Introduction
Lung cancer is one of the most common malignancies
and is the major cause of cancer-associated mortality, accounting
for ~1.38 million deaths each year (1,2). Of
all lung carcinomas, non-small cell lung cancer (NSCLC) accounts
for ~70–85% (3). Although there
have been recent advances in diagnosis and treatment, the prognosis
of lung cancer is still unfavorable and the 5-year overall survival
(OS) rate remains <15% (4,5). In
previous decades, studies reported that microRNAs (miRNAs/miRs) may
serve an important role in NSCLC pathogenesis, which provides novel
insights into disease biology (6,7).
Therefore, improved understanding of detailed mechanisms of NSCLC
with miRNAs is necessary for development of effective therapeutic
strategies.
miRNAs are a class of 20–24 nucleotide-long
non-coding RNAs, which regulate gene expression at the
post-transcriptional level through mRNA interference, and are
involved in cell development, proliferation, differentiation and
apoptosis (8,9). There are currently ~2000 miRNAs that
have been identified and this number is rapidly increasing. A large
number of miRNAs have been investigated in cancer research as
therapeutic targets, with certain miRNAs identified as being
associated with the tumor metastasis of NSCLC. For instance,
upregulation of miR-24 promotes cell proliferation by targeting
nuclear apoptosis inducing factor 1in NSCLC (10). Overexpression of miR-328 has a role
in conferring migratory potential to NSCLC cells through targeting
protein kinase C alpha (11).
miR-34c-3p functions as a tumor suppressor by inhibiting eukaryotic
initiation factor-4E expression in NSCLC (12). miR-99a suppresses the metastasis of
human NSCLC by targeting AKT serine/threonine kinase 1 (13). However, there are few studies
researching the expression of miR-124a in NSCLC and the detailed
molecular mechanism of miR-124a in NSCLC requires further
investigation.
In the present study, the differential expression of
miR-124a in NSCLC was analyzed using the GEO database and the
association between miR-124a and NSCLC was subsequently revealed.
Furthermore, the expression status of miR-124a in NSCLC was
identified by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) for a group of NSCLC biopsies. Furthermore, the
prognostic significance of NSCLC and the response to chemotherapy
was assessed.
Materials and methods
Data and date source
All miR-124a expression datasets were downloaded
from GEO (www.pubmed.com/geo). Data were
retrieved using the keywords ‘miR-124a’ and ‘NSCLC’. The first data
set, GSE10021, has samples taken from 16 human cell lines (14), and includes the following cell
lines: Human lung carcinoma A549, fibrosarcoma HT1080, cervix
carcinoma Henrietta Lacks, cervix carcinoma HeLaS3, hepatocellular
carcinoma Huh7, breast adenocarcinoma MCF7, breast adenocarcinoma
MDAMB231, embryonal kidney HEK293T, colon adenocarcinoma HT29,
hepatocellular carcinoma HepG2, neuroblastoma SKNMC, colon
adenocarcinoma Caco2, embryonal kidney HEK293 and colon carcinoma
HCT116. The second data set, GSE61741, has samples taken from
normal patients and patients with cancer (15), and it has a total of 1,049 samples
out of which 94 were normal and 15 were long-lived individuals and
940 patients had been screened for the complete miRNA repertoire.
The third data set, GSE63805, has samples taken from normal
patients and patients with lung cancer (16), and it has a total of 62 samples out
of which 31 were normal and 31 were cancerous. The last data set,
GSE17681, has samples taken from normal patients and patients with
lung cancer (17), and it has a
total of 36 samples out of which 19 were normal and 17 were
cancerous.
Clinical specimens
A total of 160 cases of surgically resected NSCLC
and 32 paired normal adjacent lung tissue samples were evaluated
for miRNA expression. These specimens were collected from patients
from the tissue bank in China-Japan Union Hospital, Jilin
University (Changchun, China) between January 2008 and December
2012. All patients gave their informed consent, and the Ethical and
Scientific Committees of Shanghai Tenth People's Hospital, Tongji
University School of Medicine (Shanghai, China) approved the study.
The tumors of NSCLC were staged based on the 7th edition of the
AJCC tumor node metastasis (TNM) staging system (18). In addition, several clinical
characteristics of these patients were assessed, including age,
gender, lymph-node metastasis, tumor differentiation, histological
subtypes, TNM stage, invasion of lung membrane, vascular invasion,
tumor size, chemotherapy, miR-124a expression status, OS and
disease-free survival (DFS). Age was stratified according to ≥60 or
<60 years. Tumor size was divided into ≥5 and <5 cm based on
the mean tumor diameter. OS represented the date of diagnosis to
the date of death, from any cause. DFS was represented the original
date of diagnosis to the first date of recurrence, or death. All
clinical data were confirmed by the patient or relatives, by
medical recording, by the social security record, or by follow-up
record.
RNA extraction and RT-qPCR
Total RNA was extracted from tissues using the
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's instructions. RNA concentration and
purity was assessed using a NanoDrop ND-1000 (Thermo Fisher
Scientific, Inc.). A total of10 ng total RNA was used for cDNA
synthesis using the Taqman MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Reverse
transcription with a miR-124a-specific primer was performed using
ABI's TaqMan MicroRNA Reverse Transcription kit, miR-124a
expression level was detected using a Taqman MicroRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.) (19). The RT-qPCR thermocycling conditions
were as follows: 94°C for 30 sec (initial denaturation), 94°C for 5
sec (denaturation) and 55°C for 30 sec (annealing), for 40 cycles.
U6 expression was used as the internal control. The following
primers were used: miR-124a forward, 5′-GGTAAGGCACGCGGT-3′, and
reverse, 5′-CAGTGCGTGTCGTGGAGT-3′; U6 forward,
5′-CTGGTTAGTACTTGGACGGGAGAC-3′, and reverse,
5′-GTGCAGGGTCCGAGGT-3′. All reactions were performed three times in
triplicate, and relative expression of miRNAs was calculated using
the standard curve and the ΔΔCq method (20).
