Introduction
Acute ischemic brain damage results in severe
neuronal loss via an inflammatory process, which predominantly
involves invading leukocytes, activated resident microglia and
macrophages (1,2). Microglia form the first line of
defense and control the immune response in the brain (3–5). It
may be speculated that the microglia serve an essential role in
forming a link between the central nervous system (CNS), a
barrier-protected organ, and the general immune system; microglia
are resident macrophages of the CNS and thus form an interface
between the neural parenchyma and the immune system (3,6).
Little is known about the microglia in the healthy CNS; however,
they are rapidly activated in acute pathological events, which may
affect the CNS.
Activated microglia have dual effects. They may
destroy invading microorganisms, remove potentially deleterious
debris, promote tissue repair by secreting wound-healing factors
and thus facilitate the return to tissue homeostasis (7,8).
However, these cells secrete large amounts of cytotoxic mediators
such as reactive oxygen intermediates (9), nitric oxide (10), proteinases, excitatory amino acids
(11) and inflammatory mediators,
including interleukin-1 (IL-1) and IL-6 (12,13).
The activation of microglia and their histological
localization is important in elucidating their roles following
ischemic insults. Our previous study demonstrated the chronological
alteration of ionized calcium-binding adapter molecule 1 (Iba-1),
which is a marker for microglia, in the hippocampal Cornu Ammonis
(CA) 1 region, induced by 5 min of transient forebrain ischemia
using Mongolian gerbils (14).
Previous research has demonstrated that microglia may be divided
into activated M1 and M2 microglia, according to their phenotypes
and polarization (15); activated
M1 microglia secrete proinflammatory cytokines and are potentially
harmful, whereas activated M2 microglia serve important roles in
repair and plasticity (15–17).
Improving our understanding of M1 microglia/macrophages is
important because this subtype predominates within injured areas,
and may accelerate neuronal damage via the release of inflammatory
mediators, including tumor necrosis factor-α and nitric oxide
(15). However, a recent study
indicated that early-stage Alzheimer's patients exhibit
M1-polarized microglia, whereas severe-stage plaques demonstrated
an M2a-polarized phenotype (18).
Notably, the concept of microglial M1 and M2 phenotypes has also
been investigated in the field of stroke research (19). Cluster of differentiation (CD) 74
is a marker for activated M1 microglia because it serves as a
chaperone for major histocompatibility complex class II (MHCII)
molecules in antigen presenting cells, and as a receptor binding
site for macrophage migration inhibitory factor (MIF). Numerous
previous in vivo studies have focused on activated M1
microglia using animal models of focal ischemia. Therefore, the
present study investigated the chronological alteration of
activated microglia expressing CD74 in the hippocampus, induced by
transient forebrain ischemia in Mongolian gerbils, which have been
used as an animal model of transient forebrain ischemia (20–23).
Materials and methods
Induction of transient cerebral
ischemia
A total of 35 male gerbils (age, 6 months; weight,
70–80 g) were obtained from Experimental Animal Center of Kangwon
National University (Chuncheon, South Korea). Gerbils were housed
in a conventional facility under stable temperature (23°C) and
humidity (60%) with a 12-h light/12-h dark cycle, and were provided
with free access to food and water. The procedures for animal
handling were in compliance with current international laws and
policies (Guide for the Care and Use of Laboratory Animals, The
National Academies Press, 8th Ed., 2011) (24), and were approved by the
Institutional Animal Care and Use Committee (IACUC) at Kangwon
National University (Chuncheon, South Korea; approval no.
KW-130424-1).
Transient cerebral ischemia surgery was performed as
previously described (25). In
brief, animals were anesthetized with a mixture of 2.5% isoflurane
(Ilsung Pharmaceuticals, Co., Ltd., Seoul, Korea) in 33% oxygen and
67% nitrous oxide. The bilateral common carotid arteries were
occluded for 5 min, and the complete interruption of blood flow was
confirmed by observation of the retinal central artery under an
ophthalmoscope. A normothermic (37±0.5°C) condition was maintained
prior to, during and following surgery, until the animals had
completely recovered from anesthesia. Sham-operated animals were
subjected to the same surgical procedures; however, the common
carotid arteries were not occluded.
Tissue processing
Tissues were collected as previously described
(26). In brief, sham-operated
(n=7) and ischemia-operated gerbils (n=28 in total; 7 gerbils/time
point) were anesthetized with pentobarbital sodium (40 mg/kg; JW
Pharmaceutical, Co., Ltd., Seoul, Korea) and perfused
transcardially with 4% paraformaldehyde 1, 3, 5 and 7 days
following reperfusion. Brain tissues were removed and serially cut
into 30-µm coronal sections.
Cresyl violet (CV) staining
To examine cellular distribution and damage, CV
staining was performed as previously described (26). In brief, CV acetate (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was dissolved at a concentration of
1.0% (w/v), and 0.28% glacial acetic acid was added. Sections were
stained and subsequently dehydrated by immersing in serial ethanol
baths.
