Introduction
Retinal and choroidal vascular diseases can be
divided into two groups: Subretinal neovascularization (NV), in
which new blood vessels penetrate the outer retina and subretinal
space (usually avascular), and retinal vascular diseases, in which
the retinal vessels undergo neovascularization or leakage (1). In the retina, new vessels branching
off from existing vessels can initially enter the retina. These
cases are termed intraretinal microvascular abnormalities (IRMAs)
(2). Unlike normal retinal
vessels, NV and IRMAs have few tight junctions, and leak plasma
into the vitreous gel and surrounding tissue (3). This causes the vitreous gel to
contract, degenerate and eventually collapse, which puts pressure
on the retina (1). Vitreous
hemorrhage and even intact NV may cause the retina to detach.
Traction retinal detachment can involve the macula, which drives
and directs vision, causing severe visual loss (4).
The pathogenesis of these vascular diseases have
been shown to be associated with hypoxia, chronic inflammation,
high levels of angiogenic factors, including stromal cell-derived
factor 1-α, vascular endothelial growth factor (VEGF), and
platelet-derived growth factor subunit B (5). In addition, inflammation is reported
to contribute substantially to several vascular events, including
the development and rupture of atherosclerotic plaques,
angiogenesis, ischemia/reperfusion damage, and formation of aortic
aneurysms (6). The infiltration of
inflammatory cells into the vascular tissues causes the release of
proteases, reactive oxygen species and cytokines, and triggers
vasoconstriction/vasodilation (7),
thrombus formation (8,9), neointimal growth (10,11),
tissue remodeling and angiogenesis (11,12).
The interleukin (IL)-17 cytokine family contains six
members (IL-17A-F) and at least five receptors (IL-17RA-E), as
reported by Moseley et al (13). IL-17, a known pro-inflammatory
cytokine exerts several biologic activities, including the
induction of prostaglandin E2 (PGE2), IL-6 and IL-8. It also
increases the expression of intercellular adhesion molecule
(ICAM)-1 in keratinocytes and fibroblasts (14–17),
and stimulates the secretion of IL-1β, stromelysin and tumor
necrosis factor (TNF)-α by macrophages (18). IL-17 receptor (IL-17R) is a type 1
transmembrane protein. It has an unusually long intracellular
domain (19). Although the
intracellular signaling pathway and pro-inflammatory function of
IL-17 are visibly similar to those of Toll and IL-1, IL-17R shares
no homology with other receptor sequences, thus IL-17, homologous
proteins and their viral homologues can be considered a novel
cytokine family (20).
Previously, it was reported that IL-17 contributes
to tumor angiogenesis by causing the proliferation and migration of
these vascular endothelial cells into tissues (21). However, the role of IL-17 in the
mediation of retinal neovascularization following severe injury
remains to be elucidated. The aim of the present study was to
investigate the role of IL-17 in angiogenesis by assessing the
effects of IL-17 during different stages of neovascularization,
including the proliferation, migration and tube formation of human
retinal endothelial cells (HRECs).
Materials and methods
Reagents and antibodies
The HRECs were purchased from Yaji Biological
Technologies (Shanghai, China). Neutralizing mouse anti-human IL-17
antibody (cat. no. NBP2-27338; clone 4H1524) and human recombinant
IL-17 protein (cat. no. NBP2-35040-25 µg) were purchased from Novus
Biologicals (Littleton, CO, USA). The cell counting kit-8 was
purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). Primers were synthesized by Shanghai Sangon Biological
Engineering Technology and Service Co., Ltd. (Shanghai, China).
Trypsin-EDTA was purchased from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). Rabbit anti-VEGF antibody (cat. no. sc-152)
was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The total RNA extraction kit (RNeasy Mini kit) and reverse
transcription kit (Ominiscript RT kit) were purchased from Qiagen
Sciences, Inc. (Frederick, MA, USA). Matrigel was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Dulbecco's modified Eagle's
medium (DMEM) was purchased from HyClone; GE Healthcare Life
Sciences (Logan, UT, USA). Fetal bovine serum was purchased from
PAA Laboratories; GE Healthcare Life Sciences).
Anti-phosphatidylinositol 3 kinase (PI3K; P110-α) antibody (cat.
no. 611399) was purchased from BD Biosciences (San Jose, CA, USA).
Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody
(cat. no. AF0006) was purchased from Beyotime Institute of
Biotechnology (Shanghai, China).
Cell culture and treatment of
HRECs
As described in detail previously (22), the HRECs were cultured at 37°C in a
humidified atmosphere of 95% air and 5% CO2 with DMEM
medium containing 10% fetal bovine serum. The cells were passaged
by trypsinization and reseeded at a 1:3 dilution. The HRECs were
split at ~90% confluence and culture media were replaced every 2–3
days.
The HRECs were divided into several groups, as
follows: Control group; IL-17 groups, to which specific
concentrations of 10, 50, 100, 200, 500 ng/ml of recombinant human
IL-17 protein (25 µg; cat. no. NBP2-35040; Novus Biologicals) were
added; and IL-17 antibodies (Abs) groups, to which IL-17 protein
(500 ng/ml) was combined with neutralizing anti-human IL-17
antibody (0.5 mg/ml, at a 1:1,100 dilution; cat. no. NBP2-27338;
Novus Biologicals). The HRECs were split at ~90% confluence and
subcultured in either 6-well or 96-well plates and incubated at
37°C with the protein and the antibody for either 12 h or 24 h
depending on the assay conditions.