Statistical analysis
All statistical data were analyzed using SPSS
software (version, 19.0; IBM SPSS; Armonk, NY, USA). Significant
differences between the two groups were evaluated using an
independent t-test. The expression of miR-124a and other
data were presented as the mean ± standard deviation. χ2
test analysis was used to assess differences in patient
characteristics. OS rates were calculated actuarially according to
the Kaplan-Meier method and results were compared with a log-rank
test. To examine which individual characteristics played important
roles in survival, univariate and multivariate Cox regression
analysis were conducted. P<0.05 was considered to indicate a
statistically significant difference and P<0.001 was considered
highly significant.
Results
Analysis of miR-124a expression using
an online database
The expression of miR-124a in 16 different cell
lines was analyzed via the GEO database (Fig. 1A), which revealed that miR-124a
exhibited relatively decreased expression in lung cancer A549
cells, when compared with most other cell lines.
miR-124a was identified as being relatively
expressed in 104 normal samples (114.41±64.55), 120 Wilms' tumor
samples (102.75±60.35), 14 gastric cancer samples (98.68±48.73), 62
lung cancer samples (89.27±45.46), 19 glioma samples (81.19±49.43),
33 melanoma samples (79.69±49.62), 20 renal cancer samples
(76.57±45.03) and 27 colon cancer samples (72.06±37.34), using the
GEO database (Fig. 1B). The
results indicated that miR-124a exhibited decreased expression in
tumor tissues, when compared with normal tissue.
A total of 30 paired lung cancer tissues were
investigated from a dataset on the GEO database (Fig. 1C). The fold-change for relative
miR-124a expression levels was calculated using the Log2 ratio of
paired tumor/normal expression, and miR-124awas observed to be
downregulated in over half of the lung cancer cases [fold change
(FC) =0.81; P=0.021].
Relative miR-124a expression in 17 lung cancer blood
samples vs. 17 adjacent normal tissues was investigated using the
GEO database (Fig. 1D). The
analysis suggested that miR-124a was downregulated in lung cancer
tissues (18.55±6.75), when compared with adjacent normal tissues
(40.76±16.48; FC=0.45; P=0.048).
miR-124a expression in NSCLC and
normal lung tissue
To validate these findings, 32 paired lung cancer
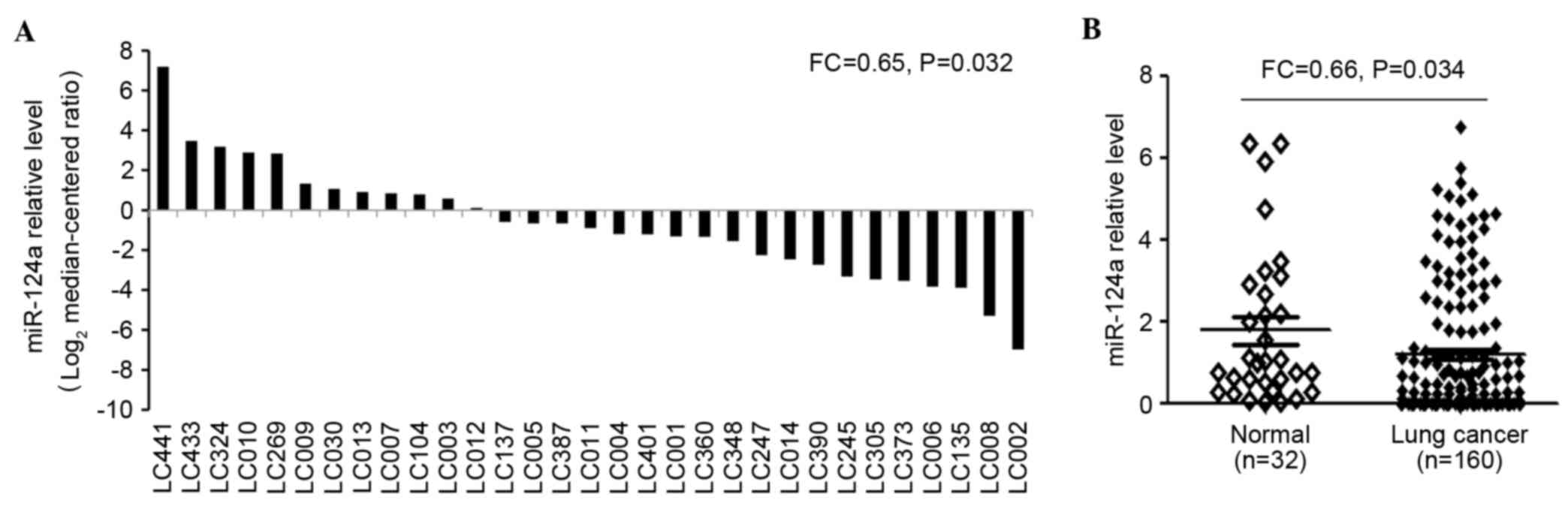
tissues were evaluated using RT-qPCR (Fig. 2A). The results demonstrated that
miR-124a was expressed at significantly decreased levels in most
lung cancer cases (FC=0.64; P=0.032). In addition, the expression
levels of miR-124a were examined in tumor (n=160) and adjacent
non-neoplastic tissues (n=32) using RT-qPCR (Fig. 2B), which demonstrated that miR-124a
expression levels were significantly decreased in NSCLC tumor
biopsies (1.14±0.85), when compared with adjacent normal tissues
(1.76±0.63; FC=0.65; P=0.034).
Association between miR-124a
expression and clinical characteristics
Univariate analysis was used to investigate the
relationship between miR-124a expression and clinical
characteristics in 160 cases of NSCLC. The results revealed that
the expression levels of miR-124a were significantly associated
with lymph node metastasis (P=0.021), tumor differentiation
(P=0.047), TNM stage (P=0.036) and diameter (P=0.026; Table I). Conversely, the results
demonstrated that age, gender, smoking history, histology, invasion
of lung membrane, vascular invasion and chemotherapy have no
significant association with miR-124a expression (P>0.05;
Table I).
 | Table I.Univariate analysis of overall
patient survival stratified by clinical characteristics. |
Table I.