Fluoro-Jade B (F-J B) staining
To examine neuronal damage/death following transient
ischemia, F-J B (a high affinity fluorescent marker for the
localization of neuronal degeneration) histofluorescence staining
was performed, according to a previously published procedure
(27). Briefly, sections were
first immersed in a solution containing 1% sodium hydroxide,
transferred to a solution of 0.06% potassium permanganate, and
subsequently transferred to a 0.0004% Fluoro-Jade B (Histo-chem,
Inc., Jefferson, AR, USA) solution. After washing, the sections
were placed on a slide warmer (~50°C), and examined using an
epifluorescent microscope (Zeiss GmbH, Jena, Germany) with blue
(450–490 nm) excitation light and a barrier filter. Digital images
of the stained hippocampus were captured with an AxioM1 light
microscope equipped with an Axiocam digital camera (both from Zeiss
GmbH), connected to a PC monitor.
Immunohistochemistry
Immunohistochemistry was performed out according to
a previously published procedure (28). In brief, sections were incubated
with mouse anti-neuronal nuclear antigen (NeuN; cat. no. MAB377;
1:1,000; EMD Millipore, Billerica, MA, USA), mouse anti-CD74 (cat.
no. MCA46R; MHCII/RT1B clone; 1:100; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) or rabbit anti-Iba-1 (cat. no. 019-19741; 1:800;
Wako Pure Chemical Industries, Ltd., Osaka, Japan) primary
antibodies overnight at 4°C, and subsequently incubated with
biotinylated goat anti-mouse immunoglobulin (Ig)-G (cat. no.
BA-9200; 1:200; Vector Laboratories, Inc., Burlingame, CA, USA) or
goat anti-rabbit IgG (cat. no. BA-1000; 1:200; Vector Laboratories,
Inc.) secondary antibodies for 2 h at room temperature, followed by
incubation with a streptavidin-peroxidase complex (cat. no.
SA-5004; 1:200; Vector Laboratories, Inc.) for 45 min at room
temperature. Immunostaining was visualized with diaminobenzidene.
Digital images of the stained tissue were captured with an AxioM1
light microscope equipped with an Axiocam digital camera, connected
to a PC monitor.
Double immunofluorescence
To examine the colocalization of CD74 and I1, the
sections were processed by double immunofluorescence staining
according to a previously published procedure (28). Briefly, sections were incubated
with mouse anti-CD74 (cat. no. MCA46R; 1:25; Bio-Rad Laboratories,
Inc.) and rabbit anti-Iba-1 (cat. no. 019-19741; 1:100; Wako Pure
Chemical Industries, Ltd.) primary antibodies overnight at 4°C, and
subsequently incubated with Cy3-conjugated donkey anti-rabbit IgG
(cat. no. 711-165-152; 1:200) and fluorescein
isothiocyanate-conjugated donkey anti-mouse IgG (cat. no.
715-096-151; 1:200) secondary antibodies (both from Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 h at
room temperature. Immunoreactions were observed under a LSM510 META
NLO confocal microscope (Zeiss GmbH).
Data analysis
Numbers of NeuN-immunoreactive and F-J B-positive
cells were analyzed according to a previously published method
(26). In brief, cells were
counted in a 250×250 µm square applied approximately at the center
of the hippocampal CA1 region, using an image analyzing system
(Optimas 6.5; CyberMetrics Corporation, Phoenic, AZ, USA). The
studied tissue sections were selected at 120-µm intervals, and cell
counts were obtained by averaging the total cell numbers of 5
sections taken from each animal per group, between 1.4 mm and 2.0
mm posterior to the bregma, as defined by the gerbil atlas
(29).
Densities of Iba-1 and CD74 immunoreactive
structures were measured as previously described (26). Images were calibrated into an array
of 512×512 pixels, corresponding to a tissue area of 250×250 µm
(primary magnification, ×40) and including the stratum pyramidale.
The densities of all Iba-1 and CD74 immunoreactive structures were
evaluated on the basis of their optical density (OD), which was
obtained following transformation of the mean gray level using the
formula: OD=log (256/mean gray level). The background OD was
determined from areas adjacent to the measured area. After the
background density was subtracted, a ratio of the image OD was
calibrated as % relative OD (ROD), using Adobe Photoshop version
8.0 (Adobe Systems, Inc., Beijing, China) and analyzed using ImageJ
version 1.50 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are presented as the mean ± standard.