Tube formation assay
A morphogenesis assay was performed on Matrigel for
assessment of the effect of IL-17 on the HRECs. The assay was
performed according to the procedure described in detail previously
(23). The Matrigel was placed
overnight in a 4°C refrigerator to thaw. Subsequently 60 µl of
Matrigel was immediately placed onto a pre-cooled 96-well plate,
which was placed for 30 min in a humidified CO2
incubator at 37°C for Matrigel to solidify. The HRECs, following
culture in different media in the presence or absence of IL-17,
were immediately seeded onto the solid Matrigel at a density of
1.5×104 cells per well. These plates were then placed at
37°C in a humidified atmosphere of 95% air and 5% CO2
for 12 h to allow the capillary-like structures to form.
Angiogenesis, the formation of capillary tubes, was assessed
following 12 h of cultivation. The tube-like capillary structures
were examined under an Olympus TMS inverted phase contrast
microscope (Olympus Corporation, Tokyo, Japan). The micrographs
were captured using an Olympus digital camera.
Cell migration assay
As described in detail previously (23), wound scratch assays were performed
to assess the effects of IL-17 on the migration of HRECs. This type
of assay is inexpensive and simple, and the experimental conditions
can be modified as required. After 24 h, the cells were seeded into
6-well plates at a density of 2.5×105 cells/well
reaching 70–80% confluence in a monolayer. The monolayer was slowly
and gently scratched perpendicularly with an unused 1 ml pipette
tip across the center of the well. The gap was equal to the outer
diameter of the end of the micropipette tip. Following the scratch
induction, the wells were washed with medium twice gently to remove
any detached cells. Fresh medium was then added to the wells. In
the experimental wells, the cells were treated with human
recombinant IL-17 protein or IL-17 Abs, whereas the control wells
were treated with PBS. The cells were grown at 37°C for 48 h,
during which images were captured under an Olympus TMS inverted
phase contrast microscope (Olympus Corporation, Tokyo, Japan) at 0,
12, 24 and 48 h. The length of the gap was evaluated quantitatively
using ImageJ software version 2.1.4.7 (http://rsb.info.nih.gov/ij/download.html). Each
assessment of each experimental group was repeated several
times.
Cell proliferation assay
As described in detail previously (24), cell proliferation was analyzed
using a CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). This kit measures the metabolic activity of
dehydrogenases using a tetrazolium salt. When dehydrogenases are
present, the tetrazolium salt produces a water-soluble, yellow
formazan. The quantity of formazan produced is proportional to the
number of living cells. It was measured using a thermo multi-scan
EX plate reader (Thermo Multiskan EX plate reader; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) The absorbance was measured 24
h following attachment of the HRECs to the plate and stimulation
with IL-17 protein or anti-IL-17 neutralizing antibody. The
inhibition rate (IR) of the proliferation of cells in the groups
were compared with the control groups.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis
RT-PCR analysis was performed as described in detail
previously (25). Total RNAs were
extracted from the HRECs using an RNeasy Mini kit (Qiagen, Inc.).
The RNA preparations were then treated with ribonuclease-free
deoxyribonuclease I (Thermo Fisher Scientific, Inc.) to remove
genomic DNA. Subsequently, 2 µg of total RNA was
reverse-transcribed at 42°C for 1 h in a 20 µl reaction mixture
with hexanucleotide random primers and mouse moloney leukemia virus
reverse transcriptase (Qiagen, Inc.). Subsequently, serial 4-fold
dilution of cDNA was amplified for GAPDH (Table I) and the level of transcribed cDNA
was estimated. Equal quantities of 2 µl of the cDNA products, 2.5
µl of buffer, 1 µl of forward primer and 1 µl of reverse primer (20
µM/ml; GeneScript, Nanjing, China), 2 µl of dNTP Mixture and 0.125
µl of TaKaRa TaqM (5 U/µ, cat. no. DR100A; TaKaRa Bio,
Inc., Kusatsu, Japan) in a final volume of 25 µl were then
amplified for identification of the target genes. Amplification was
performed using a GeneAmp® PCR System 9700
(Perkin-Elmer, Foster City, CA, USA) as follows: Denaturation at
94°C for 2 min, and the necessary number of cycles of 94°C for 30
sec, 55–58°C for 35 sec, 72°C for 35 sec, and a final extension
step at 72°C for 10 min (Table I).
These PCR products were fractionated on a 1.0% agarose gel and
visualized using ethidium bromide. The intensities of the bands
were determined and their ratios to GAPDH determined using ImageJ
version 2.1.4.7.
 | Table I.Sequences of the primers used for
reverse transcription-polymerase chain reaction analysis. |
Table I.