Univariate analysis of overall
patient survival stratified by clinical characteristics.
|
|
|
|
|
| Overall
survival |
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Variable | n | miR-124a
expression, mean ± standard deviation | P-value | Months, mean | 95% CI, mean | P-value (log-rank
test) |
|---|
| Age |
|
| ≥60 years | 97 | 1.26±0.63 | 0.281 | 22.34 | 21.06–23.17 | 0.326 |
|
| <60 years | 63 | 1.11±0.62 |
| 25.19 | 22.45–29.63 |
|
| Gender |
|
| Male | 97 | 1.19±0.61 | 0.487 | 24.76 | 22.09–26.04 | 0.168 |
|
| Female | 63 | 1.21±0.66 |
| 25.33 | 21.58–28.33 |
|
| Smoking
history |
|
| Never | 38 | 1.29±0.42 | 0.115 | 26.11 | 24.39–28.63 | 0.091 |
|
| Ever | 65 | 1.18±0.79 |
| 24.86 | 22.77–26.34 |
|
|
| Unknown | 57 | 1.26±0.67 |
| 26.34 | 24.91–28.76 |
|
| Lymphnode
metastasis |
|
|
| 0.021 |
|
| 0.039 |
|
| Negative | 91 | 1.39±0.81 |
| 26.38 | 24.73–33.59 |
|
|
| Positive | 57 | 1.11±0.13 |
| 20.44 | 17.06–24.31 |
|
|
| Unknown | 12 | 1.18±0.96 |
| 22.56 | 20.84–25.69 |
|
| Tumor
differentiation |
|
|
| 0.047 |
|
| 0.225 |
|
| Poor | 18 | 0.86±0.25 |
| 24.03 | 24.60–33.68 |
|
|
| Moderate | 84 | 1.18±0.13 |
| 26.68 | 22.28–28.34 |
|
|
| Well | 58 | 1.39±0.26 |
| 26.56 | 22.70–29.75 |
|
| Histology |
|
|
|
|
|
|
|
|
| Adenocarcinoma | 55 | 1.19±0.53 | 0.634 | 26.63 | 23.47–29.86 | 0.353 |
|
| Squamous cell
carcinoma | 105 | 1.27±0.38 |
| 25.98 | 23.33–28.74 |
|
| TNM stage |
|
|
|
|
|
|
|
|
| I–II | 104 | 1.28±0.38 | 0.036 | 27.58 | 24.19–30.36 | 0.027 |
|
| III–IV | 56 | 0.81±0.26 |
| 23.46 | 18.69–25.43 |
|
| Invasion of lung
membrane |
|
|
| 0.068 |
|
| 0.088 |
|
| Negative | 34 | 1.24±0.68 |
| 30.36 | 22.68–41.33 |
|
|
| Positive | 114 | 0.99±0.86 |
| 26.83 | 22.48–28.55 |
|
|
| Unknown | 12 | 1.18±0.37 |
| 25.43 | 22.68–26.47 |
|
| Vascular
invasion |
|
| Negative | 145 | 1.21±0.79 | 0.691 | 26.58 | 21.67–29.23 | 0.318 |
|
| Positive | 3 | 0.97±0.83 |
| 25.24 | 23.36–42.58 |
|
|
| Unknown | 12 | 1.21±0.36 |
| 24.37 | 21.48–30.59 |
|
| Chemotherapy |
|
|
|
|
|
|
|
|
| Negative | 80 | 1.17±0.36 | 0.543 | 20.58 | 18.69–24.37 | 0.029 |
|
| Positive | 69 | 1.25±0.63 |
| 26.94 | 23.06–28.94 |
|
|
| Unknown | 11 |
|
|
|
|
|
| Diameter |
|
|
|
|
|
|
|
|
| ≥5 cm | 39 | 0.88±0.35 | 0.026 | 21.93 | 18.45–26.04 | <0.001 |
|
| <5 cm | 121 | 1.36±0.62 |
| 27.86 | 25.39–29.28 |
|
Expression levels of miR-124a area
prognostic marker in NSCLC survival
Univariate analysis of OS based on patients
stratified by clinical characteristics presented in Table I. These clinical characteristics
with univariate analysis included age, gender, smoking history,
lymph-node metastasis, tumor differentiation, histology, TNM stage,
invasion of lung membrane, vascular invasion, tumor diameter and
miR-125a-3p expression. Obviously, there was a meaningful
relationship between short OS and several clinical characteristics,
including lymph-node metastasis (P=0.039), TNM stage (P=0.027),
diameter (P<0.001) and lack of treatment with chemotherapy
(P=0.029). Conclusively, it was identified that the patients who
exhibited lymph-node metastasis, high TNM stage, tumor size
exceeding 0.5 cm or those who had not received chemotherapy
exhibited increased mortality (decreased OS).