Differences between the means were statistically analyzed by
one-way analysis of variance followed by a Bonferroni's post-hoc
test, using GraphPad Prism 5.01 software (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Neuronal damage/death
CV, NeuN and F-J B staining was conducted in the CA1
hippocampal region following transient ischemia in gerbils
(Fig. 1). In the sham-operated
group, CV-positive cells and NeuN-immunoreactive neurons were
clearly observed in the stratum pyramidale of the hippocampal CA1
region (Fig. 1A, a, and D). In
contrast, no F-J B positive cells were detected in the hippocampal
CA1 region of these gerbils (Fig. 1G
and J). The 1 day after ischemic insult, the distribution
pattern of CV-positive cells and NeuN-immunoreactive neurons in the
CA1 region was similar to that in the sham-operated group (Fig. 1B, b and E), and no F-J B positive
cells were observed in the hippocampal CA1 region in this group
(Fig. 1H and J). CV-positive cells
were significantly decreased in the stratum pyramidale of the CA1
region 5 days after ischemic insult (Fig. 1C and c). Similarly, only a few NeuN
immunoreactive neurons were detected in the stratum pyramidale of
the CA1 region (Fig. 1F and J),
however, many F-J B positive cells were observed in the stratum
pyramidale of the CA1 region 5 days after ischemic insult (Fig. 1I and J). These results indicated
that transient cerebral ischemia-induced neuronal death in
pyramidal neurons of the hippocampal CA1 region occurred 5 days
after ischemic insult.
 | Figure 1.CV, NeuN and F-J B staining in the CA1
hippocampal region following transient ischemia in gerbils. (A-C,
a-c) CV, (D-F) NeuN and (G-I) F-J B staining. Staining of the
hippocampal CA1 region of (A, a, D, G) sham-operated and (B, C, b,
c, E, F, H, I) ischemia-operated gerbils was performed. CV- and
NeuN-positive cells were clearly observed in the sham-operated
group; however, F-J B positive cells were not detected. A few CV
and NeuN immunoreactive cells were present in the SP (*) of the CA1
region 5 days after ischemic insult, and several F-J B positive
cells were present in the SP at this time point. Scale bar=50 µm.
(J) Mean number of NeuN or F-J B positive cells in the CA1 region,
n=7/group. Data are presented as the mean ± standard error.
#P<0.05, vs. sham. CA, Cornu Ammonis; CV, cresyl
violet; F-J B, fluoro-Jade B; NeuN, neuronal nuclear antigen; SP,
stratum pyramidale; SO, stratum oriens; SR, stratum radiatum; Isch,
ischemia; DG, dentate gyrus. |
Iba-1 immunoreactive microglia
Iba-1 and CD74 immunoreactivity was detected in the
hippocampal CA1 region following transient ischemia in gerbils
(Fig. 2). In the sham-operated
group, Iba-1-immunoreactive microglia, in a resting form, were
distributed in all layers of the CA1 region and had a typical