Sequences of the primers used for
reverse transcription-polymerase chain reaction analysis.
| Primer Sequence
(5′→3′) | Product size
(bp) | Annealing
temperature (°C) | Cycles (n) |
|---|
| IL-17R | F:
TTGCTTTGAGCACATGCACC | 241 | 57 | 37 |
|
| R:
GAACCAGTACACCCACAGGG |
|
|
|
| VEGF | F:
TGGTCCCAGGCTGCACCCAT | 509 | 57 | 37 |
|
| R:
CGCATCGCATCAGGGGCACA |
|
|
|
| IL-1β | F:
CCACCTCCAGGGACAGGATA | 132 | 57 | 36 |
|
| R:
AACACGCAGGACAGGTACAG |
|
|
|
| IL-6 | F:
AGTGAGGAACAAGCCAGAGC | 500 | 57 | 37 |
|
| R:
AGCTGCGCAGAATGAGATGA |
|
|
|
| IL-8 | F:
GGTGCAGTTTTGCCAAGGAG | 176 | 60 | 37 |
|
| R:
TTCCTTGGGGTCCAGACAGA |
|
|
|
| ICAM-1 | F:
CCAGGAGACACTGCAGACAG | 100 | 60 | 37 |
|
| R:
CTTCACTGTCACCTCGGTCC |
|
|
|
| GAPDH | F:
ACCACAGTCCATGCCATCAC | 452 | 57 | 25 |
|
| R:
TCCACCACCCTGTTGCTGTA |
|
|
|
Western blot analysis
As described in detail previously (22), the HRECs were split at 90%
confluence, and were cultured at 37°C in a humidified atmosphere of
95% air and 5% CO2 in media, with or without IL-17 or
IL-17 Abs, for 24 h. After 24 h, the cells were harvested using
0.25% Trypsin-EDTA, washed three times with cold PBS, and
centrifuged at 4°C for 5 min at 1,200 × g. The supernatant was
discarded and lysed in 150 ml lysis buffer (10 mM KCl, 20 mM
imidazole HCl, 10 mM EGTA, 1 mM MgCl2, 10 mM NaF, 1%
Triton, 1 mM EDTA and 1 mM sodium molybdate) at pH 6.8, to which a
protease inhibitor cocktail was added (Boehringer Mannheim,
Indianapolis, IN, USA) and then sonicated. The lysate was
centrifuged at 4°C at 9,600 × g for 15 min. The samples, quantified
using NanoDrop 2000UV-Vis spectrophotometer (20 µg/each lane;
Thermo Fisher Scientific, Inc.) were boiled for 5 min and separated
using 12.5% SDS-polyacrylamide gel electrophoresis under denaturing
conditions. It was then electroblotted onto a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). These membranes were then blocked with PBS/5% nonfat dry milk
for nonspecific binding. Finally, thee membranes were incubated at
room temperature (RT) for 1 h with the following antibodies:
Anti-VEGF (cat. no. sc-152; 1:200 dilution; Santa Cruz
Biotechnology, Inc.), anti-IL-6 (cat. no. sc-7920; 1:200 dilution;
Santa Cruz Biotechnology, Inc.), anti-IL-8 (cat. no. sc-7922; 1:200
dilution; Santa Cruz Biotechnology, Inc.), anti-ICAM (cat. no.
sc-7891; 1:200 dilution; Santa Cruz Biotechnology, Inc.) and
anti-PIK3 (cat. no. 611399; 1:500 dilution; BD Biosciences)
antibodies. The immunoblot assays were then washed with PBST and
incubated at room temperature for 1 h with a horseradish
peroxidase-labeled secondary antibody (cat. no. RPN4301 or RPN4201;
at a 1:5,000 dilution; Amersham; GE Healthcare Life Sciences,
Chalfont, UK). Enhanced chemiluminescence was used to visualize the
blots (ECL Plus; Amersham; GE Healthcare Life Sciences) according
to the manufacturer's protocol. The intensities of the protein
bands were determined and their ratios to GAPDH using ImageJ
software, version 2.1.4.7.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Data were analyzed statistically as described in
detail previously (26), using
one-way analysis of variance or two-tailed Student's t-test with
statistical software (SPSS 18.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
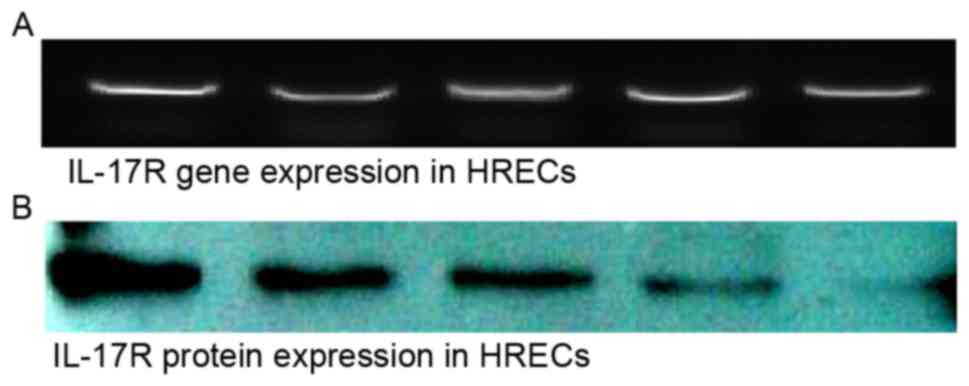
Expression of IL-17R in HRECs
The mRNA and protein expression levels of IL-17R
were detected in the HRECs. The observation of the expression of
IL-17R in the HRECs suggested the possible involvement of
IL-17/IL-17R interactions in the biological function of the HRECs
(Fig. 1).