To determine which clinical characteristics were
associated with the prognosis of NSCLC, univariate survival
analysis with Kaplan-Meier were performed. Several clinical
parameters with Kaplan-Meier survival curves presented a good
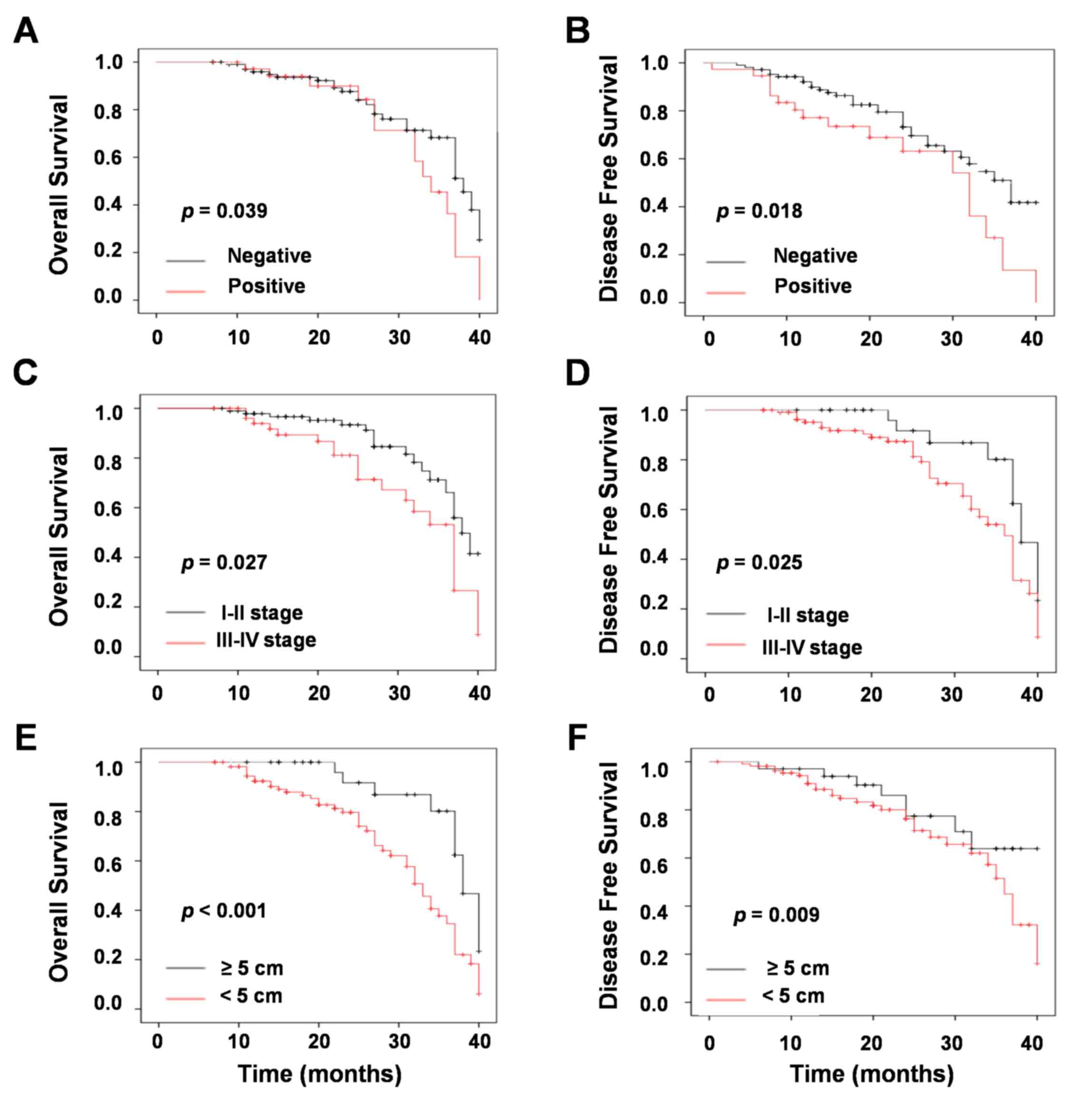
prognosis for patients with NSCLC. Negative lymph-node metastasis
was significantly associated with increased OS (P=0.039; Fig. 3A) and DFS (P=0.018; Fig. 3B) in patients with NSCLC.
Similarly, low TNM stage was positively associated with increased
OS (P=0.027; Fig. 3A) and DFS
(P=0.025; Fig. 3B). In addition,
tumor size was significantly associated with increased OS
(P<0.001; Fig. 3E) and DFS
(P=0.009; Fig. 3F).
Univariate analysis with a Cox proportional hazards
regression model was used to explore the prognosis of clinical
characteristics. The result revealed that there were four
parameters significantly associated with good prognosis, including
lymph node metastasis [P=0.03; HR=1.72 (1.18–2.96); Table II], TNM stage [P=0.02; HR=1.83
(1.56–2.64); Table II], tumor
diameter [P<0.001; HR=2.56 (2.07–5.46); Table II], as well as miR-124a expression
[P=0.023; HR=0.68 (0.52–0.73); Table
II]. There was no association between prognosis and several
parameters, including age, gender, smoking history, tumor
differentiation, histology, invasion of lung membrane or vascular
invasion. Therefore, these results suggested that miR-124a serves
an important role in NSCLC progression. In addition, it was
summarized that miR-124a is involved in the prognosis of NSCLC
patients.
 | Table II.Cox regression model analysis for
prognosis based on various clinical characteristics of patients
with non-small cell lung cancer. |
Table II.
Cox regression model analysis for
prognosis based on various clinical characteristics of patients
with non-small cell lung cancer.
|
|
|
|
| miR-124a
multivariate analysis |
|---|
|
|
|
|
|
|
|---|
| Factor | HR | 95% CI
(univariate) | P-value | HR | 95% CI
(multivariate) | P-value |
|---|
| Age | 0.96 | 0.69–1.14 | 0.45 |
|
|
|
|
| Gender | 0.87 | 0.53–1.16 | 0.18 |
|
|
|
|
| Smoking
history | 1.21 | 0.71–1.52 | 0.19 |
|
|
|
|
| Lymph-node
metastasis | 1.72 | 1.18–2.96 | 0.03 | 1.98 | 1.25–3.03 | 0.015 |
|
|
|
|
| Tumor
differentiation | 0.88 | 0.54–1.01 | 0.18 |
|
|
|
|
| Histology | 1.18 | 0.86–1.21 | 0.34 |
|
|
|
|
| TNM stage | 1.83 | 1.56–2.64 | 0.02 | 2.13 | 1.61–2.89 | 0.009 |
| Invasion of lung
membrane | 1.22 | 1.06–1.37 | 0.15 |
|
|
|
|
| Vascular
invasion | 0.98 | 0.68–1.54 | 0.31 |
|
|
|
| Diameter | 2.56 | 2.07–5.46 | <0.001 | 3.65 | 2.26–5.43 | <0.001 |
| miR-124a
expression | 0.68 | 0.52–0.73 | 0.023 |
|
|
|
To further investigate the association between
miR-124a expression and the prognosis of patients with NSCLC,
multivariate Cox proportional hazards regression analysis was
conducted. The model included all of the characteristics relative
to predicted OS in the univariate analysis of the entire patients
as presented in Table I. There
were three characteristics that presented a significant association
with prognosis, including lymph-node metastasis [P=0.015; HR=1.98
(1.25–3.03); Table II], TNM stage
[P=0.009; HR=2.13 (1.61–2.89); Table
II] and tumor diameter [P<0.001; HR=3.65 (2.26–5.43);
Table II]. Multivariable Cox
regression model analysis revealed that increased expression of
miR-124a was determined to be a predictor of increased OS in
patients with NSCLC.