ramified morphology with small areas of cytoplasm (Fig. 2A). The day after ischemic insult,
Iba-1-immunoreactive microglia demonstrated activation; the cell
bodies became hypertrophied with thickened processes (Fig. 2B) and their ROD was increased
compared with the sham-operated group. A total of 3 days after
ischemic insult, the majority of Iba-1-immunoreactive microglia
were more hypertrophied in shape and the ROD of these cells was
significantly increased, compared with those at 1 day post-ischemia
(Fig. 2C and K). After 5 days,
Iba-1-immunoreactive microglia were aggregated in the stratum
pyramidale of the CA1 region (Fig.
2D). After 7 days, the aggregation of Iba-1-immunoreactive
microglia, and their ROD, was significantly increased, compared
with 5 day post-ischemic tissues (Fig.
2E and K).
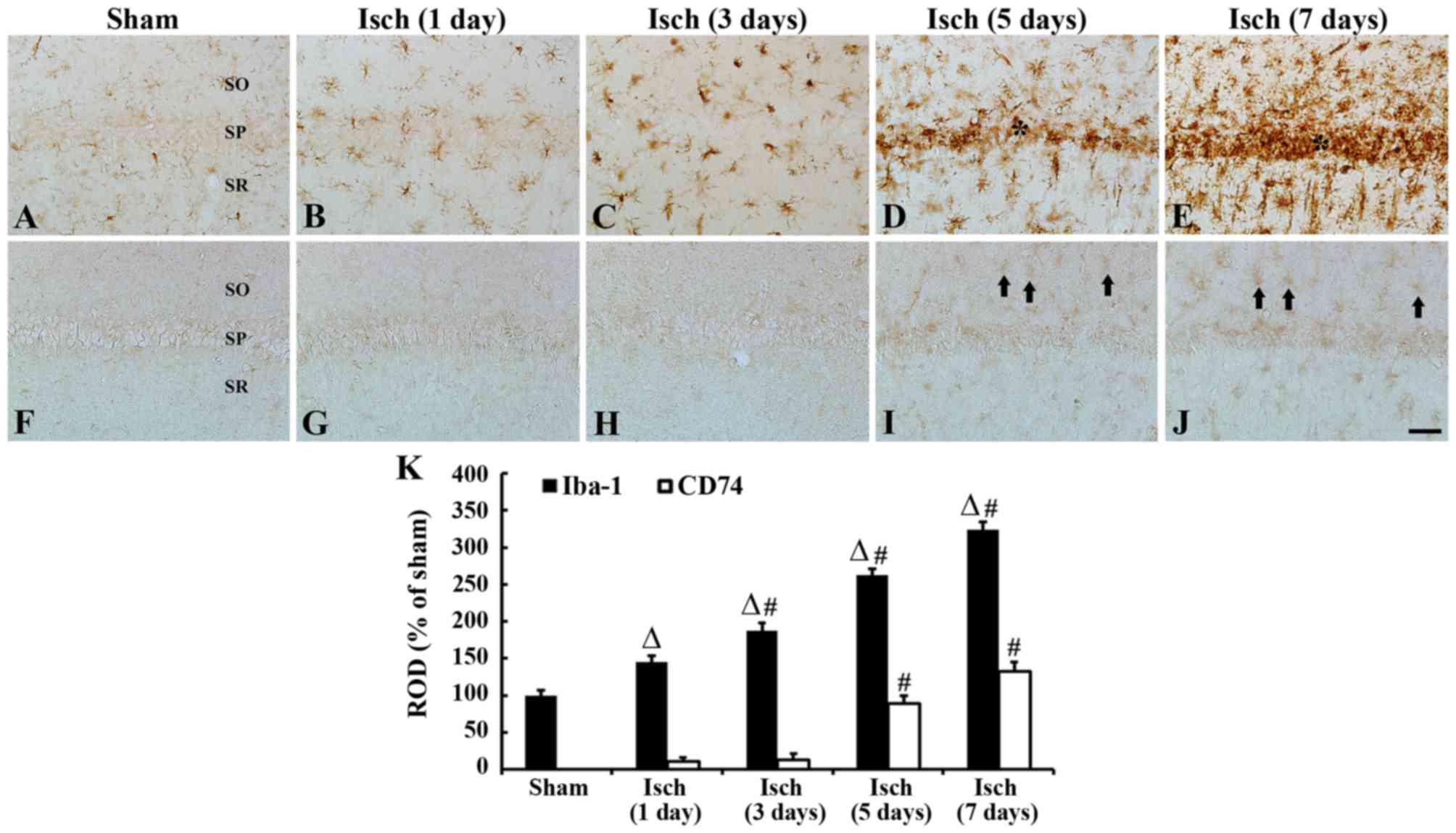 | Figure 2.Iba-1 and CD74 immunoreactivity in the
hippocampal CA1 region, following transient ischemia in gerbils.
Immunohistochemical staining of (A-E) Iba-1 and (F-J) CD74.
Staining was performed in (A and F) sham-operated and (B-E and G-J)
ischemia-operated gerbils. In the sham-operated group, only Iba-1
immunoreactive microglia were observed. After 5–7 days, numerous
Iba-1 immunoreactive microglia became aggregated in the SP (*), and
CD74-immunoreactive cells (black arrows) were scattered in the CA1
region. Scale bar=50 µm. (K) ROD as percentage of Iba-1 and CD74
immunoreactive structures in the CA1 region of the sham-and
ischemia-operated groups, n=7/group. ∆P<0.05 vs.
sham; #P<0.05, vs. the respective pre-time point
group. Data are presented as the mean ± standard error. Iba-1,
ionized calcium-binding adapter molecule 1; CD, cluster of
differentiation; CA, Cornu Ammonis; SP, stratum pyramidale; SO,
stratum oriens; SR, stratum radiatum; Isch, ischemia; ROD, relative
optical density; Isch, ischemia. |
CD74-immunoreactive microglia
In the sham-operated group, CD74 immunoreactivity
was not detected in any cells in the CA1 region (Fig. 2F). Between days 1 and 3 after
ischemic insult, very few CD74-immunoreactive cells were observed
in the CA1 region (Fig. 2G and H);
however, CD74-immunoreactive cells were detected in the strata
oriens and radiatum of the CA1 region 5 days after ischemic insult
(Fig. 2I and K). After 7 days, the
number of CD74-immunoreactive cells were further elevated in the
CA1 region, compared with those at 5 days post-ischemia (Fig. 2J and K).
Co-localization of Iba-1 and CD74
Double immunofluorescence staining indicated that
Iba-1 immunoreactivity was observed in the CA1 region of the
sham-operated group; however, no CD74 immunoreactivity was observed
in this group (Fig. 3A-C). After 7
days, CD74 immunoreactivity was observed in numerous cells in the
CA1 region, and this appeared to colocalize with Iba-1 in the
microglia (Fig. 3D-G).