Effects of IL-17 on tube formation of
HRECs
To determine whether IL-17 was involved in the
process of tube formation of HRECs, the HREC cells were grown in
96-well plates coated with Matrigel. Following 12 h of incubation,
the cells formed tubes, which showed that the HREC cells incubated
with IL-17 exhibited increased tube formation, compared with cells
in the control (Fig. 2). Tube
formation was quantified, and the results of the statistical
analysis indicated that IL-17 promoted tube formation.
Effects of IL-17 on the proliferation
of HRECs
To evaluate the effects of IL-17 on the biological
function of the HRECs, the role of IL-17 in HREC proliferation was
examined in vitro. In the presence of IL-17 or anti-IL-17
antibody, the HRECs were incubated for 24 h, following which cell
viability was examined. The proliferation rates of HRECs incubated
with IL-17 were higher, compared with that of cells in the control,
whereas the HRECs incubated with anti-human IL-17 Abs following
preconditioning with IL-17 protein exhibited a significant
reduction in cell proliferation, compared with the 100 or 500 IL-17
groups (Fig. 3). The
quantification of optical density (OD) values and statistical
analysis of IR demonstrated that IL-17 had the ability to promote
cell proliferation, however, the anti-IL-17 antibody prevented this
promotion. These data suggested that the enhancement in the
proliferation of HRECs following IL-17 treatment was responsible
for the effect of IL-17 on the promotion of HREC tube formation
in vitro.
Effects of IL-17 on cell
migration
The effects of IL-17 on HREC migration have not been
reported previously. To evaluate whether IL-17 affected the process
of HREC migration, an in vitro scratch wound assay was
performed to evaluate the migration ability of HRECs under
different concentrations of IL-17 or IL-17 antibody. As shown in
Fig. 4A, compared with the control
group, wound closure was significantly accelerated in the group
treated with IL-17, and the wound was almost closed at 24 h