Association amongst survival,
chemotherapy and miR-124a expression
Chemotherapy is a major treatment of NSCLC, thus it
was analyzed as an influential factor inOS and DFS. According to
Table III, chemotherapy was
demonstrated to significantly prolong OS (29.94±1.57 vs.
22.58±1.96; P=0.029) and DFS (26.43±3.56 vs. 20.06±3.78; P=0.032)
in the entire group. By comprehensive analysis of chemotherapy and
miR-124a expression level, it was concluded that patients who
received chemotherapy with high expression of miR-124a exhibited an
increased OS (32.78±6.96 vs. 20.43±3.58; P=0.001) and DFS
(29.33±2.65 vs. 20.06±3.98, P<0.001), compared with those who
did not receive chemotherapy with low expression of miR-124a.
 | Table III.OS and DFS of patients with non-small
cell lung cancer stratified by chemotherapy alone, or chemotherapy
and miR-124a expression. |
Table III.
OS and DFS of patients with non-small
cell lung cancer stratified by chemotherapy alone, or chemotherapy
and miR-124a expression.
|
| OS | DFS |
|---|
|
|
|
|
|---|
|
| Mean ± SD | 95% CI | P-value | Mean ± SD | 95% CI | P-value |
|---|
| Chemotherapy |
|
Positive | 29.94±1.57 | 23.06–33.94 | 0.029 | 26.43±3.56 | 25.43–28.04 | 0.032 |
|
Negative | 22.58±1.96 | 18.69–24.37 |
| 20.06±3.78 | 18.78–25.33 |
|
Chemotherapy+expression |
|
P+H | 32.78±6.96 | 26.79–36.43 | 0.001 | 29.33±2.65 | 26.04–33.41 | <0.001 |
|
N+L | 20.43±3.58 | 16.98–22.54 |
| 20.06±3.98 | 18.07–24.49 |
|
To further identify whether chemotherapy or with
miR-124a expression was associated with OS and DFS, univariate and
multivariate survival analysis with Kaplan-Meier estimates were
conducted. The result of univariate survival analysis indicated
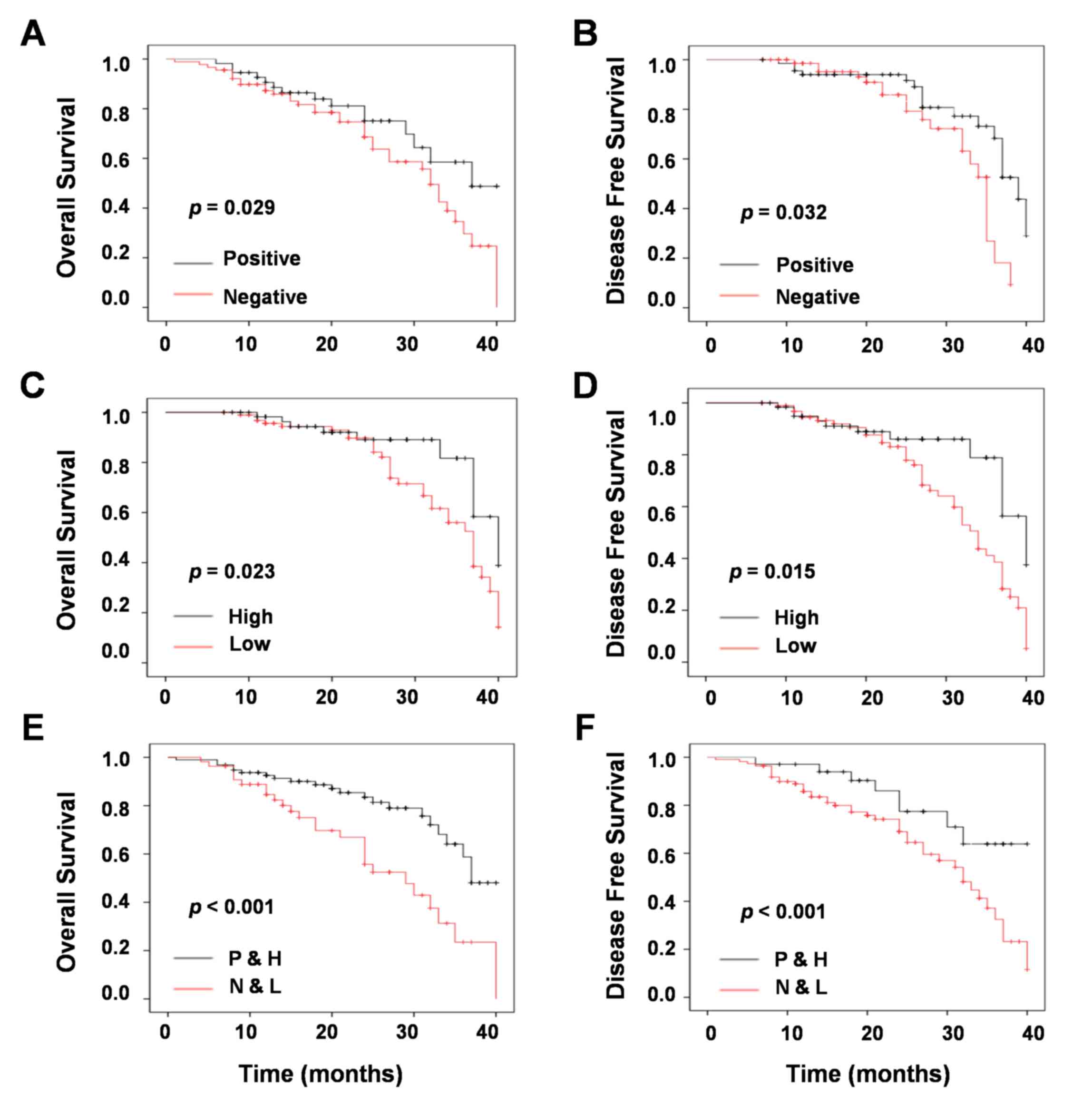
that increased OS (P=0.029; Fig.
