576–37585. 2006. View Article : Google Scholar
|
|
10
|
Tang Y, Kesavan P, Nakada MT and Yan L:
Tumor-stroma interaction: Positive feedback regulation of
extracellular matrix metalloproteinase inducer (EMMPRIN) expression
and matrix metalloproteinase-dependent generation of soluble
EMMPRIN. Mol Cancer Res. 2:73–80. 2004.
|
|
11
|
Taylor PM, Woodfield RJ, Hodgkin MN,
Pettitt TR, Martin A, Kerr DJ and Wakelam MJ: Breast cancer
cell-derived EMMPRIN stimulates fibroblast MMP2 release through a
phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene.
21:5765–5772. 2002. View Article : Google Scholar
|
|
12
|
Yanaba K, Asano Y, Tada Y, Sugaya M,
Kadono T, Hamaguchi Y and Sato S: Increased serum soluble CD147
levels in patients with systemic sclerosis: Association with
scleroderma renal crisis. Clin Rheumatol. 31:835–839. 2012.
View Article : Google Scholar
|
|
13
|
Wu J, Hao ZW, Zhao YX, Yang XM, Tang H,
Zhang X, Song F, Sun XX, Wang B, Nan G, et al: Full-length soluble
CD147 promotes MMP-2 expression and is a potential serological
marker in detection of hepatocellular carcinoma. J Transl Med.
12:1902014. View Article : Google Scholar :
|
|
14
|
Hornbeck PV: Enzyme-linked immunosorbent
assays. Curr Protoc Immunol. 110:1–23. 2015.
|
|
15
|
Moonsom S, Tayapiwatana C, Wongkham S,
Kongtawelert P and Kasinrerk W: A competitive ELISA for quantifying
serum CD147: Reduction of soluble CD147 levels in cancer patient
sera. Hybridoma (Larchmt). 29:45–52. 2010. View Article : Google Scholar
|
|
16
|
Ku XM, Liao CG, Li Y, Yang XM, Yang B, Yao
XY, Wang L, Kong LM, Zhao P and Chen ZN: Epitope mapping of series
of monoclonal antibodies against the hepatocellular
carcinoma-associated antigen HAb18G/CD147. Scand J Immunol.
65:435–443. 2007. View Article : Google Scholar
|
|
17
|
Nelson PN, Westwood OM, Jefferis R,
Goodall M and Hay FC: Characterisation of anti-IgG monoclonal
antibody A57H by epitope mapping. Biochem Soc Trans. 25:373S1997.
View Article : Google Scholar
|
|
18
|
Lipman NS, Jackson LR, Trudel LJ and
Weis-Garcia F: Monoclonal versus polyclonal antibodies:
Distinguishing characteristics, applications, and information
resources. ILAR J. 46:258–268. 2005. View Article : Google Scholar
|
|
19
|
Lane D: Antibodies: A Laboratory Manual.
Cold Spring Harbor Press; Cold Spring Harbor, N.Y: pp. 288–312.
1998
|
|
20
|
Andrew SM and Titus JA: Purification of
immunoglobulin G. Curr Protoc Immunol: Chapter. 2:Unit 2.72001.
|
|
21
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar
|
|
22
|
Chen ZN and Liu YF: Monoclonal antibody
HAb18 to human hepatoma. J Monocl Antibod. 3:15–17. 1990.(In
Chinese).
|
|
23
|
Howard GC and Bethell DR: Basic methods in
antibody production and characterization. CRC Press Ltd; Boca
Raton, Florida: pp. 1–49. 2000, View Article : Google Scholar
|
|
24
|
Verdoliva A, Basile G and Fassina G:
Affinity purification of immunoglobulins from chicken egg yolk
using a new synthetic ligand. J Chromatogr B Biomed Sci Appl.
749:233–242. 2000. View Article : Google Scholar
|
|
25
|
Hage DS: Survey of recent advances in
analytical applications of immunoaffinity chromatography. J
Chromatogr B Biomed Sci Appl. 715:3–28. 1998. View Article : Google Scholar
|
|
26
|
Hage DS and Phillips TM: Immunoaffinity
chromatographyTaylor & Francis, Handbook of Affinity
Chromatography. New York: 62006
|