post-injury. However, in the IL-17 antibody-treated group, the
wound area remained wide following preconditioning with IL-17
protein at 24 h. The migration distance of HRECs was quantified, as
shown in Fig. 4B. These data
showed that IL-17 effectively promoted the migration ability of
HRECs.
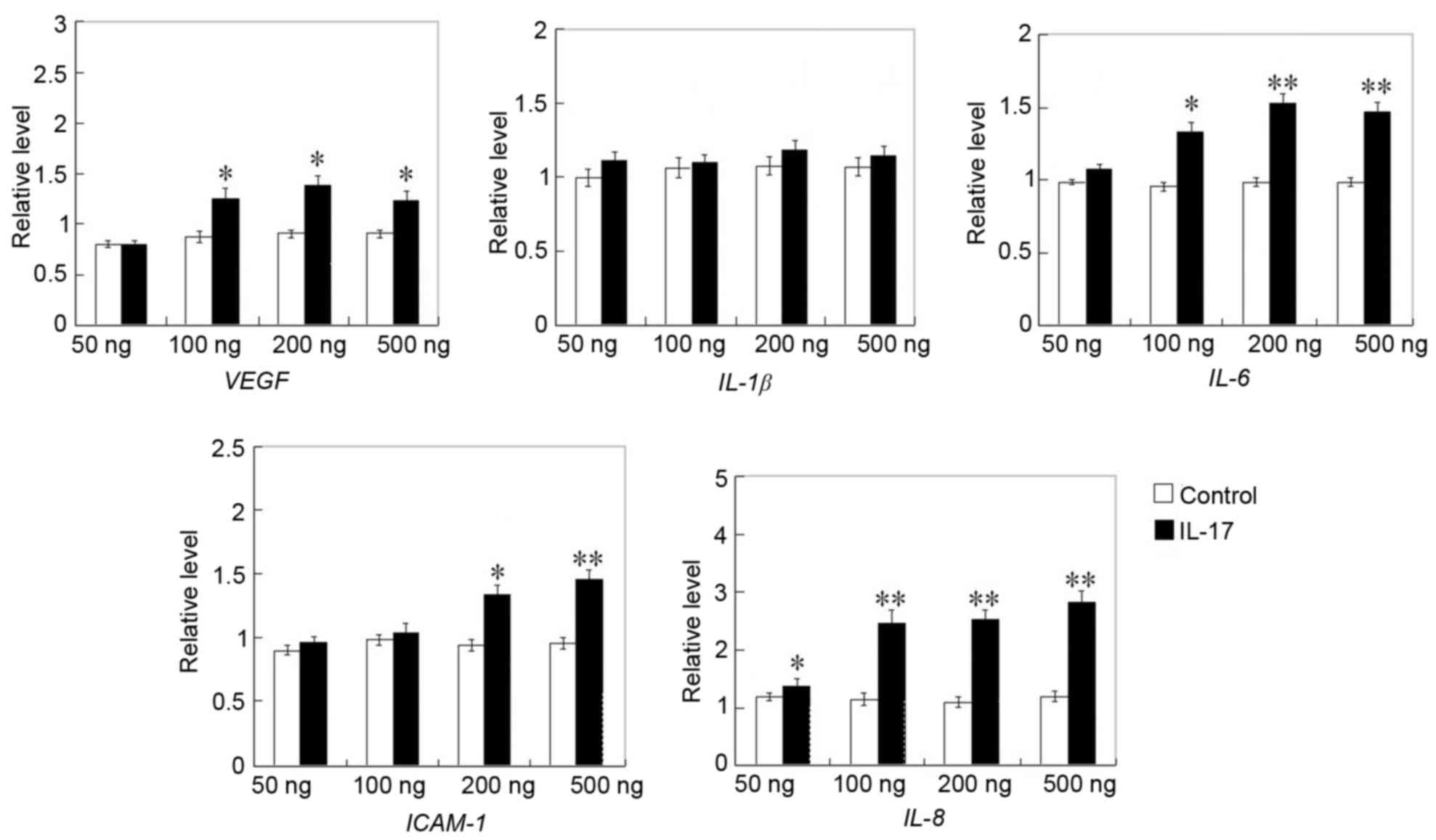
Enhanced expression of angiogenic
factors and PI3K molecules in IL-17-treated HRECs
In various situations, the outcome of angiogenic
processes is determined by the balance between anti-angiogenic and
angiogenic factors (27). In the
present study, the gene and protein expression of angiogenic
factors in HRECs were detected. Among the angiogenic-associated
factors, including VEGF, IL-8, IL-6, IL-1β and ICAM-1, which were
detected, the gene expression levels of VEGF, IL-6, IL-8 and ICAM-1
were higher in the IL-17 treated cells, compared with those in the
control groups (Fig. 5). Western
blot analysis of the protein expression of VEGF, ICAM-1, IL-6 and
IL-8 revealed that VEGF, ICAM-1, IL-6 and IL-8 were also increased
following treatment with IL-17, compared with the vehicle-treatment
cells (Fig. 6). These results
indicated that IL-17 may promote HREC tube formation, migration and
proliferation via promoting angiogenesis by enhancing the
expression of the angiogenic factors, including VEGF, ICAM-1, IL-6
and IL-8.
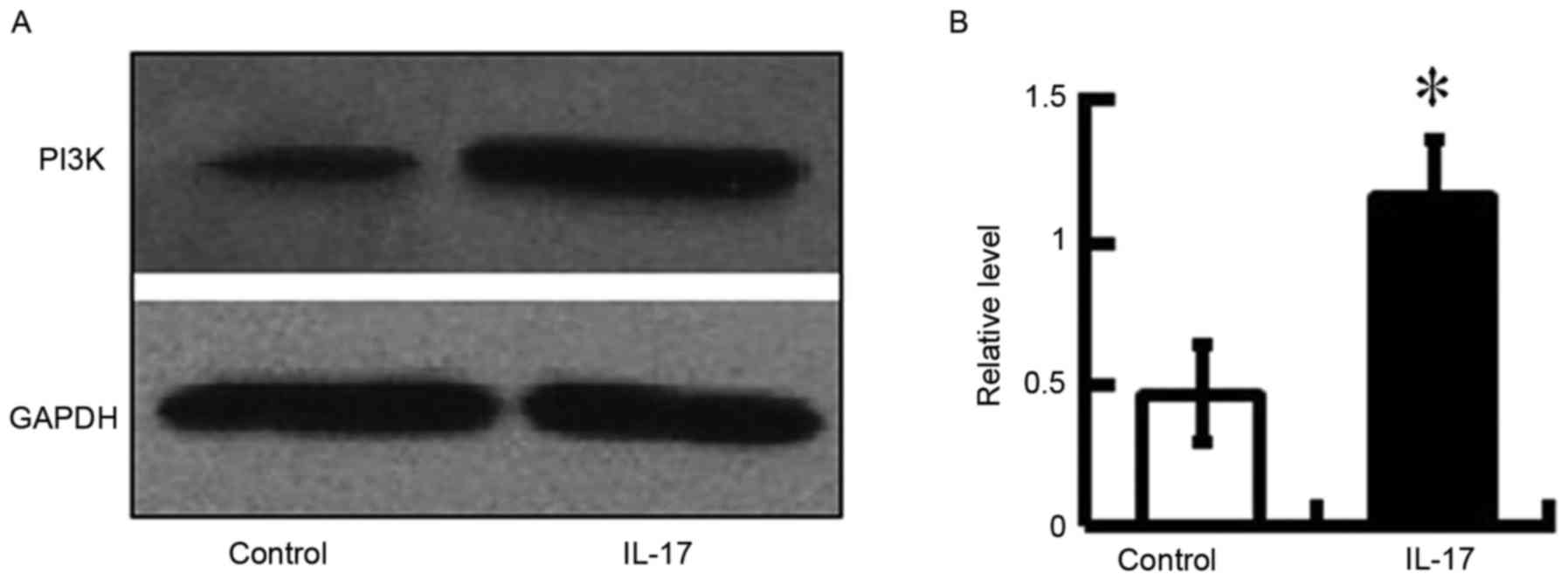
The expression of PI3K in HRECs was also examined in
the present study. The activation of PI3K is an integral component
of the VEGF signaling pathway and promotes endothelial migration
(28). The present study aimed to
determine whether IL-17 affected cell migration through the
activation of PI3K in HRECs. It was found that the expression of
PI3K was increased in the IL-17-treated mice (Fig. 7). This result suggested that IL-17
induced the activation of PI3K via the expression of VEGF. The
detection of PI3K confirmed that PI3K was activated in the cells
depending on the expression of VEGF, which was attributed to the
regulation of IL-17.