4A) and DFS (P=0.032; Fig. 4B)
was significantly associated with chemotherapy compared with the
results from untreated patients. In addition, high expression of
miR-124a increased OS (P=0.023; Fig.
4C) and DFS (P=0.015; Fig. 4D)
when compared with low expression. The result of multivariate
survival analysis demonstrated that chemotherapy with high
expression of miR-124a prolonged OS (P<0.001; Fig. 4E) and DFS (P<0.001; Fig. 4F).
Discussion
Evidence has demonstrated that miRNAs regulated cell
proliferation, cell metastasis and apoptosis at a post
transcriptional level. There are previous studies that have
reported that miRNAs serve a crucial role in the development of
chemosensitivity or chemoresistance in NSCLC (21,22).
In addition, miRNAs expression level has been identified to be
associatedwith tumor development (23). In recent years, miRNAs have been
used to detect early diagnosis, prognosis and therapeutic
evaluation (24).
miR-124a has been identified to be a novel
suppressor for cancer, and has been reported to be associated with
the suppressive effects of a variety of human cancers, including
breast, glioma, gastric cancer and colitis (25). Certain previous studies reported
that methylation of miR-124a is associated with aggressive and
advanced breast cancer disease (26,27).
Furthermore, miR-124a has been investigated to potentially inhibit
glioma cell proliferation and invasion by blocking the expression
of a particular gene (28,29). In addition, the methylation of
miR-124a was identified early in colorectal carcinogenesis
(30,31) and was epigenetically silenced in
the development of uveal melanoma (32).
In the present study, the expression of miR-124a in
160 NSCLC tissues and 32 paired normal tissues was evaluated.
Several previous papers reported that miR-124a expression level in
normal tissues is higher than those in lung cancer tissues
(33–35). Consistently, miR-124a expression
levels were lower in NSCLC tumor biopsies, when compared to
adjacent normal tissues. The OS in patients with high expression of
miR-124a was prolonged relative to patients with low expression of
miR-124a. Therefore, miR-124a may bea deregulated gene in NSCLC.
The expression levels of miR-124a were associated with clinical
characteristics, including lymph-node metastasis, tumor
differentiation, TNM stage and diameter. Frequently, lymph-node
metastasis, TNM stage, diameter and chemotherapy are associated
with a worse prognosis in patients. Therefore, there was a
significant association between miR-124a and prognosis. In
addition, there was a high expression of miR-124a identified with
chemotherapy that may increase OS. Therefore, it was concluded that
miR-124a may act as a biomarker for response to chemotherapy in
NSCLC.
Furthermore, the authors explored the biomarker role
of miR-124a in NSCLC. To the best of the authors' knowledge, it is
the first attempt to identify the status of miR-124a for
chemotherapy in NSCLC. Generally, tumor metastasis is a major cause
of high mortality of NSCLC. In recent decades, chemotherapy has
remained as the central therapeutic mainstay in metastatic lung
cancer, despite response rates being maintained at 30–40% and the
median survival being 7–12 months (36). For the past few years,
characteristic molecules began to play an important role in cancer
treatment (37). It is reported
that molecular biomarkers successfully respond to NSCLC
chemotherapy as diagnostic biomarkers and prognostic factors. For
instance, Perez-Carbonell et al (38) demonstrated that miR-320e was a
novel prognostic biomarker associated with adverse clinical outcome
in patients with stage III colorectal cancer treated with
5-FU-based adjuvant chemotherapy. In addition, miR-22, miR-24,
miR-34a and miR-638 were investigated as novel predictive
biomarkers (39,40). Furthermore, miR-200c, miR-744 and
miR-34a, have been reported to be prognostic biomarkers in
esophageal cancer, pancreatic cancer and breast cancer (41–43).
In conclusion, the present study reported the
clinical and prognostic relevance of miR-124a in patients with
NSCLC. These data revealed that the OS and DFS of patients
undergoing chemotherapy were prolonged in comparison with those not
receiving chemotherapy. Furthermore, patients who exhibited
increased expression of miR-124a with chemotherapeutic treatment
presented the longest OS and DFS, compared with those who exhibited
decreased expression of miR-124a without chemotherapy.
Conclusively, miR-124a is a predictive biomarker for the prognosis
of NSCLC with chemotherapy. In future studies, bioinformatic and
transcriptomic approaches, as well as functional analysis, will be
necessary to investigate the mechanistic role of miR-124a in NSCLC
and to confirm the present results.
Acknowledgements
The present study was partially supported by grants
from the National Natural Science Foundation of China (grant nos.
81201535, 81472202 and 81302065), Shanghai Natural Science
Foundation (grant nos. 12ZR1436000 and 16ZR1428900) and Shanghai
Municipal Commission of Health and Family Planning (grant nos.
201440398 and 201540228), Jiangxi Province Department of Science
Plan Funded Projects (grant no. 2011BBG70046), Hunan Natural
Science Fund for Distinguished Young Scholars Project (grant no.