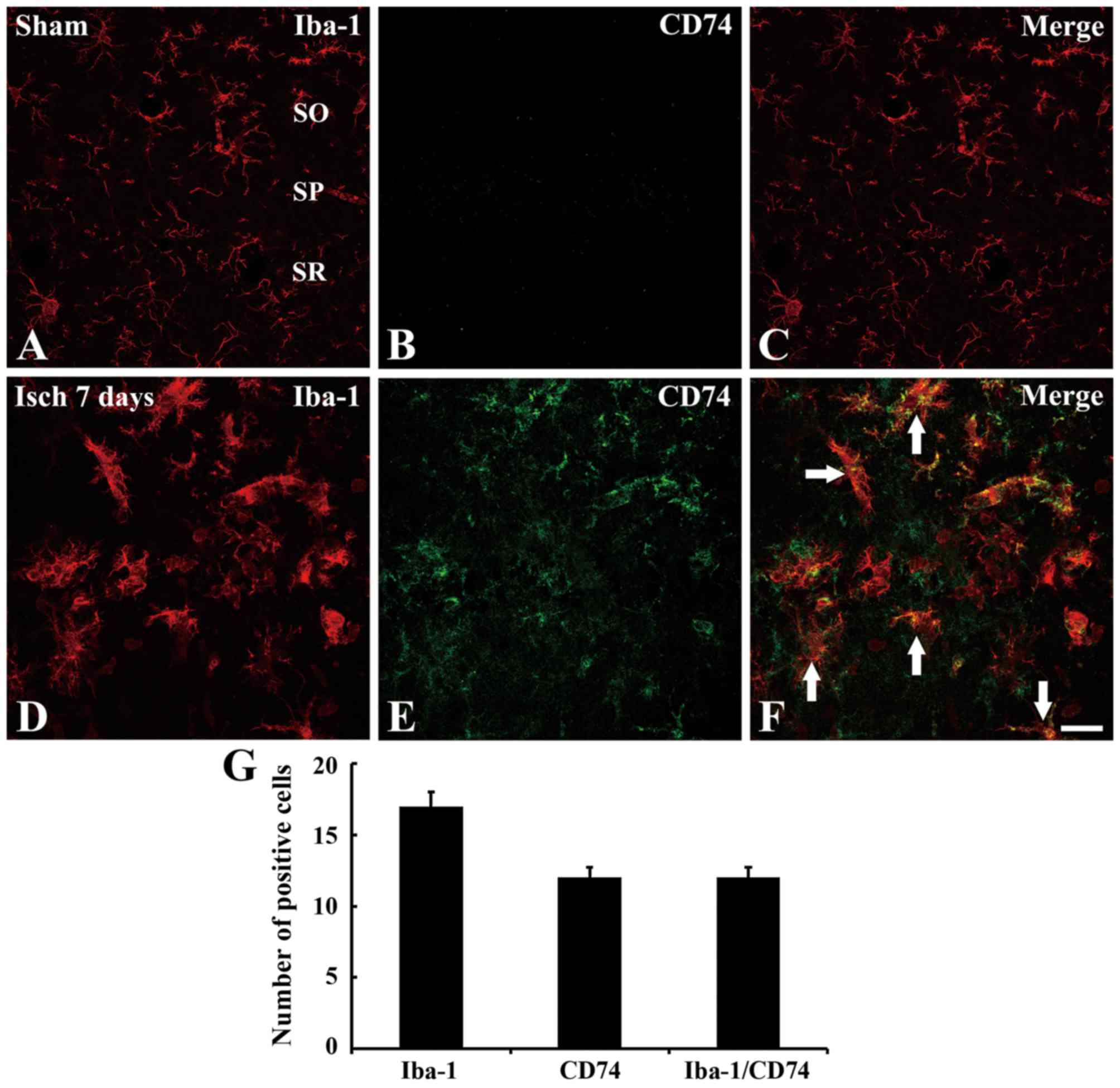 | Figure 3.Double immunofluorescence staining for
Iba-1 and CD74 in the hippocampal CA1 region. Immunofluorescence
staining in the sham group for (A) Iba-1, (B) CD74 and (C) their
merged overlay. Immunfluorescence staining in the ischemic model
group for (D) Iba-1, (E) CD74 and (F) their merged overlay. White
arrows indicate regions of Iba-1/CD74 overlap. Many of Iba-1
immunoreactive microglia demonstrate CD74 immunoreactivity in the
CA1 region. Scale bar=50 µm. (G) Mean number of Iba-1, CD74 and
Iba-1/CD74-immunoreactive cells in the CA1 region 7 days after
ischemia-reperfusion, n=7/group. Data are presented as the mean ±
standard error. Iba-1, ionized calcium-binding adapter molecule 1;
CD, cluster of differentiation; CA, Cornu Ammonis; SO, stratum
oriens; SP, stratum pyramidale; SR, stratum radiatum; Isch,
ischemia. |
Discussion
Microglial reactivity in the ischemic brain is
closely associated with the development of delayed neuronal cell
death in vulnerable regions (4,30).