Discussion
To the best of our knowledge, results on the role of
IL-17 in angiogenesis remain contradictory. In mice, tumor growth
and lung metastasis were reported to be increased in
IL-17-deficient mice (29),
suggesting that IL-17 inhibited tumor development and
neovascularization. However, there is also evidence demonstrating
that IL-17 promotes the production of pro-angiogenic factors,
including nitric oxide, hepatocyte growth factor (HGF), chemokine
(C-X-C motif) ligand (CXCL1)/KC, CXCL2/MIP-2, PGE1, PGE2 and VEGF
by rheumatoid arthritis synovial fibroblasts, and the production of
a number of these factors is further enhanced by TNF-α (30). Of note, there are data revealing
that IL-17 alone is unable to induce angiogenesis, but can
indirectly mediate human microvascular endothelial cell growth by
promoting the mitogenic activity of HGF, basic fibroblast growth
factor (bFGF) and VEGF (31,32).
However, the effects of IL-17 on ocular neovascularization require
further investigation. The present study examined HRECs and found
that the IL-17R gene and protein were expressed in HRECs, and that
the stimulation of recombinant human IL-17 protein had significant
capillary tube formation-promoting effects. It is reasonable to
suggest that IL-17 had the potential to promote the capillary tube
formation of HRECs in vitro.
As is already known, angiogenic factors, including
bFGF and VEGF, have potent efficacy in stimulating blood vessel
formation (33). The process of
angiogenesis is tightly regulated by a series of pro- and
anti-angiogenic molecules in normal physiology, and disruption of
this balance can cause serious consequences, including
neovascularization (34). These
factors are exacerbated by various cells, including fibroblasts,
macrophages and neutrophils, and by vascular endothelial cells
themselves (35). In the present
study, the gene and protein expression levels of ICAM-1, IL-1β,
IL-6, IL-8 and VEGF were detected in HRECs. It was found that the
expression levels of ICAM-1, IL-6, IL-8 and VEGF in the
IL-17-treated cells were significantly upregulated. This indicated
that IL-17 may be involved in the process of angiogenesis by
skewing the balance towards pro-angiogenesis, and thereby causing
NV (36,37).
The angiogenic cascade is a complex and multi-step
process, with endothelial cell migration and proliferation as the
initial step in angiogenesis, followed by endothelial cell
differentiation into a capillary-like network (38). In the present study, it was found
that IL-17 had the ability to promote HREC migration and
proliferation in a dose-dependent manner. Compared with the
recombinant human IL-17 protein-treated groups, treatment with
IL-17 combined with neutralizing anti-human IL-17 Abs suppressed
the migration and proliferation of HRECs. These results are
consistent with those of a previous report (39), suggesting that IL-17 exerted
angiogenic effects on HRECs.
To further examine the mechanisms underlying the
IL-17-induced mediation of HREC capillary tube formation, the role
of IL-17 on the signal expression of PI3K/Akt was evaluated. The
process of angiogenesis is associated with several signaling
pathways. The activation of PI3K/Akt in endothelial cells is a
crucial intracellular signaling step for angiogenesis (40). Several growth factors, including
bFGF and VEGF, induce angiogenesis through the activation of these
kinases (41,42). In the present study, it was found
that IL-17 promoted the expression of active phosphorylated PI3K
(43). This suggested that IL-17
had a pro-angiogenic effect via regulating the expression of VEGF
through the activation of PI3K/Akt.
In conclusion, the present study demonstrated a
novel biologic function for IL-17 on HRECs. It promoted HREC
capillary tube formation by promoting cell proliferation and
migration. These effects may have occurred through enhancing the
expression of cytokines, including VEGF and ICAM-1, and inducing
the production of several pro-angiogenic factors, leading to an
imbalance between the activators and inhibitors of angiogenesis
present within the vascular microenvironment. These findings
indicate the potential of the effect of IL-17 on angiogenesis,
which may assist in future clinical treatment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation in China (grant no. 31600736), the
Jiangsu Province's Key Provincial Talents Program (grant no.
RC2011104), the Soochow Scholar Project of Soochow University
(grant no. R5122001 to Professor Peirong Lu), the Natural Science
Foundation of Jiangsu Province of China (grant no. BK20151208) and
the Suzhou Municipal Natural Science Foundation (grant no.
SYS201448 to Dr Gaoqin Liu).
References
|
1
|
Campochiaro PA: Ocular neovascularization.