2015JJ1009) and the Jilin Provincial Science and Technology
Department (grant nos. 20130727029YY, 20140f14061GH and
20150204057SF).
References
|
1
|
Hamamoto J, Soejima K, Yoda S, Naoki K,
Nakayama S, Satomi R, Terai H, Ikemura S, Sato T, Yasuda H, et al:
Identification of microRNAs differentially expressed between lung
squamous cell carcinoma and lung adenocarcinoma. Mol Med Rep.
8:456–462. 2013.PubMed/NCBI
|
|
2
|
Meng W, Ye Z, Cui R, Perry J,
Dedousi-Huebner V, Huebner A, Wang Y, Li B, Volinia S, Nakanishi H,
et al: MicroRNA-31 predicts the presence of lymph node metastases
and survival in patients with lung adenocarcinoma. Clin Cancer Res.
19:5423–5433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tiseo M, Bordi P, Bortesi B, Boni L, Boni
C, Baldini E, Grossi F, Recchia F, Zanelli F, Fontanini G, et al:
ERCC1/BRCA1 expression and gene polymorphisms as prognostic and
predictive factors in advanced NSCLC treated with or without
cisplatin. Br J Cancer. 108:1695–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markou A, Sourvinou I, Vorkas PA, Yousef
GM and Lianidou E: Clinical evaluation of microRNA expression
profiling in non small cell lung cancer. Lung Cancer. 81:388–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitra R, Edmonds MD, Sun J, Zhao M, Yu H,
Eischen CM and Zhao Z: Reproducible combinatorial regulatory
networks elucidate novel oncogenic microRNAs in non-small cell lung
cancer. RNA. 20:1356–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie J, Yu F, Li D, Zhu X, Zhang X and Lv
Z: MicroRNA-218 regulates cisplatin (DPP) chemosensitivity in
non-small cell lung cancer by targeting RUNX2. Tumour Biol.
37:1197–1204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou YL, Xu YJ and Qiao CW: MiR-34c-3p
suppresses the proliferation and invasion of non-small cell lung
cancer (NSCLC) by inhibiting PAC1/MAPK pathway. Int J Clin Exp
Pathol. 8:6312–6322. 2015.PubMed/NCBI
|
|
8
|
Agirre X, Vilas-Zornoza A, Jiménez-Velasco
A, Martin-Subero JI, Cordeu L, Gárate L, San José-Eneriz E,
Abizanda G, Rodríguez-Otero P, Fortes P, et al: Epigenetic
silencing of the tumor suppressor microRNA Hsa-miR-124a regulates
CDK6 expression and confers a poor prognosis in acute lymphoblastic
leukemia. Cancer Res. 69:4443–4453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pierson J, Hostager B, Fan R and Vibhakar
R: Regulation of cyclin dependent kinase 6 by microRNA 124 in
medulloblastoma. J Neurooncol. 90:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao G, Liu L, Zhao T, Jin S, Jiang S, Cao
S, Han J, Xin Y, Dong Q, Liu X and Cui J: Upregulation of miR-24
promotes cell proliferation by targeting NAIF1 in non-small cell
lung cancer. Tumour Biol. 36:3693–3701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arora S, Ranade AR, Tran NL, Nasser S,
Sridhar S, Korn RL, Ross JT, Dhruv H, Foss KM, Sibenaller Z, et al:
MicroRNA-328 is associated with (non-small) cell lung cancer
(NSCLC) brain metastasis and mediates NSCLC migration. Int J
Cancer. 129:2621–2631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Wang X, Li J, Gu K, Lv L, Zhang S,
Che D, Cao J, Jin S and Yu Y: miR-34c-3p functions as a tumour
suppressor by inhibiting eIF4E expression in non-small cell lung
cancer. Cell Prolif. 48:582–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu SH, Zhang CL, Dong FS and Zhang YM:
miR-99a suppresses the metastasis of human non-small cell lung
cancer cells by targeting AKT1 signaling pathway. J Cell Biochem.
116:268–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruike Y, Ichimura A, Tsuchiya S, Shimizu
K, Kunimoto R, Okuno Y and Tsujimoto G: Global correlation analysis
for micro-RNA and mRNA expression profiles in human cell lines. J
Hum Genet. 53:515–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keller A, Leidinger P, Vogel B, Backes C,
ElSharawy A, Galata V, Mueller SC, Marquart S, Schrauder MG, Strick
R, et al: miRNAs can be generally associated with human pathologies
as exemplified for miR-144. BMC Med. 12:2242014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robles AI, Arai E, Mathé EA, Okayama H,
Schetter AJ, Brown D, Petersen D, Bowman ED, Noro R, Welsh JA, et
al: An integrated prognostic classifier for stage I lung
adenocarcinoma based on mRNA, microRNA, and DNA methylation
biomarkers. J Thorac Oncol. 10:1037–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keller A, Leidinger P, Borries A,
Wendschlag A, Wucherpfennig F, Scheffler M, Huwer H, Lenhof HP and
Meese E: miRNAs in lung cancer-studying complex fingerprints in
patient's blood cells by microarray experiments. BMC Cancer.
9:3532009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer
staging manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Acunzo M, Visone R, Romano G, Veronese A,
Lovat F, Palmieri D, Bottoni A, Garofalo M, Gasparini P, Condorelli
G, et al: miR-130a targets MET and induces TRAIL-sensitivity in
NSCLC by downregulating miR-221 and 222. Oncogene. 31:634–642.
2012.PubMed/NCBI
|
|
22
|
Garofalo M, Romano G, Di Leva G, Nuovo G,
Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, et al:
EGFR and MET receptor tyrosine kinase-altered microRNA expression
induces tumorigenesis and gefitinib resistance in lung cancers. Nat
Med. 18:74–82. 2011.PubMed/NCBI
|
|
23
|
Hou LK, Ma YS, Han Y, Lu GX, Luo P, Chang
ZY, Xie RT, Yang HQ, Chai L, Cai MX, et al: Association of
microRNA-33a molecular signature with non-small cell lung cancer
diagnosis and prognosis after chemotherapy. PLoS One.