An early and transient microglial reaction occurs throughout the
majority of the hippocampus within 24 h of ischemia, including the
hippocampal CA3 region, where no subsequent neuronal loss occurs
(31). In addition, our previous
study demonstrated chronological alterations in Iba-1-positive
microglia in the hippocampus after 5 min of transient cerebral
ischemia in gerbils (14).
However, to the best of our knowledge, few studies have
investigated the timing of microglia activation in the hippocampus,
following induction by transient cerebral ischemia.
The Iba-1 gene is located within the MHC class III
region of the brain (32), and is
specifically expressed in microglia (14,32,33).
Microglia proliferate within selectively vulnerable brain areas
with ischemia-induced neuronal cell death during the first 48 h
after ischemic injury (34,35).
Differential analysis of the M1 and M2 subtypes is important,
because M1 and M2 microglia demonstrate contradictory functions in
the inflammatory process of neurological disorders. The present
study observed a chronological change in M1 polarization (MHC-II-
and CD74-positive cells) in the hippocampal CA1 region after 5 min
of transient cerebral ischemia in gerbils. These results will
develop our understanding of the cell death process in the
hippocampus, following ischemic insults.
CD74 immunoreactivity was not observed in the
hippocampus of the sham-operated gerbils; however, CD74 was
expressed in Iba-1-immunoreactive microglia in the hippocampal CA1
region, ≥5 days after ischemic insult. In the hippocampal CA1
region, delayed neuronal death occurs several days after transient
cerebral ischemia in rodents (36–38).
In this regard, the present study observed M1 polarization after
neuronal death of CA1 pyramidal neurons. In particular, CD74
immunoreactivity gradually increased in the hippocampal CA1 region
≥5 days following ischemic insult. Notably, in a rat model of focal
cerebral ischemia induced by middle cerebral artery occlusion,
expression of the M1 marker CD16/32 was significantly increased 3
days after ischemic insult and remained elevated 14 days after
focal ischemia (16). Furthermore,
the authors reported that other M1-type genes, including inducible
nitric oxide synthase and CD11b, also gradually increased 3 days
after ischemia and remained at high levels 14 days later (16).
CD74 is also a ligand for MIF, which is released by
a variety of cell types. MIF is a proinflammatory cytokine that
binds to CD74 and sequentially activates the extracellular
signal-regulated kinase 1/2-dependent signal transduction pathway,
cell proliferation and prostaglandin E2 production (39). Overexpression of MIF significantly
decreases H2O2-induced cell death, and
knockdown of the MIF gene exacerbates neuronal damage in an animal
model of ischemic insult (23).
The present study observed an increase of CD74 immunoreactivity in
the gerbil hippocampal CA1 region; it may be hypothesized that this
increase could protect neurons from ischemic damage. However, it
was previously reported that MIF expression is decreased in the
infarct area of the mouse brain following ischemia (23). Based on these findings, the
expression pattern of CD74 may be varied in the damaged brain
tissue according to ischemic insults, with differences based on
whether the ischemia was global or focal, and the duration of the
transient ischemic insult.
In conclusion, CD74-immunoreactive activated M1
microglia were observed a few days after transient cerebral
ischemia in the gerbil hippocampal CA1 region. This observation
indicates that activated M1 microglia may be closely associated
with neuronal death in various microenvironments in the hippocampal
CA1 region following transient forebrain ischemia. Therefore, it
may be hypothesized that activated M1 microglia has potential as an
alternative target for the development of novel therapeutic
strategies for the treatment of patients with cerebral
ischemia.
Acknowledgements
This work was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and future Planning
(MSIP; grant no. NRF-2014R1A2A2A01005307), and by the Bio &
Medical Technology Development Program of the NRF, funded by the
Korean government, MSIP (grant no. NRF-2015M3A9B6066835).
References
|
1
|
Danton GH and Dietrich WD: Inflammatory
mechanisms after ischemia and stroke. J Neuropathol Exp Neurol.
62:127–136. 2003. View Article : Google Scholar
|
|
2
|
Stoll G and Jander S: The role of
microglia and macrophages in the pathophysiology of the CNS. Prog
Neurobiol. 58:233–247. 1999. View Article : Google Scholar
|
|
3
|
Kreutzberg GW: Microglia: A sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar
|
|
4
|
Lees GJ: The possible contribution of
microglia and macrophages to delayed neuronal death after ischemia.
J Neurol Sci. 114:119–122. 1993. View Article : Google Scholar
|
|
5
|
Stoll G, Jander S and Schroeter M:
Inflammation and glial responses in ischemic brain lesions. Prog
Neurobiol. 56:149–171. 1998. View Article : Google Scholar
|
|
6
|
Kato H: The role of microglia in ischemic
brain injuryInflammation and Stroke. Springer; pp. 89–99. 2001,
View Article : Google Scholar
|
|
7
|
Nakajima K, Tsuzaki N, Shimojo M, Hamanoue
M and Kohsaka S: Microglia isolated from rat brain secrete a
urokinase-type plasminogen activator. Brain Res. 577:285–292. 1992.