J Mol Med (Berl). 91:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee CS, Lee AY, Sim DA, Keane PA, Mehta H,
Zarranz-Ventura J, Fruttiger M, Egan CA and Tufail A: Reevaluating
the definition of intraretinal microvascular abnormalities and
neovascularization elsewhere in diabetic retinopathy using optical
coherence tomography and fluorescein angiography. Am J Ophthalmol.
159:101–110.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ida H, Tobe T, Nambu H, Matsumura M, Uyama
M and Campochiaro PA: RPE cells modulate subretinal
neovascularization, but do not cause regression in mice with
sustained expression of VEGF. Invest Ophthalmol Vis Sci.
44:5430–5437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teke MY, Balikoglu-Yilmaz M, Yuksekkaya P,
Citirik M, Elgin U, Kose T and Ozturk F: Surgical outcomes and
incidence of retinal redetachment in cases with complicated retinal
detachment after silicone oil removal: Univariate and multiple risk
factors analysis. Retina. 34:1926–1938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Praidou A, Androudi S, Brazitikos P,
Karakiulakis G, Papakonstantinou E and Dimitrakos S: Angiogenic
growth factors and their inhibitors in diabetic retinopathy. Curr
Diabetes Rev. 6:304–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sullivan GW, Sarembock IJ and Linden J:
The role of inflammation in vascular diseases. J Leukoc Biol.
67:591–602. 2000.PubMed/NCBI
|
|
7
|
Peitzman AB, Billiar TR, Harbrecht BG,
Kelly E, Udekwu AO and Simmons RL: Hemorrhagic shock. Curr Probl
Surg. 32:925–1002. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lassila R: Inflammation in atheroma:
Implications for plaque rupture and platelet-collagen interaction.
Eur Heart J. 14:(Suppl K). 94–97. 1993.PubMed/NCBI
|
|
9
|
Nielsen JD: The effect of antithrombin on
the systemic inflammatory response in disseminated intravascular
coagulation. Blood Coagul Fibrinolysis. 9:(Suppl 3). 11–15.
1998.PubMed/NCBI
|
|
10
|
Hansson GK: Immunological control
mechanisms in plaque formation. Basic Res Cardiol. 89:(Suppl 1).
41–46. 1994.PubMed/NCBI
|
|
11
|
Wilensky RL, March KL, Gradus-Pizlo I,
Sandusky G, Fineberg N and Hathaway DR: Vascular injury, repair,
and restenosis after percutaneous transluminal angioplasty in the
atherosclerotic rabbit. Circulation. 92:2995–3005. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mach F, Schonbeck U, Fabunmi RP, Murphy C,
Atkinson E, Bonnefoy JY, Graber P and Libby P: T lymphocytes induce
endothelial cell matrix metalloproteinase expression by a
CD40L-dependent mechanism: Implications for tubule formation. Am J
Pathol. 154:229–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moseley TA, Haudenschild DR, Rose L and
Reddi AH: Interleukin-17 family and IL-17 receptors. Cytokine
Growth Factor Rev. 14:155–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao Z, Fanslow WC, Seldin MF, Rousseau AM,
Painter SL, Comeau MR, Cohen JI and Spriggs MK: Herpesvirus Saimiri
encodes a new cytokine, IL-17, which binds to a novel cytokine
receptor. Immunity. 3:811–821. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fossiez F, Djossou O, Chomarat P,
Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E,
Saeland S, et al: T cell interleukin-17 induces stromal cells to
produce proinflammatory and hematopoietic cytokines. J Exp Med.
183:2593–2603. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao Z, Painter SL, Fanslow WC, Ulrich D,
Macduff BM, Spriggs MK and Armitage RJ: Human IL-17: A novel
cytokine derived from T cells. J Immunol. 155:5483–5486.
1995.PubMed/NCBI
|
|
17
|
Aarvak T, Chabaud M, Miossec P and Natvig
JB: IL-17 is produced by some proinflammatory Th1/Th0 cells but not
by Th2 cells. J Immunol. 162:1246–1251. 1999.PubMed/NCBI
|
|
18
|
Jovanovic DV, Di Battista JA,
Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F and
Pelletier JP: IL-17 stimulates the production and expression of
proinflammatory cytokines, IL-beta and TNF-alpha, by human
macrophages. J Immunol. 160:3513–3521. 1998.PubMed/NCBI
|
|
19
|
Numasaki M, Fukushi J, Ono M, Narula SK,
Zavodny PJ, Kudo T, Robbins PD, Tahara H and Lotze MT:
Interleukin-17 promotes angiogenesis and tumor growth. Blood.
101:2620–2627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang SH, Park H and Dong C: Act1 adaptor
protein is an immediate and essential signaling component of
interleukin-17 receptor. J Biol Chem. 281:35603–35607. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suryawanshi A, Veiga-Parga T, Reddy PB,
Rajasagi NK and Rouse BT: IL-17A differentially regulates corneal
vascular endothelial growth factor (VEGF)-A and soluble VEGF
receptor 1 expression and promotes corneal angiogenesis after
herpes simplex virus infection. J Immunol. 188:3434–3446. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Z, Liu G, Xiao Y and Lu P:
Adrenomedullin22-52 suppresses high-glucose-induced migration,
proliferation, and tube formation of human retinal endothelial
cells. Mol Vis. 20:259–269. 2014.PubMed/NCBI
|
|
23
|
Liu G, Zhang W, Xiao Y and Lu P: Critical
role of IP-10 on reducing experimental corneal neovascularization.