12:e01704312017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Faversani A, Amatori S, Augello C, Colombo
F, Porretti L, Fanelli M, Ferrero S, Palleschi A, Pelicci PG,
Belloni E, et al: miR-494-3p is a novel tumor driver of lung
carcinogenesis. Oncotarget. 8:7231–7247. 2017.PubMed/NCBI
|
|
25
|
Zhou Q, Long L, Zhou T, Tian J and Zhou B:
Demethylation of MicroRNA-124a genes attenuated proliferation of
rheumatoid arthritis derived fibroblast-like synoviocytes and
synthesis of tumor necrosis factor-α. PLoS One. 11:e01642072016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ben Gacem R, Ben Abdelkrim O, Ziadi S, Ben
Dhiab M and Trimeche M: Methylation of miR-124a-1, miR-124a-2, and
miR-124a-3 genes correlates with aggressive and advanced breast
cancer disease. Tumour Biol. 35:4047–4056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang YJ, Wang QY, Zhou CX, Yin QQ, He M,
Yu XT, Cao DX, Chen GQ, He JR and Zhao Q: MiR-124 targets Slug to
regulate epithelial-mesenchymal transition and metastasis of breast
cancer. Carcinogenesis. 34:713–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tivnan A, Zhao J, Johns TG, Day BW,
Stringer BW, Boyd AW, Tiwari S, Giles KM, Teo C and McDonald KL:
The tumor suppressor microRNA, miR-124a, is regulated by epigenetic
silencing and by the transcriptional factor, REST in glioblastoma.
Tumour Biol. 35:1459–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu SH, Jiang XJ, Xiao GL, Liu DY and Yuan
XR: miR-124a restoration inhibits glioma cell proliferation and
invasion by suppressing IQGAP1 and β-catenin. Oncol Rep.
32:2104–2110. 2014.PubMed/NCBI
|
|
30
|
Deng G, Kakar S and Kim YS: MicroRNA-124a
and microRNA-34b/c are frequently methylated in all histological
types of colorectal cancer and polyps, and in the adjacent normal
mucosa. Oncol Lett. 2:175–180. 2011.PubMed/NCBI
|
|
31
|
Ueda Y, Ando T, Nanjo S, Ushijima T and
Sugiyama T: DNA methylation of microRNA-124a is a potential risk
marker of colitis-associated cancer in patients with ulcerative
colitis. Dig Dis Sci. 59:2444–2451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, He D, Dong XD, Dong F, Wang J,
Wang L, Tang J, Hu DN, Yan D and Tu L: MicroRNA-124a is
epigenetically regulated and acts as a tumor suppressor by
controlling multiple targets in uveal melanoma. Invest Ophthalmol
Vis Sci. 54:2248–2256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He T, Feng G, Chen H, Wang L and Wang Y:
Identification of host encoded microRNAs interacting with novel
swine-origin influenza A (H1N1) virus and swine influenza virus.
Bioinformation. 4:112–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim YH, Lee WK, Lee EB, Son JW, Kim DS and
Park JY: Combined effect of metastasis-related MicroRNA, miR-34 and
miR-124 family, methylation on prognosis of non-small-cell lung
cancer. Clin Lung Cancer. 18:e13–e20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao
R and Cui L: The tumor suppressor miR-124 inhibits cell
proliferation by targeting STAT3 and functions as a prognostic
marker for postoperative NSCLC patients. Int J Oncol. 46:798–808.
2015.PubMed/NCBI
|
|
36
|
Paz-Ares L, de Marinis F, Dediu M, Thomas
M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et
al: Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): A double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SH, Jung SH, Kim TM, Rhee JK, Park HC,
Kim MS, Kim SS, An CH, Lee SH and Chung YJ: Whole-exome sequencing
identified mutational profiles of high-grade colon adenomas.
Oncotarget. 8:6579–6588. 2017.PubMed/NCBI
|
|
38
|
Perez-Carbonell L, Sinicrope FA, Alberts
SR, Oberg AL, Balaguer F, Castells A, Boland CR and Goel A:
MiR-320e is a novel prognostic biomarker in colorectal cancer. Br J
Cancer. 113:83–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Franchina T, Amodeo V, Bronte G, Savio G,
Ricciardi GR, Picciotto M, Russo A, Giordano A and Adamo V:
Circulating miR-22, miR-24 and miR-34a as novel predictive
biomarkers to pemetrexed-based chemotherapy in advanced non-small
cell lung cancer. J Cell Physiol. 229:97–99. 2014.PubMed/NCBI
|
|
40
|
Wang F, Lou JF, Cao Y, Shi XH, Wang P, Xu
J, Xie EF, Xu T, Sun RH, Rao JY, et al: miR-638 is a new biomarker
for outcome prediction of non-small cell lung cancer patients
receiving chemotherapy. Exp Mol Med. 47:e1622015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanaka K, Miyata H, Yamasaki M, Sugimura
K, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M and
Doki Y: Circulating miR-200c levels significantly predict response
to chemotherapy and prognosis of patients undergoing neoadjuvant
chemotherapy for esophageal cancer. Ann Surg Oncol. 20:(Suppl 3).
S607–S615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyamae M, Komatsu S, Ichikawa D,
Kawaguchi T, Hirajima S, Okajima W, Ohashi T, Imamura T, Konishi H,
Shiozaki A, et al: Plasma microRNA profiles: Identification of
miR-744 as a novel diagnostic and prognostic biomarker in
pancreatic cancer. Br J Cancer. 113:1467–1476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Frères P, Josse C, Bovy N, Boukerroucha M,
Struman I, Bours V and Jerusalem G: Neoadjuvant chemotherapy in
breast cancer patients induces miR-34a and miR-122 expression. J
Cell Physiol. 230:473–481. 2015. View Article : Google Scholar : PubMed/NCBI
|