View Article : Google Scholar
|
|
8
|
Vaca K and Wendt E: Divergent effects of
astroglial and microglial secretions on neuron growth and survival.
Exp Neurol. 118:62–72. 1992. View Article : Google Scholar
|
|
9
|
Colton CA and Gilbert DL: Production of
superoxide anions by a CNS macrophage, the microglia. FEBS Lett.
223:284–288. 1987. View Article : Google Scholar
|
|
10
|
Han HS, Qiao Y, Karabiyikoglu M, Giffard
RG and Yenari MA: Influence of mild hypothermia on inducible nitric
oxide synthase expression and reactive nitrogen production in
experimental stroke and inflammation. J Neurosci. 22:3921–3928.
2002.
|
|
11
|
Stumm R, Culmsee C, Schafer MK,
Krieglstein J and Weihe E: Adaptive plasticity in tachykinin and
tachykinin receptor expression after focal cerebral ischemia is
differentially linked to gabaergic and glutamatergic
cerebrocortical circuits and cerebrovenular endothelium. J
Neurosci. 21:798–811. 2001.
|
|
12
|
Maeda Y, Matsumoto M, Hori O, Kuwabara K,
Ogawa S, Yan SD, Ohtsuki T, Kinoshita T, Kamada T and Stern DM:
Hypoxia/reoxygenation-mediated induction of astrocyte interleukin
6: A paracrine mechanism potentially enhancing neuron survival. J
Exp Med. 180:2297–2308. 1994. View Article : Google Scholar :
|
|
13
|
Suzuki S, Tanaka K, Nogawa S, Nagata E,
Ito D, Dembo T and Fukuuchi Y: Temporal profile and cellular
localization of interleukin-6 protein after focal cerebral ischemia
in rats. J Cereb Blood Flow Metab. 19:1256–1262. 1999. View Article : Google Scholar
|
|
14
|
Hwang IK, Yoo KY, Kim DW, Choi SY, Kang
TC, Kim YS and Won MH: Ionized calcium-binding adapter molecule 1
immunoreactive cells change in the gerbil hippocampal CA1 region
after ischemia/reperfusion. Neurochem Res. 31:957–965. 2006.
View Article : Google Scholar
|
|
15
|
Kigerl KA, Gensel JC, Ankeny DP, Alexander
JK, Donnelly DJ and Popovich PG: Identification of two distinct
macrophage subsets with divergent effects causing either
neurotoxicity or regeneration in the injured mouse spinal cord. J
Neurosci. 29:13435–13444. 2009. View Article : Google Scholar :
|
|
16
|
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen
S, Gao Y and Chen J: Microglia/macrophage polarization dynamics
reveal novel mechanism of injury expansion after focal cerebral
ischemia. Stroke. 43:3063–3070. 2012. View Article : Google Scholar
|
|
17
|
Perego C, Fumagalli S and De Simoni MG:
Temporal pattern of expression and colocalization of
microglia/macrophage phenotype markers following brain ischemic
injury in mice. J Neuroinflammation. 8:1742011. View Article : Google Scholar :
|
|
18
|
Sudduth TL, Schmitt FA, Nelson PT and
Wilcock DM: Neuroinflammatory phenotype in early Alzheimer's
disease. Neurobiol Aging. 34:1051–1059. 2013. View Article : Google Scholar
|
|
19
|
Frieler RA, Meng H, Duan SZ, Berger S,
Schütz G, He Y, Xi G, Wang MM and Mortensen RM: Myeloid-specific
deletion of the mineralocorticoid receptor reduces infarct volume
and alters inflammation during cerebral ischemia. Stroke.
42:179–185. 2011. View Article : Google Scholar
|
|
20
|
Liu YR, Lei RY, Wang CE, Zhang BA, Lu H,
Zhu HC and Zhang GB: Effects of catalpol on ATPase and amino acids
in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci.
35:1229–1233. 2014. View Article : Google Scholar
|
|
21
|
Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S,
Xie N, Wang L, Chen T, Shaw C and Cai J: Donepezil attenuates
hippocampal neuronal damage and cognitive deficits after global
cerebral ischemia in gerbils. Neurosci Lett. 510:29–33. 2012.
View Article : Google Scholar
|
|
22
|
Qi J, Li Y, Zhang H, Cheng Y, Sung Y, Cao
J, Zhao Y and Wang F: A novel conjugate of low-molecular-weight
heparin and Cu, Zn-superoxide dismutase: Study on its mechanism in
preventing brain reperfusion injury after ischemia in gerbils.
Brain Res. 1260:76–83. 2009. View Article : Google Scholar
|
|
23
|
Zhang YB, Kan MY, Yang ZH, Ding WL, Yi J,
Chen HZ and Lu Y: Neuroprotective effects of N-stearoyltyrosine on
transient global cerebral ischemia in gerbils. Brain Res.