Curr Eye Res. 40:891–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chao TI, Xiang S, Chen CS, Chin WC, Nelson
AJ, Wang C and Lu J: Carbon nanotubes promote neuron
differentiation from human embryonic stem cells. Biochem Biophys
Res Commun. 384:426–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu P, Li L, Liu G, van Rooijen N, Mukaida
N and Zhang X: Opposite roles of CCR2 and CX3CR1 macrophages in
alkali-induced corneal neovascularization. Cornea. 28:562–569.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu G, Lu P, Li L, Jin H, He X, Mukaida N
and Zhang X: Critical role of SDF-1α-induced progenitor cell
recruitment and macrophage VEGF production in the experimental
corneal neovascularization. Mol Vis. 17:2129–2138. 2011.PubMed/NCBI
|
|
27
|
Gyenge M, Amagase K, Kunimi S, Matsuoka R
and Takeuchi K: Roles of pro-angiogenic and anti-angiogenic factors
as well as matrix metalloproteinases in healing of NSAID-induced
small intestinal ulcers in rats. Life Sci. 93:441–447. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhuang Z Xiao-qin, Hu H, Tian SY, Lu ZJ,
Zhang TZ and Bai YL: Down-regulation of microRNA-155 attenuates
retinal neovascularization via the PI3K/Akt pathway. Mol Vis.
21:1173–1184. 2015.PubMed/NCBI
|
|
29
|
Kryczek I, Wei S, Szeliga W, Vatan L and
Zou W: Endogenous IL-17 contributes to reduced tumor growth and
metastasis. Blood. 114:357–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakashio A, Fujita N and Tsuruo T:
Topotecan inhibits VEGF- and bFGF-induced vascular endothelial cell
migration via downregulation of the PI3K-Akt signaling pathway. Int
J Cancer. 98:36–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryu S, Lee JH and Kim SI: IL-17 increased
the production of vascular endothelial growth factor in rheumatoid
arthritis synoviocytes. Clin Rheumatol. 25:16–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Honorati MC, Neri S, Cattini L and
Facchini A: Interleukin-17, a regulator of angiogenic factor
release by synovial fibroblasts. Osteoarthritis Cartilage.
14:345–352. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uno K, Hayashi H, Kuroki M, Uchida H,
Yamauchi Y, Kuroki M and Oshima K: Thrombospondin-1 accelerates
wound healing of corneal epithelia. Biochem Biophys Res Commun.
315:928–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martínez A: A new family of angiogenic
factors. Cancer Lett. 236:157–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sakaguchi I, Ikeda N, Nakayama M, Kato Y,
Yano I and Kaneda K: Trehalose 6,6′-dimycolate (Cord factor)
enhances neovascularization through vascular endothelial growth
factor production by neutrophils and macrophages. Infect Immun.
68:2043–2052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Edelman JL, Castro MR and Wen Y:
Correlation of VEGF expression by leukocytes with the growth and
regression of blood vessels in the rat cornea. Invest Ophthalmol
Vis Sci. 40:1112–1123. 1999.PubMed/NCBI
|
|
37
|
Lai CM, Spilsbury K, Brankov M, Zaknich T
and Rakoczy PE: Inhibition of corneal neovascularization by
recombinant adenovirus mediated antisense VEGF RNA. Exp Eye Res.
75:625–634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Griffioen AW and Molema G: Angiogenesis:
Potentials for pharmacologic intervention in the treatment of
cancer, cardiovascular diseases, and chronic inflammation.
Pharmacol Rev. 52:237–268. 2000.PubMed/NCBI
|
|
39
|
Krstić J, Jauković A, Mojsilović S,
Ðorđević IO, Trivanović D, Ilić V, Santibañez JF and Bugarski D: In
vitro effects of IL-17 on angiogenic properties of endothelial
cells in relation to oxygen levels. Cell Biol Int. 37:1162–1170.
2013.PubMed/NCBI
|
|
40
|
Wu LW, Mayo LD, Dunbar JD, Kessler KM,
Baerwald MR, Jaffe EA, Wang D, Warren RS and Donner DB: Utilization
of distinct signaling pathways by receptors for vascular
endothelial cell growth factor and other mitogens in the induction
of endothelial cell proliferation. J Biol Chem. 275:5096–5103.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dimmeler S and Zeiher AM: Akt takes center
stage in angiogenesis signaling. Circ Res. 86:4–5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsubaki M, Yamazoe Y, Yanae M, Satou T,
Itoh T, Kaneko J, Kidera Y, Moriyama K and Nishida S: Blockade of
the Ras/MEK/ERK and Ras/PI3K/Akt pathways by statins reduces the
expression of bFGF, HGF and TGF-β as angiogenic factors in mouse
osteosarcoma. Cytokine. 54:100–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bellacosa A, Testa JR, Staal SP and
Tsichlis PN: A retroviral oncogene, akt, encoding a
serine-threonine kinase containing an SH2-like region. Science.
254:274–277. 1991. View Article : Google Scholar : PubMed/NCBI
|