1287:146–156. 2009. View Article : Google Scholar
|
|
24
|
Institute of Laboratory Animal Research,
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals, National Research Council, . Guide for the care
and use of laboratory animals. 8th. National Academies Press;
Washington, DC: pp. 2202011
|
|
25
|
Lee CH, Park JH, Yoo KY, Choi JH, Hwang
IK, Ryu PD, Kim DH, Kwon YG, Kim YM and Won MH: Pre- and
post-treatments with escitalopram protect against experimental
ischemic neuronal damage via regulation of BDNF expression and
oxidative stress. Exp Neurol. 229:450–459. 2011. View Article : Google Scholar
|
|
26
|
Park JH, Shin BN, Chen BH, Kim IH, Ahn JH,
Cho JH, Tae HJ, Lee JC, Lee CH, Kim YM, et al: Neuroprotection and
reduced gliosis by atomoxetine pretreatment in a gerbil model of
transient cerebral ischemia. J Neurol Sci. 359:373–380. 2015.
View Article : Google Scholar
|
|
27
|
Schmued LC and Hopkins KJ: Fluoro-Jade B:
A high affinity fluorescent marker for the localization of neuronal
degeneration. Brain Res. 874:123–130. 2000. View Article : Google Scholar
|
|
28
|
Lee CH, Park JH, Cho JH, Ahn JH, Yan BC,
Lee JC, Shin MC, Cheon SH, Cho YS, Cho JH, et al: Changes and
expressions of Redd1 in neurons and glial cells in the gerbil
hippocampus proper following transient global cerebral ischemia. J
Neurol Sci. 344:43–50. 2014. View Article : Google Scholar
|
|
29
|
Loskota WJ, Lomax P and Verity MA: A
stereotaxic atlas of the mongolian gerbil brain (Meriones
unguiculatus). Mich Ann Arbor Science. 1974.
|
|
30
|
Yan BC, Park JH, Ahn JH, Choi JH, Yoo KY,
Lee CH, Cho JH, Kim SK, Lee YL, Shin HC and Won MH: Comparison of
glial activation in the hippocampal CA1 region between the young
and adult gerbils after transient cerebral ischemia. Cell Mol
Neurobiol. 32:1127–1138. 2012. View Article : Google Scholar
|
|
31
|
Finsen BR, Jørgensen MB, Diemer NH and
Zimmer J: Microglial MHC antigen expression after ischemic and
kainic acid lesions of the adult rat hippocampus. Glia. 7:41–49.
1993. View Article : Google Scholar
|
|
32
|
Imai Y, Ibata I, Ito D, Ohsawa K and
Kohsaka S: A novel gene iba1 in the major histocompatibility
complex class III region encoding an EF hand protein expressed in a
monocytic lineage. Biochem Biophys Res Commun. 224:855–862. 1996.
View Article : Google Scholar
|
|
33
|
Ito D, Imai Y, Ohsawa K, Nakajima K,
Fukuuchi Y and Kohsaka S: Microglia-specific localisation of a
novel calcium binding protein, Iba1. Brain Res Mol Brain Res.
57:1–9. 1998. View Article : Google Scholar
|
|
34
|
Gehrmann J, Bonnekoh P, Miyazawa T,
Hossmann KA and Kreutzberg GW: Immunocytochemical study of an early
microglial activation in ischemia. J Cereb Blood Flow Metab.
12:257–269. 1992. View Article : Google Scholar
|
|
35
|
Gehrmann J, Bonnekoh P, Miyazawa T,
Oschlies U, Dux E, Hossmann KA and Kreutzberg GW: The microglial
reaction in the rat hippocampus following global ischemia:
Immuno-electron microscopy. Acta Neuropathol. 84:588–595. 1992.
View Article : Google Scholar
|
|
36
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar
|
|
37
|
Miyawaki S, Imai H, Hayasaka T, Masaki N,
Ono H, Ochi T, Ito A, Nakatomi H, Setou M and Saito N: Imaging mass
spectrometry detects dynamic changes of phosphatidylcholine in rat
hippocampal CA1 after transient global ischemia. Neuroscience.
322:66–77. 2016. View Article : Google Scholar
|
|
38
|
Pulsinelli WA, Brierley JB and Plum F:
Temporal profile of neuronal damage in a model of transient
forebrain ischemia. Ann Neurol. 11:491–498. 1982. View Article : Google Scholar
|
|
39
|
Leng L, Metz CN, Fang Y, Xu J, Donnelly S,
Baugh J, Delohery T, Chen Y, Mitchell RA and Bucala R: MIF signal
transduction initiated by binding to CD74. J Exp Med.
197:1467–1476. 2003. View Article : Google Scholar :
|

















